Abstract
Background & objectives:
TNF-α is an adipocytokine that has been implicated in the development of insulin resistance. Dysregulation of TNF-α production has been implicated in a variety of human diseases including type 2 diabetes mellitus. We aimed to find out the association of TNF-α levels with insulin resistance, body mass index and waist hip ratio; and to elicit its role with respect to duration of the disease, if any.
Methods:
50 type-2 diabetic patients attending Narayana Medical Hospital, Nellore, were studied. Body mass index and Waist hip ratio were calculated. Homeostasis model assessment method was used to calculate insulin resistance (HOMA IR) and per cent β cell function (HOMA B). Insulin was estimated by chemiluminescence method and TNF-α by ELISA method. The subjects were arbitrarily categorized into three groups based on duration of diabetes. Group 1 included subjects with diabetes of less than 5 yr duration, group 2 included diabetics of 6-10 yr duration and group 3 greater than 10 yr duration.
Results:
Our study revealed a significant correlation between TNF-α levels and BMI (P=0.006), the correlation being stronger in males when compared to females. A significant correlation was found between per cent β cell function and TNF-α (P=0.008). TNF-α correlated significantly with HOMA IR, HOMA B and insulin, in group 2 diabetes.
Interpretation & conclusions:
Our results suggest the possible role of TNF-α in the pathogenesis of type-2 diabetes mellitus and the importance of reducing obesity to prevent elevated levels of the cytokine and related complications.
Keywords: Beta cell function, Obesity, TNF-α, Type- 2 diabetes mellitus
Tumour necrosis factor alpha (TNF-α) is an adipocytokine involved in systemic inflammation and stimulates the acute phase reaction1. TNF-α is primarily secreted by macrophages, and also by a broad variety of other cells including adipocytes2,3. TNF-α inhibits insulin transduction, and has an effect on glucose metabolism4,5. Disturbances in the TNF-α metabolism have been implicated in metabolic disorders, such as obesity and insulin resistance6, indicating that perturbations of TNF-α metabolism may affect the onset of type 2 diabetes mellitus and the progression of the disease. However, the role of TNF-α in the duration and progression of the disease is not clear. The aim of this study was to investigate the levels of TNF-α in type 2 diabetes mellitus (T2DM) and to analyze its association with beta cell function, insulin resistance and body mass index (BMI) and its role with respect to duration of diabetes.
Material & Methods
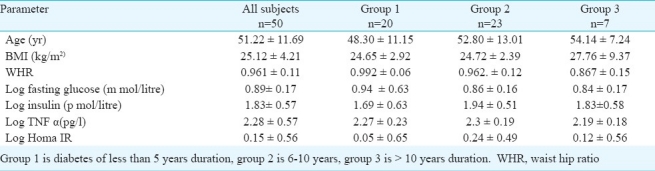
A total of 50 (male=28, female=22) diabetic subjects with mean age 51.2 11.7 yr were selected from the outpatient Department of General Medicine, Narayana Medical College and Hospital, Nellore, Andhra Pradesh (Table I). The study was conducted over a four month period (April - July 2010). The study protocol was approved by the Institutional Ethics Committee. Informed written consent was obtained from all subjects. A detailed medical history was taken and physical examination was done on all subjects.
Table I.
Characteristics of study subjects and groups
Inclusion criteria: (i) Diabetic subjects of either sex between 35-70 yr. (ii) The subjects who used only sulphonylureas in the past and currently not on any oral hypoglycaemic agents for a minimum period of 6 months were only chosen. This is to avoid any effect of the drugs on insulin secretion by pancreatic beta cells.
Studies have shown that sulphonylureas only modestly affect inflammatory markers in patients with type 2 diabetes mellitus7–10. So far only metformin and the thiazolidinediones have been shown to be directly anti-inflammatory11.
Exclusion criteria: (i) Patients on insulin or any other drugs that would affect glucose homeostasis. (ii) Clinically significant hepatic, neurological, endocrinological, or other major systemic disease, including malignancy. (iii) Patients with complications of diabetes like diabetic neuropathy, nephropathy, retinopathy, etc., based on clinical and laboratory investigations. (iv) Patients with the habit of smoking and alcohol were also excluded from the study.
Anthropometric measurements: Height, waist and hip circumference were noted using a measuring tape (to the nearest 0.1 cm), with the subjects wearing light clothes and no shoes. Waist circumference was measured at the midpoint between lower border of the rib cage and the iliac crest . Hip circumference was measured at the level of trochanter. Waist hip ratio (WHR) was calculated as waist circumference divided by hip circumference. Weight was measured to the nearest 0.1 kg using a mechanical weighing machine. BMI, defined as mass in kilograms divided by the square of height in meters, was calculated.
