Abstract
BACKGROUND:
Annually, millions of children are exposed to anesthetic agents that cause apoptotic neurodegeneration in immature animals. To explore the possible significance of these findings in children, we investigated the association between exposure to anesthesia and subsequent (1) learning disabilities (LDs), (2) receipt of an individualized education program for an emotional/behavior disorder (IEP-EBD), and (3) scores of group-administered achievement tests.
METHODS:
This was a matched cohort study in which children (N = 8548) born between January 1, 1976, and December 31, 1982, in Rochester, Minnesota, were the source of cases and controls. Those exposed to anesthesia (n = 350) before the age of 2 were matched to unexposed controls (n = 700) on the basis of known risk factors for LDs. Multivariable analysis adjusted for the burden of illness, and outcomes including LDs, receipt of an IEP-EBD, and the results of group-administered tests of cognition and achievement were outcomes.
RESULTS:
Exposure to multiple, but not single, anesthetic/surgery significantly increased the risk of developing LDs (hazard ratio: 2.12 [95% confidence interval: 1.26–3.54]), even when accounting for health status. A similar pattern was observed for decrements in group-administered tests of achievement and cognition. However, exposure did not affect the rate of children receiving an individualized education program.
CONCLUSIONS:
Repeated exposure to anesthesia and surgery before the age of 2 was a significant independent risk factor for the later development of LDs but not the need for educational interventions related to emotion/behavior. We cannot exclude the possibility that multiple exposures to anesthesia/surgery at an early age may adversely affect human neurodevelopment with lasting consequence.
WHAT'S KNOWN ON THIS SUBJECT:
Exposure to virtually all anesthetic drugs has been shown to cause neurodegeneration in young animals. Studies of learning and cognition in children exposed to anesthesia and surgery have been few, have relied on single outcome measures, and have not controlled for comorbidity.
WHAT THIS STUDY ADDS:
In this study of children exposed to anesthesia/surgery before the age of 2, multiple group and individual measures of learning and behavior are examined by using a matched design with adjustment for comorbidity using 2 separate methods.
Exposure of developing animal brains to anesthetics may cause neurodegenerative changes with adverse effects on learning and behavior.1–8 Implicated drugs include N-methyl-d-aspartate glutamate receptor agonists and γ-aminobutyric acid antagonists. When these drugs, including virtually all anesthetics, are administered to young animals (including primates), neurodegeneration results.2,4,5,7,9,10 Few studies provide insight into the potential clinical significance of these observations.11–13 Recently, we reported an association between multiple, but not single, exposures to anesthesia/surgery before the age of 4 and subsequent learning disabilities (LDs).14 In other clinical studies broad measures have been examined of behavior and learning using group-administered tests of achievement, teacher rating scales, or diagnostic codes, with varying results.11–13 In no study to date have multiple types of outcomes been examined simultaneously to determine which may be associated with exposures to anesthesia/surgery. Also, the need for anesthesia may be merely a marker for other factors, such as the condition necessitating surgery or coexisting disease that could confound the relationship between anesthesia/surgery and neurodevelopmental outcomes. As noted in letters, editorials, and reviews,15–17 this is a potential weakness of all observational studies (including our previous study).
In this study, we used a population-based birth cohort and employed a matched cohort design that included adjustment for health status, taking advantage of access to complete medical charts for all members of the cohort. We examined LDs, the receipt of an individualized education program (IEP) for disorders of emotion/behavior or speech-language, and group achievement tests to determine if an association exists between exposure to anesthesia/surgery during the first 2 years of life and the development of these outcomes. In particular, we sought to determine if the effects of multiple exposures on LDs observed in our previous analysis persisted when differences in health status were accounted for, and whether group-administered achievement tests and an indicator of emotional/behavioral disorders also were affected.
MATERIALS AND METHODS
The Mayo Clinic and Olmsted Medical Center institutional review boards approved this study. A previously described birth cohort of children born in Rochester, Minnesota, was used to identify cases and controls.18–20 The cohort includes all children (n = 8548) born between January 1, 1976, and December 31, 1982, to mothers residing in the townships comprising Independent School District 535. Children who emigrated or died before the age of 5 (n = 2830), were severely mentally retarded (n = 19) (full range IQ < 50 or neurodevelopmentally unable to test), or did not provide research authorization (n = 342) were excluded.19 Of the 19 with severe mental retardation, all but 1 were known to have profound neurodevelopmental deficits before exposure to anesthesia and surgery (1 had an intraoperative stroke). All schools (public, private, home) gave permission to access complete records for every child including results of all individually administered tests of intelligence (Wechsler Intelligence Scale for Children) and academic achievement (Woodcock-Johnson Battery), all group-administered tests of achievement (California Achievement Test [CAT]) and cognitive ability (Test of Cognitive Skills [TCS]), as well as educational and socioeconomic information.
