Abstract
Background:
Abelmoschus esculentus L. belonging to the family Malvaceae is a kind of one year herbage plant, which is one of the most important vegetables widely grown in Nigeria for its tender fruits and young leaves. It's easy to be cultivated and grows well in both tropical and temperate zones, that is, it is widely planted from Africa to Asia, South European to America. A new flavonol glycoside characterized as 5,7,3′,4′-tetrahydroxy-4′′-O-methyl flavonol -3-O-β-D- glucopyranoside (1) has been isolated from the fruit of A. esculentus together with one known compound 5,7,3′,4′-tetrahydroxy flavonol -3-O-[β-D-glucopyranosyl-(1→6)]-β-D-glucopyranoside (2). The structure of the new compound was elucidated on the basis of its spectral data, including 2-D NMR and mass (MS) spectra. The antioxidant activities of the isolated compounds 1 and 2 were evaluated by 2 assays, the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and ferric reducing antioxidant power (FRAP). The present work deals with the isolation, identification and antioxidant activity of the two compounds.
Materials and Methods:
The compounds were isolated by Diaion HP-20, Sephedex LH-20 column chromatography methods, their structures were identified by physicochemical properties and spectroscopic analysis. The antioxidant activities of the isolated compounds 1 and 2 were evaluated by two assays, e.g., DPPH and FRAP.
Results:
Two flavonol glycosides have been isolated from the fruit of Abelmoschus esculentus L. for the first time, and the compound 1 was a new compound, the compound 2 was isolated from the plant for the first time.
Conclusion:
The results show that the two flavonol glycosides have strong ability for scavenging DPPH and FRAP free radical by the experiment of antioxidant activities, so A. esculentus may be a natural antioxidants resource.
Keywords: Abelmoschus esculentus L, antioxidant activity, flavonoid glycoside, HRESIMS, NMR
INTRODUCTION
Along with the discovery of its nutrition, A. esculentus is widely cultivated in north and south China recent years.[1] It had been the first choice vegetable for the Olympic athlete in Peking Olympic Games.[2] It is also known to have significant amounts of phenols and flavonoids. Earlier researches[3,4] had already reported the presence of hyperoside, quercetin, coumarin scopoletin, uridine, phenylalanine, and so on in this plant. We have isolated a new flavonoid glycoside named 5,7,3′,4′-tetrahydroxy-4′′-O-methyl flavonol-3-O-β-D-glucopyranoside and a known flavonoid glycoside 5,7,3′,4′-tetrahydroxy flavonol -3-O-[β-D-glucopyranosyl-(1→6)]-β-D- glucopyranoside [Figure 1] from it. Both compound 1 and compound 2 are reported for the first time in this plant. Their structures were established by spectral (1H NMR, 13C NMR, DEPT 135, DEPT 90, HSQC, HMBC), and HRESIMS data. The antioxidant activities of the two compounds have been evaluated by the methods of DPPH and FRAP assays.
Figure 1.
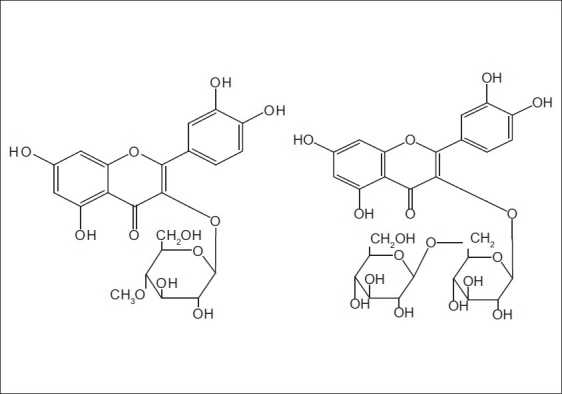
The structures of compound 1 and compound 2
MATERIALS AND METHODS
Experimental instruments
Melting points were determined on an WRS-1B melting point apparatus. Optical rotation was measured using a W22-2(A) polarimeter. 1H NMR, 13C NMR, DEPT, HSQC, and HMBC data were measured on a Bruker DPX-400 spectrometer with CD3OD as solvent and TMS as internal standard (400 MHz for 1H NMR and 100 MHz for 13C NMR). HRESI MS spectra was obtained using WATERSZQ 2000. All solvents used were of analytical grade (Shanghai Chemical Reagent Company Ltd.). Precoated silica gel GF254 plates (Qingdao Haiyang Chemical Company Ltd.) were used for TLC. Sephadex LH-20 (Pharmacia Bioteck Inc.), Diaion HP-20 (Tosoh Corp.) were used for column chromatography. Infinite M200 Universal Microplate Spectrophotometer (Swiss Tecan company, Swiss) was used to measure the absorbance (DPPH and FARP assays).