Biochemical assays: Blood samples were obtained by venipuncture after overnight fasting. The serum was separated and stored at -20°C. Glucose was measured using glucose oxidase method by automated chemical analyzer, Humaster-300 (GmbH, Germany). Serum insulin was determined utilizing chemiluminescence immunoassay, method (Human GmbH, Germany). Serum TNF-α was quantified using sandwich ELISA kit from eBioscience, Bender Med Systems, Austria, which has an inter-assay coefficient of variation of 7.5-10.4 per cent and a lower limit of detection of 0.5 pg/ml.
Homeostatic Model Assessment (HOMA) method, which has been validated as a reliable measure of insulin sensitivity in vivo in humans12, was used to estimate insulin resistance (HOMA IR) and % beta cell functioning (HOMA β). The subjects were arbitrarily categorized into three groups based on duration of diabetes. Group 1 included subjects with diabetes of less than 5 yr duration, group 2 included diabetics of 6-10 yr duration and group 3 greater than 10 yr duration.
Statistical analysis: Statistical analysis was performed using SPSS software (Version 12.0). Distributions of continuous variables were tested for normality, and, if appropriate, the natural log transformations of skewed variables were used in subsequent analyses. All analysis was two tailed and P<0.05 was considered as statistically significant. Spearman's correlation was used to estimate the association between the variables.
Results & Discussion
We found a strong correlation between BMI and TNF-α (P =0.006), (Table II) which is in agreement with earlier studies13,14. Bertin et al15 detected a correlation between TNF-α and BMI with indices of intra-abdominal fat tissue, but not with glycaemia or total amount of fatty mass in the body. Mishima et al14 found that serum TNF-α concentration in obese persons with type 2 diabetes mellitus depends on the degree of their insulin resistance but does not depend on BMI.
Table II.
Spearman's correlation analysis of data
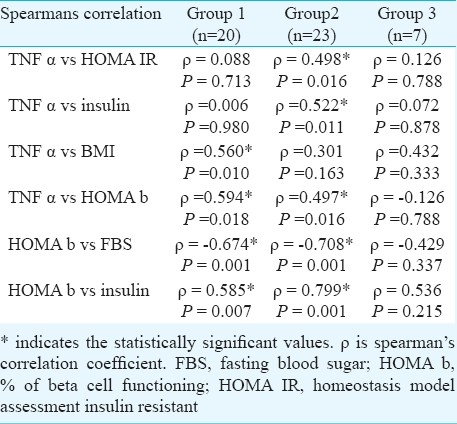
The lack of correlation with WHR suggests that TNF-α expression is not tightly linked to differences in distribution of body fat. However, previous studies have indicated the importance of regional fat deposition as a determinant of increased risk for insulin resistance and T2DM16. Further clinical studies in larger groups will be necessary to address this issue. Skewed data of variables was log transformed.
There was a significant correlation between TNF-α and insulin resistance in group 2 whereas TNF-α was significantly correlated with BMI in group 1. A significant negative correlation of HOMA b with fasting blood sugar (FBS) and a positive correlation with insulin levels in groups 1 and 2 was observed (Table II).
The loss of correlation between HOMA β with fasting sugar and insulin in group 3 when compared to group 1 and 2 indicates the progressive loss of beta cell function as the disease progresses. Thus, a better percentage of beta cell functioning implies a relative early stage of the disease.
The significant correlation of TNF-α with HOMA β and insulin may indicate the compensatory overfunctioning of beta cells to nullify the insulin resistance produced by TNF-α in the peripheral tissues. Therefore, intervention of TNF-α, before the progressive loss of beta cell function, may yield promising results in the treatment of diabetes.
Miyazaki et al17, have concluded that TNF-α increased before the onset of diabetes and further increase was not associated with insulin resistance. But Bluher et al18 reported no role of TNF-α in the genesis of early stages of insulin resistance. They attributed the genesis of insulin resistance to non-esterified fatty acids in particular18. Demirbas et al19 showed that in patients with hypertension serum TNF-α concentration increased together with increase in concentrations of insulin, and HOMA IR. No correlations were found between insulin resistance and TNF-α.
Our limited data suggest that TNF-α may play a potentially important pathophysiological role in the development of insulin resistance, particularly in males and in people with high BMI. Longitudinal studies with larger sample size need to be carried out to address this issue.
Similarly, it will be critical to assess in the future the effect of neutralizing TNF-α activity on the reversibility of the inflammatory responses and on the progression of diabetes related complications.