All cohort members who underwent a procedure requiring general anesthesia before their second birthday (cases) were identified and matched with 2 unexposed controls from the cohort. In this manuscript we use term “exposure to anesthesia,” “anesthesia/surgery” or simply “exposure” as a description of the use of general anesthesia for either surgical or diagnostic procedures. Cases were matched to controls on the basis of factors known to influence the incidence of LDs, including gender, mother's education (<12, 12, or >12 years), birth weight (< 2500 or ≥2500 g), gestational age (<32, 32–36, or ≥37 weeks), and birth date ±12 months. Exact matching was required with the exception of gestational age for which criteria were relaxed allowing those with gestational age of 32 to 36 weeks to be matched with either of the other categories.
Health Status
Two methods were used to quantify health status: the American Society of Anesthesiologists Physical Status (ASA-PS) and the Johns Hopkins adjusted clinical groups (ACG) Case-Mix System.21–24 The ASA-PS was assigned irrespective of the need for surgery on the basis of health data available at the age of 5 years. On the basis of review of the complete medical chart, assignment (unblinded) was performed by 2 pediatric anesthesiologists (Drs Flick and Wilder); a third (Dr Warner) adjudicated discrepancies. The ACG Case-Mix System uses International Classification of Diseases, Ninth Revision, codes. Codes for each child entered up to and during the fifth year of life were assigned by computer to 1 of 32 unique morbidity clusters designated as aggregated diagnostic groups (ADGs) on the basis of criteria including duration of care, severity, diagnostic certainty, etiology, and need for subspecialty care.
Outcomes
Need for Individualized Educational Program
By law, schools are required to provide IEPs to those qualifying for special education because of problems with development, learning, and behavior.25 The need for an IEP has been used as a measure of learning and behavior in a variety of settings.26,27 School records were abstracted to determine those children for whom an IEP was developed for disorders of emotion/behavior (IEP-EBD). Indications for an IEP-EBD include mood disorders such as anxiety and depression, unusual behavior patterns, and aggression and impulsivity. The receipt of an IEP for speech/language (IEP-SL) was also noted.
Learning Disabilities
LDs, including subtypes (reading, written language, mathematics), were identified using the results of individually administered tests of achievement and research criteria detailed in previous reports.18,20,28 Children considered to have LDs met research criteria before the age of 19 for at least 1 LD subtype by any 1 of 3 formulas.18,20,28
Group-Administered Achievement Tests
Achievement and cognition were assessed using results from the last available group-administered CAT (including subscales for written language, mathematics, phonics, and spelling) and TCS (total cognitive scale, memory subscale).29 Scores were expressed as age-related percentile ranks derived from a national normative sample.
Statistical Analyses
The primary risk factor of interest was exposure to general anesthesia under the age of 2. For IEP and LDs, each child was at risk from birth until meeting criteria. Cumulative incidence rates were calculated using the method of Kaplan and Meier with censoring at date of emigration, death, last follow-up, or 19 years old. For analysis purposes, frequency-matched strata were created by combining matched sets with identical values for factors known to influence the incidence of LDs: gender, mother's education (<12, 12, or >12 years), birth weight (<2500 or ≥2500 g) and gestational age (<32, 32–36, or ≥37 weeks).30 Separate analyses were performed for each LD and IEP outcome using stratified proportional hazards regression to assess whether exposure to anesthesia/surgery (none, 1, 2, or more) was a risk factor. Group-administered achievement tests expressed as percentiles were transformed to z scores using a probit transformation and analyzed using mixed linear models with random effects used to accommodate the correlation of outcomes within strata. To account for differences in comorbidities between exposed and unexposed groups, 2 additional analyses were performed. One analysis included ASA-PS as a covariate and the other adjusted for ADG morbidity clusters. To avoid overfitting in the ADG-adjusted analysis, covariates were included for the ADG morbidity clusters that differed significantly (P < .01) between exposed and unexposed groups. In all cases, the P value for the 2 degrees of freedom test comparing the given outcome across the 3 exposure groups is reported. The effect estimates and 95% confidence intervals (CIs) are presented for comparisons of single and multiple exposure groups versus no exposure. Two-tailed P values of <.05 were considered significant. Analyses were performed by using SAS 8.2 (SAS Institute, Inc, Cary, NC).
