Abstract
Background & objectives:
Premature ovarian failure (POF) is defined as the cessation of ovarian function under the age of 40 yr and is characterized by amenorrhoea, hypoestrogenism and elevated serum gonadotrophin levels. The cause of POF remains undetermined in majority of the cases. This study was aimed to investigate the type and frequency of cytogenetic abnormalities in patients with idiopathic POF and also to study the role of oxidative stress in such cases.
Methods:
Seventy five women with idiopathic POF were included in this study. Chromosome analysis was done in peripheral blood lymphocytes by conventional GTG banding to identify numerical or structural abnormalities. Cytogenetically normal cases were investigated for reactive oxygen species (ROS) levels in their blood by luminol-chemiluminescence assay.
Results:
Eighteen chromosomal anomalies were identified in POF patients (24%). Majority of the cases were found to have X-chromosome abnormalities (28%). Overall median ROS range was found to be significantly higher (P<0.01) in POF patients [50480 (120,132966) RLU/min] compared to controls [340 (120,5094) RLU/min]. Among these, 50 per cent of the POF patients had higher ROS levels, 20 per cent had medium elevation and 30 per cent were found to have normal values comparable to controls.
Interpretation & conclusions:
X-chromosome anomalies were found to be the major contributor of POF. Oxidative stress may be the underlying aetiology in idiopathic premature ovarian failure. Thus the results of this study highlight the role of cytogenetic abnormalities and supraphysiological levels of ROS in causation of idiopathic POF. But the role of oxidative stress needs to be confirmed by other studies on patients from different geographical areas and from different ethnicities.
Keywords: Cytogenetic, female infertility, premature ovarian failure, reactive oxygen species
Premature ovarian failure (POF) is defined as the cessation of ovarian function under the age of 40 yr and is characterized by amenorrhoea, hypoestrogenism and elevated serum gonadotrophin concentration1. Follicle stimulating hormone (FSH) levels greater than 40 mIU/ml is indicative of ovarian failure2. POF is a heterogeneous disorder affecting one per 1000 women by 30 yr of age and affects one per 100 women by 40 yr accounting for 10 per cent of ovulatory female sterility3. A wide spectrum of pathogenic mechanisms may lead to the development of POF [autoimmune, metabolic (galactosaemia), infectious (mumps), oxidative stress and iatrogenic (anti-cancer treatment)], but chromosomal or genetic factors are most important as their presence affects subsequent management1,3. The age of menopause in an individual is determined by both genetic and environmental factors4. The idiopathic form of POF can show sporadic and familial forms.
Observation of familial cases with POF indicates the role of genetic aberrations in its pathogenesis5. The familial form of POF is rare, representing 4 to 31 per cent of all cases of POF5. A careful family history can identify other affected female members in as many as 30 per cent of cases whose relatives can then be offered genetic counselling6. Genetic causes of POF probably comprise about one third to one half of all cases7. Chromosomal defects are frequently seen, especially in young women with POF, involving the X-chromosome8. Defects of the X chromosome associated with POF include complete deletion of one X (Turner syndrome), trisomy X or partial defects in form of deletions or X-autosome translocations9. Despite the involvement of several genetic loci10, the cause of POF remains undetermined in majority of cases.
X chromosome defects may lead to the deletion or disruption of genes which are critical for ovarian function10. The deleterious effect on ovarian function results from X breakpoints that fall on the long arm between Xq13 and Xq26, the ‘critical region’ for normal ovarian function9,11. Structurally and functionally, two intact X-chromosomes are required for normal ovarian function and haplo-insufficiency results in accelerated apoptosis of germ cells12. Several genes responsible for oogenesis are present on the critical region of X chromosome and interrupted by balanced translocations which may lead to POF13,14.
The role of oxidative stress (OS) in pathogenesis of POF has not been studied extensively. In a recent study15 it was reported that administration of coenzyme Q in POF patients with high ROS levels improves the embryo quality. High superoxide ion levels lead to a decrease in the bioavailability of nitric oxide and an increase in reactive oxygen species levels and oxidative stress16. As compared to spermatozoa, female germ cells develop under hypoxic condition in the ovarian cortex, however exposure to supraphysiological levels of reactive oxygen species (ROS) are detrimental to developing oogonia3. In another study17 it was suggested that increased production of ROS contributes to oophoritis associated with premature ovarian insufficiency. High ROS levels induce mitochondrial DNA alterations and lead to mitochondria dysfunction18. This could lead to low production of ATP due to impaired oxidative phosphorylation and thus to impaired oogenesis, low oocyte number and POF.