In conclusion, the current observations support the hypotheses that TNF-α may be involved in the aetiology of insulin resistance in type 2 diabetes mellitus
Acknowledgments
The study was done as a part of Short Term Studentship, STS 2010, Indian Council of Medical Research (ICMR), New Delhi. The study was supported financially by the Management of Narayana Medical College, Nellore, Andhra Pradesh.
Footnotes
Conflicts of interest: None.
References
- 1.Moller DE. Potential role of TNF alpha in the pathogenesis of insulin resistance and type 2 diabetes. Ternds Endocrinol Metab. 2000;11:212–7. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Cerami A. The biology of cachectin/TNF-α primary mediator of thel host response. Ann Rev Immunol. 1989;7:625–55. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 3.Giemeno RE, Klaman LD. Adipose tissue as an actibe endocrine organ;recent advances. Curr Opinion Pharmacol. 2005;5:122–8. doi: 10.1016/j.coph.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Zou C, Shao J. Role of adipocytokines in obesity- associated insulin resistance. J Nutr Biochem. 2008;19:277–86. doi: 10.1016/j.jnutbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phsosphorylation of Ser (307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 6.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin- dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72:96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- 7.Kassem SA, Raz I. Is there evidence that oral hypoglycemic agents reduce cardiovascular morbidity or mortality? No. Diabetes Care. 2009;32(Suppl 2):s337–40. doi: 10.2337/dc09-S335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, et al. Targeting C- reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 9.Pfutzner A, Marx N, Lubben G, Langenfeld M, Walcher D, Konnad T, et al. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control:results from the pioneer study. J Am Coll Cardiol. 2005;45:1925–31. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 10.Akbar DH. Effect of metformin and sulfonylurea on C- reactive protein level in well- controlled type 2 diabetics with metabolic syndrome. Endocrine. 2003;20:215–8. doi: 10.1385/ENDO:20:3:215. [DOI] [PubMed] [Google Scholar]
- 11.Yudkin JS, Panahloo A, Stehouwer C, Emeis JJ, Bulmer K, Mohamed-Ali V, et al. The influence of improved glycaemic control with insulin and sulphonylureas on acute phase and endothelial markers in type II diabetic subjects. Diabetologia. 2000;43:1099–106. doi: 10.1007/s001250051500. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Gwozdziewiczova S, Lichnovska R, Ben Yahia R, Chlup R, Hrebicek J. TNFα in the development of insulin resistance and other disorders in metabolic syndrome. Biomed Pap Med Fac Uni Palacky Olmouc Czech Repub. 2005;149:109–17. [PubMed] [Google Scholar]
- 14.Mishima Y, Kuyama A, Tada A, Takahashi K, Ishioka T, Kibata M. Relationship between tumor necrosis factor-alpha and insulin resistance in obese men with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2001;52:119–23. doi: 10.1016/s0168-8227(00)00247-3. [DOI] [PubMed] [Google Scholar]
- 15.Bertin E, Nguyen P, Guenounocu M, Durlach V, Potron G, Leutenegger M. Plasma levels of tumor necrosis factor-alpha (TNF-alpha) are essentially dependent on visceral fat amount in type 2 diabetic patients. Diabetes Metab. 2000;26:178–82. [PubMed] [Google Scholar]
- 16.Bjorntorp P. The association between obesity, adipose tissue distribution and diseases. Acta Medica Scand. 1992;222:121–34. doi: 10.1111/j.0954-6820.1987.tb05935.x. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki Y, Pipek R, Mandarino LJ, DeFronzo RA. Tumor necrosis factor alpha and insulin resistance in obese type 2 diabetic patients. Int J Obes Relat Metab Disord. 2003;27:88–94. doi: 10.1038/sj.ijo.0802187. [DOI] [PubMed] [Google Scholar]
- 18.Bluher M, Kratzsch J, Paschke R. Plasma levels of tumor necrosis factor-alpha, angiotensin II, Growth harmone, and IGF- 1 are not elevated in insulin-resistant obese individuals with impaired glucose tolerance. Diabetes Care. 2001;24:328–34. doi: 10.2337/diacare.24.2.328. [DOI] [PubMed] [Google Scholar]
- 19.Demirbas B, Guler S, Cakir B, Culha C, Aral Y. Plasma tumor necrosis factor-alpha levels and insulin resistance in nondiabetic hypertensive subjects. Horm Res. 2002;58:283–6. doi: 10.1159/000066447. [DOI] [PubMed] [Google Scholar]