Plant material
The whole plant of A. esculentus was collected from the botanical garden of Zhejiang Agriculture and Forestry University, China. It was identified as A. esculentus of Malvaceae family by professor Lu-huan Lou Zhejiang Agriculture and Forestry University, and the specimen were deposited in our laboratory.
Extraction and isolation
Air-dried and powdered fruits of A. esculentus were extracted with 70% methanol and this methanolic extract was successively extracted with petroleum ether and EtOAc. The concentrated water-soluble fraction was subjected to column chromatography over Diaion HP-20. The elute with MeOH:H2O (2:8) was combined and concentrated to powder by the rotary evaporator under 50 °C. After dissolved with distilled water, the solution was subjected to column chromatography over Sephadex LH-20, eluting with gradient MeOH-H2O. Compound 1 (100 mg) and compound 2 (2g) were obtained in MeOH-H2O (2:8) and (1:1).
RESULTS AND DISCUSSIONS
The structural elucidation of compound 1
The 1H NMR spectrum of compound 1 in CD3OD showed a 1,3,4-trisubstituted phenyl moiety by the signals of δ 7.577 (dd, 1H, J = 8.4 Hz, 1.2 Hz), δ 7.699 (d, 1H, J = 1.2 Hz), and δ 6.858 (d, 1H, J = 8.4 Hz), two isolated protons at δ 6.189 (s, 1H) and δ 6.381 (s, 1H), as well as signals assignable to one anomeric protons at δ 5.250 (d, 1H, J = 7.6 Hz) of glucopyranoside, and three isolated protons δ 3.684 (s, 3H) characteristic peaks of 4΄΄methyl together with 6 other glycosyl protons between δ 3.199--3.719.
The 13C NMR and DEPT spectra of compound 1 displayed 22 carbon signals which consisted of characteristic including 15 of flavonol aglycone, 1 methoxyl and 6 of one glycosyl moieties [Table 1, Figure 1]. The above spectral data revealed that compound 1 is a flavone glycoside with β-sugar units. The 1H and 13C NMR spectral data were unambiguously assigned by HMBC and HSQC experiments. While the signals assigned to sugar units revealed the presences of a 3-substituted β-D-glucopyranosyl units.
Table 1.
The spectral data of compound 1
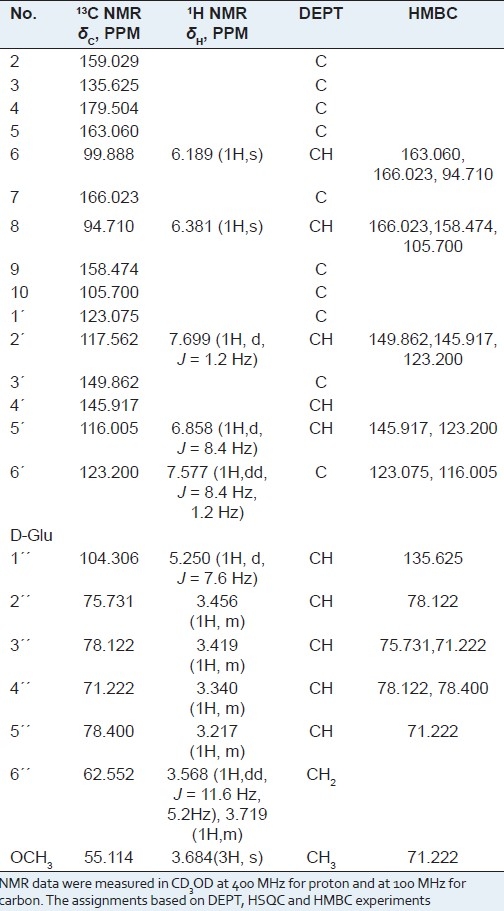
In the HMBC 13C--1H correlations of compound 1 [Figure 2], long range correlations from H-1” δ 5.250 to C-3 δ 135.625 uneqivocally established that a β-D-glucopyranosyl moiety was located at C-3 aglycone, Methyl H δ 3.684 (s, 3H) was attached to Glu-C4 of β-D-glucopyranosyl, δ 3.456 (Glu-H2) was correlated with δ 104.306 (Glu-C 1), accordingly the structure of compound 1 was determined as 5,7,3′,4′-tetrahydroxy -4′′ -O-methyl flavonol -3-O-β-D- glucopyranoside.
