Abstract
Background:
Plants of Labiatae are used in traditional medicine and phytotherapy. Rosmarinic acid (RA) is a phenolic compound which is found in many genus of Labiatae and exhibits important biological activities.
Materials and Methods:
In this investigation, RA contents of 29 species of Labiatae named Salvia officinalis, Salvia limbata, Salvia virgata, Salvia hypoleuca, Salvia macrosiphon, Salvia choloroleuca, Melissa officinalis, Origanum vulgare, Lavandula angustifolia, Rosmarinus officinalis, Thymus daenensis, Thymus citriodorous, Thymus pubescens, Thymus vulgaris, Zataria multiflora, Mentha piperita, Mentha pulegium, Mentha longifolia, Mentha spicata, Mentha aquatica, Mentha crispa, Perovskia artemisoides, Zhumeria majdae, Satureja hortensis, Satureja khuzistanica, Satureja bachtiarica, Satureja atropatana, Satureja mutica and Satureja macrantha were determined by using high-performance liquid chromatographic method.
Results:
The results showed that RA content in different species of Labiatae was 0.0-58.5 mg g-1 of dried plants. The highest amount of RA was found in Mentha species especially M. spicata.
Conclusion:
M. spicata can be considered as a new source of rosmarinic acid .
Keywords: High-performance liquid chromatographic, Labiatae, Mentha spicata, rosmarinic acid
INTRODUCTION
Plants of Labiatae family have been used in traditional medicine for exhaustion, weakness, depression, memory enhancement, circulation improvement, strengthening of fragile blood vessels,[1] inflammation, infection,[2] indigestion and gastritis.[3] Researchers have proved that these plants are source of compounds with antioxidant,[4] anti-inflammatory,[5] anti-allergic,[6] anti-depression,[7] anti-hyperglycemic[8] and antimicrobial[9–11] properties. These activities are mostly related to their phenolic compounds content especially rosmarinic acid (RA), an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid [Figure 1][1] which was isolated for the first time from Rosmarinus officinalis L. leaves and later found in other species of Labiatae and Boraginaceae. RA has interesting properties which has led to a broad range of applications from food preservatives to cosmetics.[12] Different studies have shown that antioxidant activity of RA is more than vitamine E[13] or Trolox.[14] RA has been reported to have some biological activities in vitro such as antiviral properties[15] including anti-HIV-1,[16] antibacterial, antioxidant, anti-carcinogenic,[17] and anti-allergic activities.[18] In vivo studies have shown that RA exhibit anti-allergic,[19,20] anti-thrombotic,[21] and anti-carcinogenic[22,23] properties as well. This compound is also efficient against peroxidative damage to biomembranes.[24] Nowadays, many products have been prepared from RA in pharmaceutical, cosmetic, and food industries. RA can be found in many plants but usually rosemary plant is used as the major source. This matter has caused to increase of demand and price of the plant.[25] Therefore, finding other plants containing high amount of RA is very important to introduce as new sources. This compound has been reported to occur in several taxonomically non-related families of the plant kingdom, but it is found abundantly in Labiatae.[26] In this investigation, in order to mark the best source of RA in Labiatae plants which grow in Iran, RA contents of 29 plants have been determined by using High-performance liquid chromatographic (HPLC) method. All of the plants are used as medicinal herbs or in food industries in different pats of Iran.
Figure 1.
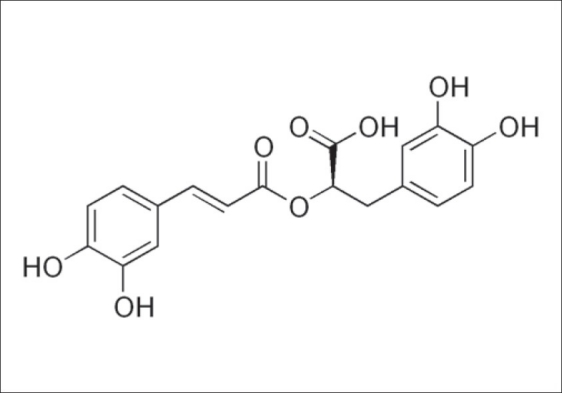
The structure of rosmarinic acid
MATERIALS AND METHODS
Plant material
Aerial parts of Salvia officinalis L., Salvia limbata C. A. Mey., Salvia virgata Jacq., Salvia hypoleuca Benth., Salvia macrosiphon Boiss., Salvia choloroleuca Rech. f. and Aell., Melissa officinalis L., Origanum vulgare L., Lavandula angustifolia Mill., Rosmarinus officinalis L., Thymus daenensis Celak, Thymus citriodorous (Pers.) Schreb., Thymus pubescens Boiss. and Kotschy ex Celak, Thymus vulgaris L., Zataria multiflora Boiss., Mentha piperita L., Mentha pulegium L., Mentha longifolia (L.) Huds., Mentha spicata L., Mentha aquatica L., Mentha crispa L., Perovskia artemisoides Boiss, Zhumeria majdae Rech., Satureja hortensis L., Satureja khuzistanica Jamzad, Satureja bachtiarica Bunge, Satureja atropatana Bunge, Satureja mutica Fisch. and C. A. Mey., and Satureja macrantha C. A. Mey., were collected from their growing area of Iran [Table 1] during flowering stage in summer 2008. Herbarium specimens were kept at the Herbarium of the Medicinal Plants Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences.