RESULTS
Among 5357 children in the cohort, 350 underwent procedures that required general anesthesia before the age of 2, including 286 exposed once and 64 exposed more than once. The mean ± SD duration of anesthetic exposure was 133 ± 239 minutes (median: 75 minutes). The most frequent anesthetic was a combination of halothane (87.5%) and nitrous oxide (88.1%). A summary of the types of surgical procedures has been published previously.14 ASA-PS was higher in exposed compared with nonexposed children (P < .001) (Table 1), as were several of the 32 ADG clusters (Supplemental Table 5).
TABLE 1.
Birth Characteristics and ASA-PS at the Age of 5 Years
| Characteristic | No Anesthesia (n = 700) | Anesthesia (n = 350) | P |
|---|---|---|---|
| Gender, n (%) | 1.000 | ||
| Male | 478 (68) | 239 (68) | |
| Female | 222 (32) | 111 (32) | |
| Birth weight, n (%) | 3417.5 ± 622.5 | 3395.5 ± 690.4 | .603 |
| <2500 g | 60 (9) | 30 (9) | |
| ≥2500 g | 640 (91) | 320 (91) | |
| Gestational age, n (%) | 39.8 ± 2.3 | 39.7 ± 2.7 | .488 |
| <32 wk | 10 (1) | 14 (4) | |
| 32–36 wk | 49 (7) | 16 (5) | |
| ≥37 wk | 641 (92) | 320 (91) | |
| Mother's education, n (%) | 1.000 | ||
| <12 y | 38 (5) | 19 (5) | |
| 12 y | 244 (35) | 122 (35) | |
| >12 y | 418 (60) | 209 (60) | |
| ASA-PS at age 5, n (%) | <.001 | ||
| 1 | 646 (92) | 284 (81) | |
| 2 | 51 (7) | 55 (16) | |
| 3 | 3 (0) | 11 (3) |
By design, exposed and unexposed groups were matched on gender, birth weight, gestational age, and mother's education.
Among the 350 children exposed to anesthesia/surgery, 81 developed LDs before the age of 19 (55 reading, 63 mathematics, 64 written language). Among 700 unexposed controls, 138 developed LDs (95 reading, 108 mathematics, 11 written language). The estimated cumulative incidence of LDs at 19 years was 21.3% for unexposed controls, 23.6% for those exposed once, and 36.6% for those with multiple exposures (Fig 1A). From the analysis not including adjustment for comorbidities, the incidence of LDs differed significantly across exposure groups (P = .006) (Table 2) with multiple exposures being a risk factor for LDs (hazard ratio [HR]: 2.16 [95% CI: 1.35–3.46]. This finding remained consistent in both analyses that included adjustment for comorbidities (ie, using ASA-PS or ADG clusters). A similar pattern was observed when LDs for reading, mathematics, and written language were analyzed separately. In all cases, the CI for the HR associated with multiple exposures excluded unity (Table 2). In all analyses, a single exposure was not a risk factor for LDs.
FIGURE 1.
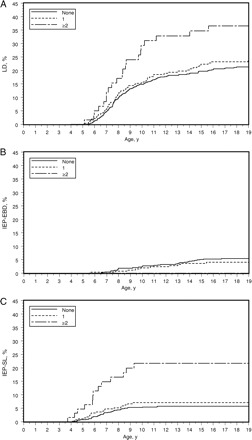
The estimated cumulative percentage of children with LDs (A), IEP-EBDs (B), and IEP-SLs (C) are shown separately for those with 0, 1, and multiple exposures to anesthesia before the age of 2 years.
TABLE 2.
Association of Anesthetic Exposure Before the Age of 2 Years With LDs
| No. of Exposures to Anesthesia | No Covariate Adjustmenta |
ASA-PS Adjusteda |
ADG Adjusteda |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Mathematics, reading, or written language LDs | .006 | .015 | .016 | |||
| 0 (n = 700) | Referent | Referent | Referent | |||
| 1 (n = 286) | 1.10 (0.82–1.50) | 1.09 (0.80–1.48) | 1.06 (0.77–1.46) | |||
| ≥2 (n = 64) | 2.16 (1.35–3.46) | 2.11 (1.27–3.49) | 2.12 (1.26–3.54) | |||
| Mathematics LDs | 019 | .094 | .051 | |||
| 0 (n = 700) | Referent | Referent | Referent | |||
| 1 (n = 286) | 1.08 (0.76–1.53) | 1.05 (0.74–1.49) | 0.98 (0.68–1.42) | |||
| ≥2 (n = 64) | 2.12 (1.26–3.57) | 1.87 (1.06–3.29) | 1.97 (1.11–3.50) | |||
| Reading LDs | .063 | .123 | .049 | |||
| 0 (n = 700) | Referent | Referent | Referent | |||
| 1 (n = 286) | 1.08 (0.75–1.56) | 1.06 (0.74–1.54) | 1.01 (0.68–1.50) | |||
| ≥2 (n = 64) | 1.99 (1.12–3.53) | 1.89 (1.03–3.49) | 2.14 (1.15–4.01) | |||
| Written language LDs | .067 | .072 | .101 | |||
| 0 (n = 700) | Referent | Referent | Referent | |||
| 1 (n = 286) | 1.09 (0.78–1.53) | 1.08 (0.77–1.51) | 0.99 (0.69–1.43) | |||
| ≥2 (n = 64) | 1.88 (1.10–3.21) | 1.93 (1.10–3.40) | 1.84 (1.03–3.31) | |||
Analyses were performed by using stratified proportional hazards regression. Three models were fit for each LD outcome: the first model did not adjust for any covariates; the second model included ASA-PS as a covariate; and the third model included ADG morbidity clusters as covariates.