Determining the chromosome constitution or karyotype of idiopathic POF patients is the first step in identification of genetic origin of POF. The percentage of chromosomal abnormalities in POF varies significantly in different studies due to different inclusion criteria and population enrolled for study. This study was aimed to investigate the type and frequency of cytogenetic abnormalities and to assess the ROS levels in cytogenetically normal idiopathic POF patients.
Material & Methods
Seventy five consecutive women with idiopathic POF were selected over a 2 years period from January 2008 to July 2010, and 50 women who had two children (enrolled in family planning OPD and had regular menses with no history of infertility or autoimmune disease) were enrolled as controls. The criterion considered for inclusion as a POF patient was absence of menstruation along with a FSH concentration of >40mIU/ml. Physical and clinical examination was done to identify secondary sexual characteristics or any syndromic features. All the patients having primary amenorrhea were included in the study. A complete gynaecological, occupational and medical history was taken from each patient. Patients having secondary amenorrhea including pregnancy were excluded.
The patients were referred from Department of Obstetrics and Gynaecology to Laboratory for Molecular Reproduction and Genetics Department of Anatomy, All India Institute of Medical Sciences, New Delhi, for genetic analysis. The study was approved by institutional review board (IRB#IRB00006862; All India Institute of Medical Sciences, Delhi, India). The study protocol was approved by institutional review board, and all participants gave their written informed consent. All cases which were found be cytogenetically normal were assessed for ROS levels.
Conventional cytogenetic analysis
Chromosome preparation19: In POF patients, chromosome analysis was done to identify any numerical or structural chromosomal aberrations. Lymphocyte cultures were set up and chromosomes were analyzed by G banding. Heparinized blood (5 ml) was drawn and kept in an upright position at 37°C for 30 min. This helps in the separation of plasma from red blood cells. The plasma and the settled lymphocyte (PLS, plasma lymphocyte suspension) in buffy coat was tapped gently and mixed together. The needle was bent and 0.5 ml of PLS was transferred into a sterile culture vial containing 5 ml of media RPMI-1640 and 0.2 ml phytohaemagglutinin (PHA; Gibco). The cultures were incubated for 72 h at 37°C. After 70 h of incubation, 0.1 ml (0.2%) of colcemid (Gibco) was added to the cultures. At 72 h the samples were washed for removing colcemid and centrifuged at 67 g for 10 min. The supernatant was discarded and freshly prepared pre-warmed hypotonic solution (0.56% KCl) was added and incubated for 20-25 min at 37°C. The cell suspension was centrifuged again and after discarding the supernatant, freshly prepared chilled carnoy's fixative (methanol:acetic acid/3:1) was added to the cell pellet slowly. At least three changes of fixative were given till the pellet became pale. Two drops of cell suspension were dropped from a height of 30-100 cm on a clean wet slide.
G-banding20: Giemsa staining of chromosome preparation after proteolytic enzyme treatment revealed G-banding. The 3 days old matured unstained chromosome preparations were flooded with 0.25 per cent Trypsin for 10-15 sec, the slides were rinsed in phosphate buffer saline pH 7.4. The slides were stained in 2 per cent Gimesa stain for 5-7 min and were washed in distilled water. Metaphases were analyzed using cytovision software (Applied Imaging, USA) (Zeiss Microscope, Olympus, Japan) and were classified according to ISCN 200521. At least 20 metaphases in each patient were analyzed and karyotyped. In case of abnormal karyotype more metaphases (~100) were examined.