Figure 2.
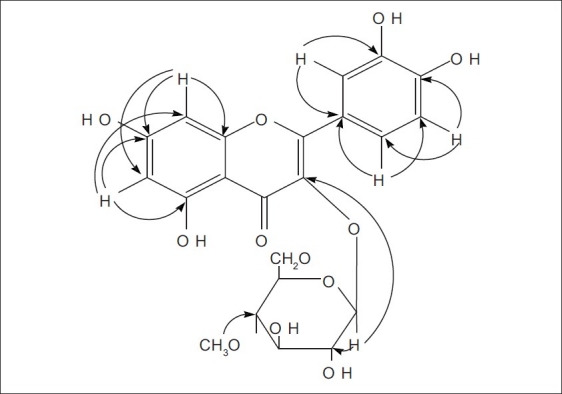
The Key HMBC correlations of compound 1
NMR data of compound 1 and compound 2
The molecular formula of compound 1 was determined to be C22H22O12 by HRESIMS analysis and the m/z of [M + H]+ is 479.1187 (calcd 479.1189). 1H NMR (400 MHz, CD3OD): δ 7.699 (d,1H, J = 1.2 Hz), 7.577 (dd, 1H, J = 8.4 Hz, 1.2 Hz), 6.858 (d, 1H, J = 8.4 Hz), 6.381 (s, 1H), 6.189 (s, 1H), 5.250 (d, 1H, J = 7.6 Hz), 13C NMR (100 MHz, CD3 OD): δ 179.504 (C-4), 166.023 (C-7), 163.060 (C-5), 159.029 (C-2), 158.474 (C-9), 149.862 (C-3′), 145.917 (C-4′), 135.625 (C-3), 123.200 (C-6′), 123.075 (C-1′), 117.562 (C-2′), 116.005 (C-5′), 105.700 (C-10), 104.306 (C-1′′), 99.888 (C-6), 94.710 (C-8), 78.400 (C-5′′), 78.122 (C-3′′), 75.731 (C-2′′), 71.122 (C-4′′), 62.552 (C-6′′), 55.114 (C-4′′ -OCH3). The structure of compound 1 was determined as 5,7,3′,4′-tetrahydroxy-4′′-O-methyl-3-O-β-D-glucopyranoside flavonol. Its structure can be seen Figure 1.
The molecular formula of compound 2 was determined to be C27H30O17[5] 1H NMR (400 MHz, CD3OD): δ 7.690 (d, 1H, J = 2 Hz), 7.656 (dd, 1H, J = 8.4 Hz, 2.4 Hz), 6.858 (d, 1H, J = 8.4 Hz), 6.390 (s, 1H), 6.188 (s, 1H), 5.234 (d, 1H, J = 7.6 Hz), 4.143 (d, 1H, J = 7.6 Hz), 13C NMR (100 MHz, CD3OD): δ 179.5396 (C-4), 165.987 (C-7), 163.006 (C-5), 158.877 (C-2), 158.472 (C-9), 149.842 (C-3′), 145.912 (C-4′), 135.592 (C-3), 123.533 C-6′), 123.093 (C-1′), 117.521 (C-2′), 116.078 (C-5′), 105.757 (C-10), 104.583 (C-1′′), 103.984 (C-1′′′),99.888 (C-6), 94.824 (C-8), 77.992 (C-5′′), 77.872 (C-5′′′), 77.773 (C-3′′), 77.601 (C-3′′′), 75.747 (C-2′′), 75.082 (C-2′′′), 71.313 (C-4′′), 71.284 (C-4′′′), 69.569 (C-6′′), 62.507 (C-6′′′). The spectral data showed basically agreement with the literature, the compounds 2 is 5,7,3′,4′-tetrahydroxy-3-O-[β-D- glucopyranosyl-(1→6)]-β-D-glucopyranoside flavonol. Its structure can be seen Figure 1.