Among the 350 children exposed to anesthesia/surgery, 32 had an IEP-SL and 10 had an IEP-EBD. Among the 700 unexposed controls, 39 had an IEP-SL and 34 had an IEP-EBD (Fig 1 B and C). From the stratified proportional hazards regression analysis, multiple exposures were a risk factor for subsequent IEP-SL in all models (HR: 4.76 [95% CI: 2.48–9.12] not including covariates for comorbidities). A single exposure was not associated with need for an IEP-SL and no associations were found between exposure and IEP-EBD (Table 3).
TABLE 3.
Association of Anesthetic Exposure Before Age 2 With Need for IEPs
| No. Exposures to Anesthesia | No Covariate Adjustment |
ASA-PS Adjusted |
ADG Adjusted |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| IEP-EBD | ||||||
| 0 (n = 700) | Referent | .619 | Referent | .606 | Referent | .285 |
| 1 (n = 286) | 0.70 (0.35–1.42) | 0.70 (0.34–1.41) | 0.54 (0.25–1.16) | |||
| ≥2 (n = 64)a | 0.00 | 0.00 | 0.00 | |||
| IEP-SL | <.001 | .003 | <.001 | |||
| 0 (n = 700) | Referent | Referent | Referent | |||
| 1 (n = 286) | 1.21 (0.70–2.10) | 1.16 (0.67–2.02) | 0.92 (0.50–1.69) | |||
| ≥2 (N = 64) | 4.76 (2.48–9.12) | 3.49 (1.70–7.16) | 4.16 (1.96–8.87) | |||
Analyses were performed by using stratified proportional hazards regression. Three models were fit for each IEP outcome: the first model did not adjust for any covariates; the second model included ASA-PS as a covariate; and the third model included ADG morbidity clusters as covariates.
No children having multiple anesthetic exposures were identified as having an IEP-EBD.
For the group-administered TCS, the total cognitive score and memory subscale differed significantly across exposure groups with multiple exposures associated with significantly lower scores (Table 4). When health status measured by either method was added to the model, the P value for the overall comparison across exposure groups was not significant, but the specific comparison of multiple exposures versus no exposure was significant (CI excluded 0) in nearly all models. For the group-administered test of achievement (CAT), multiple exposures were associated with significantly lower scores only for mathematics.
TABLE 4.
Association of Anesthetic Exposure Before the Age of 2 Years With Group-Administered Ability and Achievement Test Scores (TOC and CAT)a
| No. of Exposures | No Covariate Adjustment |
ASA-PS Adjusted |
ADG Adjusted |
|||
|---|---|---|---|---|---|---|
| Parameter Estimate (95% CI) | P | Parameter Estimate (95% CI) | P | Parameter Estimate (95% CI) | P | |
| Total cognitive score | .032 | .180 | .105 | |||
| 0 (n = 529) | Referent | Referent | Referent | |||
| 1 (n = 200) | −0.05 (−0.21 to 0.12) | −0.04 (−0.21 to 0.12) | −0.04 (−0.21 to 0.13) | |||
| ≥2 (n = 40) | −0.44 (−0.76 to −0.11) | −0.33 (−0.68 to 0.02) | −0.38 (−0.72 to −0.03) | |||
| Memory subscale score | .049 | .124 | .083 | |||
| 0 (n = 526) | Referent | Referent | Referent | |||
| 1 (n = 200) | −0.04 (−0.20 to 0.13) | −0.03 (−0.20 to 0.13) | −0.04 (−0.21 to 0.13) | |||
| ≥2 (n = 40) | −0.41 (−0.73 to −0.08) | −0.37 (−0.72 to −0.01) | −0.39 (−0.74 to −0.05) | |||
| Mathematics total score | .016 | .087 | .023 | |||
| 0 (n = 638) | Referent | Referent | Referent | |||
| 1 (n = 244) | 0.05 (−0.09 to 0.19) | 0.06 (−0.08 to 0.20) | 0.06 (−0.09 to 0.21) | |||
| ≥2 (N = 54) | −0.35 (−0.62 to −0.09) | −0.27 (−0.55 to 0.01) | −0.34 (−0.62 to −0.06) | |||
| Reading total score | .255 | .406 | .290 | |||
| 0 (n = 646) | Referent | Referent | Referent | |||
| 1 (n = 247) | 0.01 (−0.12 to 0.14) | 0.01 (−0.12 to 0.14) | 0.01 (−0.13 to 0.14) | |||
| ≥2 (n = 55) | −0.20 (−0.44 to 0.04) | −0.17 (−0.42 to 0.09) | −0.19 (−0.45 to 0.06) | |||