ROS measurement: One ml of fresh blood was centrifuged at 300 g for 7 min and the plasma was removed and transferred into a microcentrifuge tube. To 400 μl of the plasma 10 μl of 5M luminol (5-amino-2,3,-dihydro-1,4-phthalazinedione; Sigma, USA), was added to the mixture and served as a probe. Levels of ROS were assessed by measuring the luminol-dependant chemiluminescence with the luminometer (Sirius, Berthold) in the integrated mode for 15 min. The results were expressed as median (minimum, maximum) relative light unit/minute (RLU/min) per 400 μl of blood plasma.
Statistical analysis: The significance difference in ROS levels between patients and controls was found by applying Mann-Whitney test. The values were expressed as median (minimum, maximum) range. FSH levels, age and height were compared by one-way ANOVA. P<0.005 was considered as significant. The statistical analyses were performed using Stata 9.0 version (StataCorp LP, TX, USA).
Results
A total of 75 cases (19.27±1.72) yr and 50 female controls (18.98±1.54) yr were analysed. Eighteen chromosome variations were identified in POF patients (24%) by conventional cytogenetic analysis of 50 G-banded well spread metaphases. X-chromosome variations were found in 21 (28%) cases. 45,XO/46,XX mosaicisms or other complex mosaicisms involving X chromosome were also observed in patients. 45,XO chromosome complement was found in 11 (14.66%) cases whereas 8 (10.66%) were found to be Turner mosaic. Three cases (4%) were found to harbour autosomal chromosome abnormality. Chromosomal complement of patients with their clinical phenotype is shown in Table I and Fig.
Table I.
Clinical phenotype of POF patients with their chromosome complement

Fig.
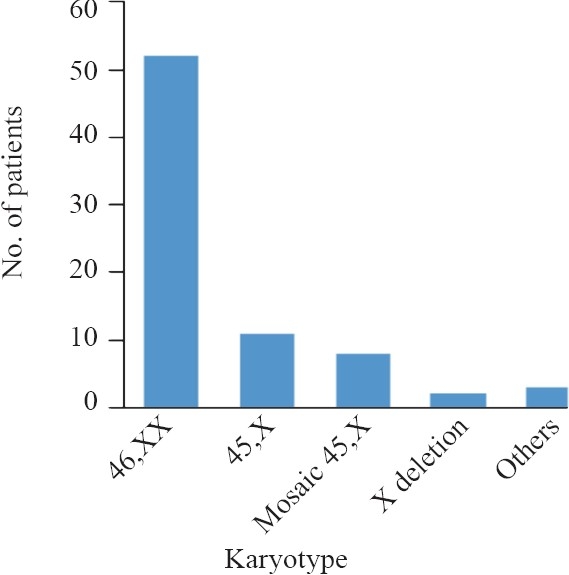
Karyotype distribution of POF patients.
ROS levels were assessed in all cytogenetically normal cases and controls. Overall median ROS range was found to be significantly higher (P<0.05) in POF patients 50480 (120, 132966) RLU/min when compared to controls [340 (120, 5094) RLU/min]. However, 50 per cent of the POF patients had very high ROS levels, 20 per cent had medium elevation and 30 per cent were found to have normal values comparable to controls.
The mean values of the FSH, age and height of the patients and controls are shown in Table II. The Table describes the values according to the karyotype distribution of the patients. A significant difference (P<0.01) in mean FSH level was found between Turner and other POF patients. Whereas patients (other than 45,XO) harbouring X-chromosome abnormalities had higher (P<0.001) FSH levels than patients having normal karyotype. The mean height was found to be significantly lower (P<0.001) in POF patients as compared to controls. 45,XO patients showed significantly (P<0.001) low mean height as compared to other POF patients. No family showing blepharophimosis or with conditions related to cancer cluster nor families presenting cases having fragile X syndrome or other forms of mental retardation were found.
Table II.