Determination of the antioxidant activity of compound 1 and 2
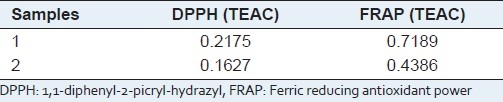
The scavenging effect of the two compounds on DPPH radical was monitored as described by Hale[6] with some modifications. Briefly, test the scavenging ratio of the sample and trolox on DPPH at the same time, and then find a suitable concentration range of the trolox and its scavenging percentage, build a linear regression equation [Table 2] between the trolox concentration and its scavenging percentage, calculate the trolox equivalent antioxidant capacity (TEAC) through the equation according to the scavenging percentage of the sample solution to DPPH radical solution [Table 3].
Table 2.
Linear regression equation of 1,1-diphenyl-2-picryl-hydrazyl and ferric reducing antioxidant power assays

Table 3.
Antioxidant activities of compound 1 and 2 (mg Trolox/mg DW)
Ferric reducing antioxidant power of the two compounds was monitored as described by Pulido et al[7] with some modifications. Mix 200 μl FRAP regent[8] with 100 μL Trolox solution, and then let the mixture react under 40 °C for an hour without illumination. Read the absorbance at 593 nm of it through the Infinite M 200 and measure the absorbance of reagent blank. Calculate a linear regression equation between Trolox solution concentrations and the absorbance at 593 nm [Table 2]. At the same time make 100 μl sample solution react with 200 μl FRAP regent as the above treatment to Trolox solution, calculate the Trolox equivalent antioxidant capacity (TEAC) of the sample through the regression equation according to the absorbance at 593 nm of the sample and FRAP regent mixture [Table 3].
The antioxidant activity of each compound was evaluated by measuring the ability of the isolated flavonol glycoside to scavenge the free radicals of DPPH and reducing ferric in vitro [Table 3]. The tests were carried out according to previous methods.[6–9]
From Tables 2 and 3, it is obvious to see that compound 1 and 2 have the ability to scavenging DPPH and FRAP free radical. The scavenging ability of DPPH and FRAP free radical increases along with the rise of sample concentration.
CONCLUSION
The two flavonol glycosides were isolated and elucidated from the fruits of A. esculentus. Compound 1 was a new compound, and compound 2 was isolated from the plant for the first time. The results show that the two flavonol glycosides have the strong ability for scavenging DPPH and FRAP free radical by the experiment of antioxidant activities.
ACKNOWLEDGMENTS
We are grateful to Professor Janxun Kang and Shaomin Wang, Analysis and Testing Center, Zhengzhou University, for performing for the NMR and HRESIMS data. We also thank Zhejiang Provincial Key Laboratory for Modern Silvicultural Technology, Zhejiang Agriculture and Forestry University for performing Universal Microplate Spectrophotometer.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Camciuc M, Deplagne M, Vilarem G, Gaset A. Okra—Abelmoschus esculentus L.(Moench) a crop with economic potential for set aside acreage in France. J Industrial Crops and Products. 1998;7:257–64. [Google Scholar]
- 2.Kolawole OF, Bukola SO. Effect of Processing Methods on Physical, Chemical, Rheological, and Sensory Properties of Okra (Abelmoschus esculentus) J Food Bioprocess Technol. 2010;3:387–94. [Google Scholar]
- 3.Bandyukova VA, Ligai LV. A chemical investigation of the fruit of Abelmoschus esculentus. J Chem Nat Compd. 1987;23:376–7. [Google Scholar]
- 4.Lu J, Huanfen L, Linlin J. Chemical constituents in n-butanol extract of Abelmoschus esculentus. J Chin Tradit Herb Drugs. 2011;41:1771–3. [Google Scholar]
- 5.Budzianowski J. Six flavonol glucuronides from Tulipa gesneriana. J Phytochem. 1991;30:1679–82. [Google Scholar]
- 6.Hale AL, Reddivari L, Nzaramba MN, Bamberg JB, Miller JC. Interspecific variability for antioxidant activity and phenolic content among solanum species. Am J Potato Res. 2008;85:332–41. [Google Scholar]
- 7.Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 8.Siddhuraju P, Manian S. The antioxidant activity and free radical scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds. J Food Chem. 2007;105:950–8. [Google Scholar]
- 9.Ani V, Varadaraj MC, Akhilender NK. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L) J Eur Food Res Technol. 2006;224:109–15. [Google Scholar]