| Phonics subscale score | .789 | .946 | .754 | |||
| 0 (n = 613) | Referent | Referent | Referent | |||
| 1 (n = 232) | 0.02 (−0.12 to 0.15) | 0.02 (−0.12 to 0.16) | 0.05 (−0.10 to 0.19) | |||
| ≥2 (n = 52) | −0.08 (−0.34 to 0.18) | −0.01 (−0.28 to 0.26) | −0.04 (−0.31 to 0.24) | |||
| Written language total score | .189 | .481 | .302 | |||
| 0 (n = 627) | Referent | Referent | Referent | |||
| 1 (n = 242) | 0.02 (−0.11 to 0.15) | 0.03 (−0.11 to 0.16) | 0.04 (−0.10 to 0.18) | |||
| ≥2 (n = 54) | −0.22 (−0.46 to 0.03) | −0.14 (−0.40 to 0.12) | −0.17 (−0.43 to 0.09) | |||
| Spelling subscale score | .425 | .526 | .299 | |||
| 0 (n = 624) | Referent | Referent | Referent | |||
| 1 (n = 238) | 0.04 (−0.10 to 0.18) | 0.04 (−0.10 to 0.18) | 0.04 (−0.11 to 0.18) | |||
| ≥2 (n = 53) | −0.15 (−0.41 to 0.12) | −0.12 (−0.40 to 0.16) | −0.19 (−0.47 to 0.09) | |||
Results from the last available test were used for each scale. For the 7 scores included, the median age at the time of the last available test ranged from 11.4 to 12.7 years and did not differ significantly between those exposed to anesthesia versus not.
National percentile scores were transformed to z scores by using a probit transformation and analyzed using a mixed linear model. Three models were fit for each scale: the first model did not adjust for any covariates; the second model included ASA-PS as a covariate; and the third model included ADG morbidity clusters as covariates.
DISCUSSION
The major finding of this study is that, after adjustment for health status and matching for other factors associated with LDs, exposure to anesthesia/surgery before the age of 2 was a risk factor for the development of LDs and the need for an IEP for speech/language impairment in children with multiple, but not single, exposures. A similar pattern of results was observed for most scales of group-administered tests of cognitive ability and achievement, but not the need for an IEP for emotional/behavioral disorders.
Although γ-aminobutyric acid agonists and N-methyl-d-aspartate antagonists can produce widespread apoptotic neurodegeneration in young animals, human significance is unknown. Available studies analyze existing data sets, DiMaggio et al11 examined Medicaid patients undergoing hernia repair and found, compared with an unexposed cohort, an increased risk (HR: 2.3) of diagnoses consistent with behavioral abnormalities. In contrast, Bartels et al12 analyzed data from the Netherlands Twin Registry and reported that group achievement test scores did not differ between exposed and unexposed co-twins. In a pilot study, Kalkman et al13 found a nonsignificant increase in “deviant behavior” among children exposed before 24 months of age.
In the current study we used a birth cohort that is unique in that all members reside in the same community, attended the same schools, and received virtually all of their health care at Mayo Clinic or Olmsted Medical Center.18,20,28,31,32 The construction of this cohort, including potential effects of migration have been described previously.19 Comparisons of children who left the community before the age of 5 with those remaining in the cohort suggests that they do not differ in important aspects.19 The current work incorporated several key features. First, a matched cohort design was employed using children matched for factors known to be associated with LDs. Second, health status using 2 methods was used as an adjuster. Finally, this represents the only study that examined multiple outcomes, including individually administered tests of achievement (used to diagnose LDs), group-administered achievement tests, and assessment of behavioral disorders.