Mean values of FSH, age and height of the POF patients

Discussion
In this study chromosomal abnormalities were found in 24 per cent of POF patients. The frequency of sex chromosome abnormalities was found to be 28 per cent, similar to that reported3,8,9. The involvement of the X chromosome may not be limited to POF but may influence the broader spectrum of menopausal age22. In this study, X chromosome structural anomalies were found in 8 per cent of the POF patients (three cases of Xq deletions, one case of ring X chromosome and two cases of iso X-chromosome). One of three patient showed deletion in Xq13.3-q21.1 which involved the critical region. Deletions involving Xq region are known to cause amenorrhoea between the age of 16 and 21 yr because of haplo-insufficiency of genes resulting in accelerated apoptosis of germ cells8,11. In the presence of only one X chromosome as in Turner's syndrome, ovarian follicles degenerate by birth due to accelerated atresia of germ cells which may be the result of a lack of diploid dosage of one or more vital genes, both alleles of which are active in oogenesis12. Gonadal dysfunction due to chromosomal abnormalities can occur for several reasons commonly linked to gene expression being disrupted by the rearrangement14. Our findings related to breakpoints falling within a region of repetitive elements in Xq are in favour of alterations in chromosome meiotic pairing or position effect. Some microdeletions may be escaped by routine cytogenetic analysis. For detecting these microdeltions comparative genome hybridization (CGH) would be ideal method.
Two cases of dicentric isochromosome X, dic (Xp-), with premature ovarian failure have been reported23. Turner's syndrome associated with X ring chromosome, r(X) is rare and there has been no report of pregnancy in Turner's syndrome with 45,X/46,Xr(X)/46,XX mosaicism except in one study24. We identified a patient with 46,Xr(X)/46,XX,i(2q)/46,X mosaicism. The physical and clinical characteristics of the patient were similar to Turner's syndrome. Very few cases with r(X) chromosome have been reported in the literature25 and as it is present with Turner cell line the correlation of r(X) and ovarian phenotype in patient could be suggestive only. Chromosome 2 contains the gene for FSH and leutinizing hormone (LH) receptors and mutation in FSH receptor gene in a Finnish family presented several cases of primary amenorrhoea26. Chromosome abnormalities involving chromosomes 9 and 2 have not been reported in POF, though inv(9) has been previously reported in female infertility and other congenital anomalies26. The mechanisms of origin of inversions 9 are highly complex. It was not possible to study the karyotype of family members of these patients’ pedigrees in order to exclude a polymorphic inheritance pattern of these chromosome variants.
In this study ROS levels were found to be significantly higher in cytogenetically normal POF cases as compared to controls. Supraphysiological free radicals damage both nuclear and mtDNA and induce mutations, and result in decreased ATP production18,28,29. The pathological role of ROS has been demonstrated in various female reproductive disorders such as endometriosis, polycystic ovarian disease, spontaneous abortions, hydatidiform mole and pre-eclampsia30. Oxidative stress induces accumulation of lipid peroxide products (MDA, acetaldehyde, etc.) and may also alter protein structure which may change its function and cause chemotaxis of inflammatory cytokines.
Though physiological free radicals promote oogenesis and follicle formation, excess ROS levels adversely affects female reproductive events30. In a study from our laboratory we found increased number of nucleotide alterations in mtDNA and raised ROS levels in POF cases compared to age matched controls18. Mitochondrial mutations adversely affect ATP production but produce high ROS levels18,28 which further induce nucleotide alterations. Low ATP levels may impair oogenesis and result in accelerated apoptosis of germ cells. The mammalian oocyte and embryo are very sensitive to oxidative stress and though physiological levels subserve several important functions, high levels impair oocyte maturation31. It has been reported that oocytes maintain a delicate balance of pro and anti-oxidants to minimize oxidative stress28. High ROS levels may thus induce mtDNA damage and results in nuclear DNA fragmentation and increased apoptosis32. Accelerated apoptosis may result in premature cessation of ovarian function.
Women with POF have a 5 per cent chance of spontaneous conception after diagnosis, as in some cases hormone levels fluctuate and return to biochemical normality5. The diagnosis of POF may have a deleterious psychological impact and the emotional importance of the condition is often underestimated. In case where POF can be diagnosed at an early age the patient can be counselled for ova cryopreservation to be used later for ART. Cytogenetic analysis should be considered for women presenting with unexplained POF, even when there are no other clinical features suggestive of chromosomal abnormality. In clinical cytogenetics, the precise identification of a chromosomal abnormality is a key factor when considering genotype-phenotype correlation and with advancement of new high resolution techniques like CGH microdeletions can also be detected.
In conclusion, we identified 28 per cent cases with X chromosome abnormalities in a small population of idiopathic POF patients and 50 per cent cytogenetically normal cases had raised ROS levels. Women having POF before the age of 30 should undergo cytogenetic and ROS level analysis. Since a large number of idiopathic cases have raised ROS levels, such cases if diagnosed at an early stage, can be administered antioxidants which may prevent mt and nuclear DNA damage and can also delay or slow down germ cell apoptosis. Thus, cytogenetic and oxidative stress analyses are important tools in the evaluation of idiopathic cases of POF.
References
- 1.Santoro N. Mechanisms of premature ovarian failure. Ann Endocrinol. 2003;64:87. [PubMed] [Google Scholar]
- 2.Hickey M, Balen A. Menstrual disorders in adolescence: investigation and management. Hum Reprod Update. 2003;9:493–504. doi: 10.1093/humupd/dmg038. [DOI] [PubMed] [Google Scholar]
- 3.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–6. [PubMed] [Google Scholar]
- 4.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83:1875–80. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 5.Conway GS, Kaltsas G, Patel A, Davies MC, Jacobs HS. Characterization of idiopathic premature ovarian failure. Fertil Steril. 1996;64:337–41. doi: 10.1016/s0015-0282(16)58095-9. [DOI] [PubMed] [Google Scholar]
- 6.van Kasteren YM, Hundscheid RD, Smits AP, Cremers FP, van Zonneveld P, Braat DD. Familial idiopathic premature ovarian failure: an overrated and underestimated genetic disease? Hum Reprod. 1999;14:2455–9. doi: 10.1093/humrep/14.10.2455. [DOI] [PubMed] [Google Scholar]
- 7.Santoro N. Research on the mechanisms of premature ovarian failure. J Soc Gynecol Investig. 2001;8:S10–2. doi: 10.1016/s1071-5576(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 8.Shelling AN. X chromosome defects and premature ovarian failure. Aust N Z J Med. 2000;30:5–7. doi: 10.1111/j.1445-5994.2000.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 9.Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- 10.Cordts EB, Christofolini DM, Dos Santos AA, Bianco B, Barbosa CP. Genetic aspects of premature ovarian failure: a literature review. Arch Gynecol Obstet. 2001;283:635–43. doi: 10.1007/s00404-010-1815-4. [DOI] [PubMed] [Google Scholar]
- 11.Therman E, Laxova R, Susman B. The critical region on the human Xq. Hum Genet. 1990;85:455–61. doi: 10.1007/BF00194216. [DOI] [PubMed] [Google Scholar]
- 12.Loughlin SA, Redha A, McIver J, Boyd E, Carothers A, Connor JM. Analysis of the origin of Turner's syndrome using polymorphic DNA probes. J Med Genet. 1991;28:156–8. doi: 10.1136/jmg.28.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davison RM, Fox M, Conway GS. Mapping of the POF1 locus and identification of putative genes for premature ovarian failure. Mol Hum Reprod. 2000;6:314–8. doi: 10.1093/molehr/6.4.314. [DOI] [PubMed] [Google Scholar]
- 14.Sala C, Arrigo G, Torri G. Eleven X chromosome breakpoints associated with premature ovarian failure (POF) map to a 15-Mb YAC contig spanning Xq21. Genomics. 1997;40:123–31. doi: 10.1006/geno.1996.4542. [DOI] [PubMed] [Google Scholar]
- 15.Bentov Y, Esfandiari N, Burstein E, Casper RF. The use of mitochondrial nutrient to improve the outcome of infertility treatment in older patients. Fertil Steril. 2010;93:272–5. doi: 10.1016/j.fertnstert.2009.07.988. [DOI] [PubMed] [Google Scholar]
- 16.Lu B, Poirier C, Gaspar T, Gratzke C, Harrison W, Busija D, et al. A mutation in the inner mitochondrial membrane peptidase 2-like gene (Immp2l) affects mitochondrial function and impairs fertility in mice. Biol Reprod. 2008;78:601–10. doi: 10.1095/biolreprod.107.065987. [DOI] [PubMed] [Google Scholar]