The ASA-PS, first described as a means of stratifying health status,23,33–36 was determined for all children at the age of 5 years to ensure that classification reflected health status immediately before the period of risk (school entry). Because no measurement of health status is widely accepted in children, we also used the Hopkins ACG system. This method, originally designed for children, has been used to predict costs, use, mortality, and morbidity in this and other populations.21,22,37–39 As all diagnostic codes were available for cohort members, application of Hopkins ACG software provided a consistent means of stratifying health status. Not surprisingly, those exposed were more likely to have a higher ASA-PS and frequency of ADG assignment. However, for all analyses, the hazard ratios were similar for the model adjusted only for the matching variables and when adjusting for either ASA-PS or ADGs. Thus, we identified no evidence that differences in health status contributed to the higher frequency of LDs observed among those with multiple exposures in this or our previous analysis. The rates of LDs observed in this study are consistent with those found in previous published incidence studies of LDs that used this same cohort and definitions of LDs.18,20,28,40
We also analyzed outcomes beyond the occurrence of any LD, including LD subtypes (reading, written language, and mathematics), group-administered tests of ability and achievement, and the need for IEP-SLs and IEP-EBDs. The rationale for this extended analysis included determining whether (1) a similar pattern of results observed for LDs (diagnosed using individually administered tests) would be present in group-administered tests and (2) there was a differential effect of anesthetic and surgical exposure on outcomes such as learning versus behavior or a predominance of 1 type of LD verses another. The pattern of injury identified in animals is variable, and it is not yet possible to predict what specific defects in learning or behavior would arise in humans with similar injury, making a more careful definition of a phenotype potentially associated with exposure to anesthesia/surgery of particular interest.
In previous studies end points have been used that are nonspecific and include both behavior and learning with both positive and negative findings.11–13 In the current study, using well defined measures, we found a differential effect such that outcomes related to learning (LDs and IEP-SL) were positive and that related to behavior (IEP-EBD) was negative. This finding suggests that if multiple exposures to anesthesia/surgery have neurodevelopmental effects, these may be limited to impairment of speech and language (perhaps reflecting effects on learning and memory), rather than the general category of developmental disorders (which include behavioral disorders). However, this can be only a provisional conclusion because the receipt of an IEP-EBD has limitations as an indicator of behavioral disorders. Children requiring such services tend to exhibit more severely disruptive behaviors, so that children who have more subtle behavioral disorders (or whose disorders can be successfully treated) may not require an IEP-EBD. Thus, this analysis cannot exclude the possibility other behavioral outcomes could be affected by anesthesia/surgery. In general, a similar pattern of results was observed in most group achievement test scores as was seen for LDs, although the comparison of multiple exposures versus no exposure was often not statistically significant. This may reflect that not all members of the cohort received every group test, such that power to detect effects is lessened, or that group-administered tests are less sensitive than individually administered tests in detecting the association. Nonetheless, we find little evidence of a differential pattern of effect on the various domains measured by these instruments. The only possible exception is the phonics subscale of the CAT, in which the parameter estimate for the effect of multiple exposure compared with no exposure was close to 0 (see Table 3). We also find no evidence of differential effects on the 3 forms of LD, although there is considerable overlap between these 3 (ie, children are frequently diagnosed with multiple LD subtypes). The profound effect of multiple exposures on the need for a speech/language IEP is consistent with the increase in LDs diagnosed by research criteria.
This study has several limitations as described in previous publications,14,41 including the use of a cohort exposed to an anesthetic (halothane) that, although implicated, is no longer in widespread use and may be associated with adverse effects that are different (eg, bradycardia) or more pronounced (eg, hypotension) than those seen with modern anesthetic agents.42,43 It is also possible that the observed effects result from effects other than those related to anesthesia including hypoxia, hyperoxia, or hypocapnia associated with monitoring that was less comprehensive than used currently (eg, pulse oximetry). In addition, this study, like others, cannot distinguish between effects of anesthesia per se and surgery. Despite adjustment for comorbidity the need for surgery cannot be separated from exposure to anesthesia in this and other studies. During the period examined, the community comprising the Rochester schools was primarily white and had a higher income that the national average which may limit the ability to generalize these results to the US population. Unique to this report, migration of cohort members (a potential source of bias in all population studies) has been carefully examined and reported in a separate publication.19 Also unique to this study was the use of 2 comprehensive independent methods to control for health status. However, it is still possible that these measures did not capture all potential confounders related to health status.14 Finally, different types of outcomes (continuous versus binary) were evaluated for group-administered and individually administered tests. This limits our ability to assess a differential pattern of effect and discern whether group-administered tests may be more or less sensitive compared with individually administered tests.
CONCLUSIONS
This population-based birth cohort analyzed using a matched cohort design including health status as a covariate, repeated exposure to anesthesia/surgery before the age of 2 years was a significant independent risk factor for the later development of LDs and the receipt of IEPs related to speech and language, but not behavior. A similar pattern was observed in group-administered achievement tests of cognition and achievement. There is no evidence that single exposures to anesthesia/surgery are associated with any adverse outcome examined. Although this and other observational studies have limitations, prospective studies are currently under way and will be welcome. At this point we cannot exclude the possibility that multiple exposures to anesthesia/surgery may adversely affect neurodevelopment.
Supplementary Material
ACKNOWLEDGMENTS
This work was carried out under US Food and Drug Administration contract HHSF223200810037C and was supported by Rochester Epidemiology Project grant R01 AG034676 from the National Institute of Aging (Bethesda, MD).
We thank our colleagues Bob A. Rappaport, MD, Wendy R. Sanhai, PhD, and Chekesha S. Clingman, PhD, LCDR, USPHS, at the US Food and Drug Administration for their support and their commitment to protecting the public's health; the late Leonard T. Kurland, MD, (epidemiologist, Mayo Clinic) for his vision in initiating the Rochester Epidemiology Project; Candice Klein, BS (clinical research coordinator, Mayo Clinic); Peg Farrell, RN (data abstractor) and other members of the Learning Disability team for data collection; Independent School District 535; the Reading Center/Dyslexia Institute of Minnesota for collaboration; data analyst Andrew Hanson, BA (statistical program analyst, Mayo Clinic); and the Anesthesia Clinical Research Unit, Mayo Clinic (Cynthia Medcalfe, RT assistant; Nora Feher, research coordinator; Melissa Passe, RRT-research coordinator; and Shonie Buenvenida, RN study coordinator) for help in abstracting the data.
All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
COMPANION PAPER: A companion to this article can be found on page e1268, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2011-2489.
- LD
- learning disability
- IEP
- individualized education program
- CAT
- California Achievement Test
- TCS
- Test of Cognitive Skills
- ASA-PS
- American Society of Anesthesiologists Physical Status
- ACG
- adjusted clinical group
- ADG
- aggregated diagnostic group
- IEP-EBD
- IEP for disorders of emotion/behavior
- IEP-SL
- IEP for speech/language
- CI
- confidence interval
- HR
- hazard ratio
REFERENCES
- 1.Wang C, Sadovova N, Fu X, et al. The role of the N-methyl-d-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132(4):967–977 [DOI] [PubMed] [Google Scholar]
- 2.Slikker W, Jr, Zou X, Hotchkiss CE, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98(1):145–158 [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Slikker W., Jr Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system. Anesth Analg. 2008;106(6):1643–1658 [DOI] [PubMed] [Google Scholar]
- 4.Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12(4):488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jevtovic-Todorovic V, Benshoff N, Olney JW. Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats. Br J Pharmacol. 2000;130(7):1692–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young C, Jevtovic-Todorovic V, Qin YQ, et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146(2):189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74 [DOI] [PubMed] [Google Scholar]
- 8.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol. 2008;18(2):198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou X, Patterson TA, Divine RL, et al. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27(7):727–731 [DOI] [PubMed] [Google Scholar]
- 11.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21(4):286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12(3):246–253 [DOI] [PubMed] [Google Scholar]
- 13.Kalkman CJ, Peelen L, Moons KG, et al. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110(4):805–812 [DOI] [PubMed] [Google Scholar]
- 14.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen TG, Flick R. Anesthetic effects on the developing brain: insights from epidemiology. Anesthesiology. 2009;110(1):1–3 [DOI] [PubMed] [Google Scholar]
- 16.Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387–1390 [DOI] [PubMed] [Google Scholar]
- 17.Raghunathan K, Schwartz DA, Connelly NR. The need for perspective. Anesthesiology. 2009;111(6):1386; author reply 1386–1387 [DOI] [PubMed] [Google Scholar]
- 18.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth cohort, 1976–1982, Rochester, Minn. Mayo Clin Proc. 2001;76(11):1081–1092 [DOI] [PubMed] [Google Scholar]
- 19.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Potential influence of migration bias in birth cohort studies. Mayo Clin Proc. 1998;73(11):1053–1061 [DOI] [PubMed] [Google Scholar]