- 17.Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig. 2001;8(Suppl):S40–2. doi: 10.1016/s1071-5576(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 18.Kumar M, Pathak D, Kriplani A, Ammini AC, Talwar P, Dada R. Nucleotide variations in mitochondrial DNA and supra-physiological ROS levels in cytogenetically normal cases of premature ovarian insufficiency. Arch Gynecol Obstet. 2010;282:695–705. doi: 10.1007/s00404-010-1623-x. [DOI] [PubMed] [Google Scholar]
- 19.Rooney DE, Czepulkowski BH. Human Cytogenetics. Oxford Press, UK: IRL Press; 1994. [Google Scholar]
- 20.Sumner AT. The nature and mechanisms of chromosome banding. Cancer Genet Cytogenet. 1982;6:59–87. doi: 10.1016/0165-4608(82)90022-x. [DOI] [PubMed] [Google Scholar]
- 21.Mitelman F, editor. ISCN: An international system for human cytogenetic nomenclature. Basel: Karger; 2005. [Google Scholar]
- 22.van Asselt K, Kok HS, Putter H, Wijmenga C, Peeters PH, van der Schouw YT, et al. Linkage analysis of extremely discordant and concordant sibling pairs identifies quantitative trait loci influencing variation in human menopausal age. Am J Hum Genet. 2004;74:444–53. doi: 10.1086/382136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu MH, Tzeng CC, Kuo PL. Dicentric isochromosome X with premature ovarian failure: report of two cases. J Formos Med Assoc. 1993;92:848–50. [PubMed] [Google Scholar]
- 24.Taga M, Minaguchi H, Saotome K. Two pregnancies in a 45,X/46,Xr(X)/46,XX Turner mosaic patient. A case report. Gynecol Obstet Invest. 1996;42:206–8. doi: 10.1159/000291958. [DOI] [PubMed] [Google Scholar]
- 25.Blumenthal AL, Allanson JE. Turner syndrome in a mother and daughter: r(X) and fertility. Clin Genet. 2008;52:187–91. doi: 10.1111/j.1399-0004.1997.tb02543.x. [DOI] [PubMed] [Google Scholar]
- 26.Aittomaki K, Herva R, Stenman UH, Juntunen K, Ylostalo P, Hovatta O, et al. Clinical features of primary ovarian failure caused by a point mutation in the follicle-stimulating hormone receptor gene. J Clin Endocrinol Metab. 1995;81:3722–6. doi: 10.1210/jcem.81.10.8855829. [DOI] [PubMed] [Google Scholar]
- 27.Davalos IP, Rivas F, Ramos AL, Galaviz C, Sandoval I, Rivera H. Inv [9] (p24q13) in three sterile brothers. Ann Genet. 2000;43:51–4. doi: 10.1016/s0003-3995(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 28.Venkatesh S, Deecaraman M, Kumar R, Shamsi MB, Dada R. Reactive oxygen species, its role in the pathogenesis of mitochondrial DNA (mtDNA) mutations in male infertility. Indian J Med Res. 2009;129:127–37. [PubMed] [Google Scholar]
- 29.Kumar R, Venkatesh S, Kumar M, Tanwar M, Shasmsi MB, Kumar R, et al. Oxidative stress and sperm mitochondrial DNA mutation in idiopathic oligoasthenozoospermic men. Indian J Biochem Biophys. 2009;46:172–7. [PubMed] [Google Scholar]
- 30.Murphy AA, Palinski W, Rankin S, Morales AJ, Parthasarathy S. Evidence for oxidatively modified lipid-protein complexes in endometrium and endometriosis. Fertil Steril. 1998;69:1092–4. doi: 10.1016/s0015-0282(98)00087-9. [DOI] [PubMed] [Google Scholar]
- 31.Harvey AJ, Kind KL, Thompson JG. Redox regulation of early embryo development. Reproduction. 2002;123:479–86. doi: 10.1530/rep.0.1230479. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesh S, Kumar M, Sharma A, Kriplani A, Ammini AC, Talwar P, et al. Oxidative stress and ATPase6 mutation is associated with primary ovarian insufficiency. Arch Gynecol Obstet. 2010;282:313–8. doi: 10.1007/s00404-010-1444-y. [DOI] [PubMed] [Google Scholar]