- 20.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Math learning disorder: incidence in a population-based birth cohort, 1976–82, Rochester, Minn. Ambul Pediatr. 2005;5(5):281–289 [DOI] [PubMed] [Google Scholar]
- 21.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29(5):452–472 [DOI] [PubMed] [Google Scholar]
- 22.Starfield B, Weiner J, Mumford L, Steinwachs D. Ambulatory care groups: a categorization of diagnoses for research and management. Health Serv Res. 1991;26(1):53–74 [PMC free article] [PubMed] [Google Scholar]
- 23.Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–284 [Google Scholar]
- 24.American Society for Anesthesiologists. ASA Physical Status Classification System. 2010. Available at: www.asahq.org/clinical/physicalstatus.htm Accessed January 10, 2011
- 25.American Academy of Pediatrics, Committee on Children with Disabilities. The pediatrician's role in development and implementation of an Individual Education Plan (IEP) and/or an Individual Family Service Plan (IFSP). Pediatrics. 1999;104(1 pt 1):124–127 [DOI] [PubMed] [Google Scholar]
- 26.Adams HR, Szilagyi PG, Gebhardt L, Lande MB. Learning and attention problems among children with pediatric primary hypertension. Pediatrics. 2010;126(6). Available at: www.pediatrics.org/cgi/content/full/126/6/e1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine TP, Liu J, Das A, et al. Effects of prenatal cocaine exposure on special education in school-aged children. Pediatrics. 2008;122(1). Available at: www.pediatrics.org/cgi/content/full/122/1/e83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katusic SK, Colligan RC, Weaver AL, Barbaresi WJ. The forgotten learning disability: epidemiology of written-language disorder in a population-based birth cohort (1976–1982), Rochester, MN. Pediatrics. 2009;123(5):1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CAT/5: California Achievement Tests [technical bulletin]. 4th ed Monterey, CA: CTB/Macmillan/McGraw-Hill; 1993 [Google Scholar]
- 30.Jewell NP. Statistics for Epidemiology. Boca Raton, FL: Chapman and Hall/CRC; 2004 [Google Scholar]
- 31.Sprung J, Flick RP, Wilder RT, et al. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111(2):302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St Sauver JL, Katusic SK, Barbaresi WJ, Colligan RC, Jacobsen SJ. Boy/girl differences in risk for reading disability: potential clues? Am J Epidemiol. 2001;154(9):787–794 [DOI] [PubMed] [Google Scholar]
- 33.Owens WD. American Society of Anesthesiologists Physical Status Classification System in not a risk classification system. Anesthesiology. 2001;94(2):378. [DOI] [PubMed] [Google Scholar]
- 34.Bunchungmongkol N, Somboonviboon W, Suraseranivongse S, Vasinanukorn M, Chau-in W, Hintong T. Pediatric anesthesia adverse events: the Thai Anesthesia Incidents Study (THAI Study) database of 25 098 cases. J Med Assoc Thai. 2007;90(10):2072–2079 [PubMed] [Google Scholar]
- 35.Gobbo Braz L, Braz JR, Modolo NS, do Nascimento P, Brushi BA, Raquel de Carvalho L. Perioperative cardiac arrest and its mortality in children: a 9-year survey in a Brazilian tertiary teaching hospital. Paediatr Anaesth. 2006;16(8):860–866 [DOI] [PubMed] [Google Scholar]
- 36.D'Errico C, Voepel-Lewis TD, Siewert M, Malviya S. Prolonged recovery stay and unplanned admission of the pediatric surgical outpatient: an observational study. J Clin Anesth. 1998;10(6):482–487 [DOI] [PubMed] [Google Scholar]
- 37.Weiner JP, Dobson A, Maxwell SL, Coleman K, Starfield B, Anderson GF. Risk-adjusted Medicare capitation rates using ambulatory and inpatient diagnoses. Health Care Financ Rev. 1996;17(3):77–99 [PMC free article] [PubMed] [Google Scholar]
- 38.Carlsson L, Borjesson U, Edgren L. Patient based ‘burden-of-illness’ in Swedish primary health care: applying the Johns Hopkins ACG case-mix system in a retrospective study of electronic patient records. Int J Health Plann Manage. 2002;17(3):269–282 [DOI] [PubMed] [Google Scholar]
- 39.Leibson CL, Katusic SK, Barbaresi WJ, Ransom J, O'Brien PC. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA. 2001;285(1):60–66 [DOI] [PubMed] [Google Scholar]
- 40.Katusic SK, Colligan RC, Beard CM, et al. Mental retardation in a birth cohort, 1976–1980, Rochester, MN. Am J Ment Retard. 1996;100(4):335–344 [PubMed] [Google Scholar]
- 41.Kopp VJ. Hyperoxia in pediatric anesthesia: time for reconsideration? Anesthesiology. 2009;111(6):1383; author reply 1383–1384 [DOI] [PubMed] [Google Scholar]
- 42.Uemura E, Bowman RE. Effects of halothane on cerebral synaptic density. Exp Neurol. 1980;69(1):135–142 [DOI] [PubMed] [Google Scholar]
- 43.Uemura E, Levin ED, Bowman RE. Effects of halothane on synaptogenesis and learning behavior in rats. Exp Neurol. 1985;89(3):520–529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