Table 1.
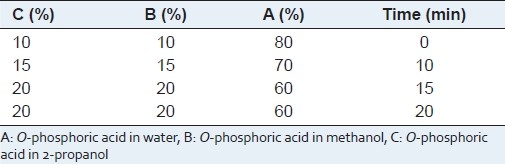
Gradient time program for analysis of rosmarinic acid in Labiatae plants
Chemicals
Methanol (HPLC grade), 2-propanol (analytical grade), O-phosphoric acid (analytical grade) were purchased from Merck (Germany). The standard of rosmarinic acid was prepared from Aldrich (Germany). The water used in HPLC and for sample preparation was produced with a Purelab UHQ (ELGA) with a resistivity over 18 MΩ·cm.
Instrumentation
A Waters high performance liquid chromatograph system comprising vacuum degasser, quaternary pump, auto sampler and a waters 2996 diode array detector was used. UV spectra were collected across the range of 200--900 nm extracting 330 nm for chromatograms. The column, an ACE 5 C18, (250 × 4.6 mm) was maintained at 30 °C. Mobile phase used for separation was the mixture of 0.085% O-phosphoric acid in water (A), 0.085% O-phosphoric acid in methanol (B), and 0.085% O-phosphoric acid in 2-propanol (C) in gradient mode [Table 1]. The flow rate, detection wavelength and sample injection volume were 1.0 ml min-1, 330 nm, and 20 μl, respectively.[27] The chromatographic peak of rosmarinic acid was confirmed by comparing the retention time and UV spectra with that of related to the reference standard. Quantization was performed by using calibration curve of rosmarinic acid.
Preparation of standard solutions
Stock standard solution was prepared accurately by weighing 10 mg of rosmarinic acid reference standard into 10 ml volumetric flask and dissolving in water: methanol: 2-propanol (each one contained 0.085% O-phosphoric acid) (80:10:10) with the aid of ultrasonic. Serial dilutions (1-150 μg/ml) were made from stock solution.
Sample preparation
Milled and powdered samples (200 mg) were accurately weighed into a 25-ml tube, and extracted with 25 ml of the same solvent system for preparing standard solutions, during 30 min by ultrasonic. The resulting mixture was centrifuged at 4500 r/min for 5 min, and the supernatant transferred to a 100-ml volumetric flask. The residual solid was extracted for two more times with 25 ml of the same solvent mixture by ultrasonic, and centrifuged as above. The supernatants were combined, and diluted to 100 ml with the same solvent mixture. Each sample was extracted three times and injected (three times) to HPLC for analysis.
RESULTS AND DISCUSSION
Several mobile phases including methanol, water, acetonitrile, 2-propanol, THF and TFA in different combinations were tested. Finally, it was found that a 0.085% O-phosphoric acid in water: 0.085% O-phosphoric acid in methanol: 0.085% O-phosphoric acid in 2-propanol in gradient mode in 20 min [Table 1] gave the best separation.[27] After comparison between C8 and C18 columns, the best separation efficacy was obtained by using C18 column. HPLC chromatogram of Mentha spicata sample and UV spectrum of RA in 11.16 min obtained from PDA detector have been shown in Figures 2 and 3, respectively. Comparison between purity threshold and purity angle reported in em-power software showed that the method is specific for rosmarinic acid and reported peak is completely separated from other interfering compounds. The linear relationship between detector response and different concentrations of rosmarinic acid (eight levels) was confirmed in range of 1-150 μg/ml with correlation coefficient of 0.9983 and equation of y = 45337×-19410. In order to obtain the best recovery and peak shape of rosmarinic acid, different solvents and extraction methods were examined. Methanol, methanol followed by CCl4, methanol:water, methanol followed by hexane and water:methanol:2-propanol (each one contained 0.085% O-phosphoric acid) were used to investigate the effect of solvents on the RA extraction. Moreover, the effect of extraction time on the content of RA was studied (data was not shown). Finally, water:methanol:2-propanol (each one contained 0.085% O-phosphoric acid) (80:10:10) and ultrasonic for 30 min in three repeats were selected as the best parameters for RA extraction method.
Figure 2.
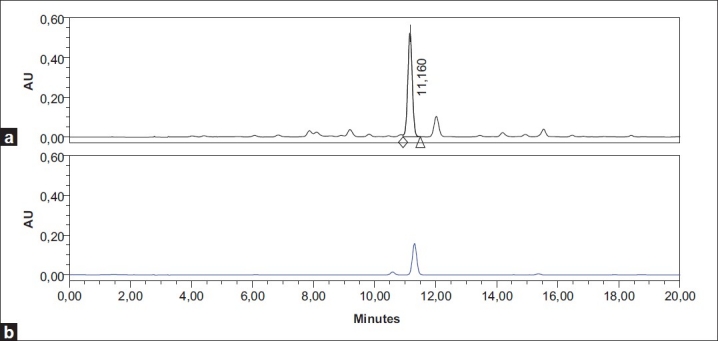
High-performance liquid chromatographic Chromatograms of (a) Mentha spicata extract and (b) rosmarinic acid standard solution
Figure 3.
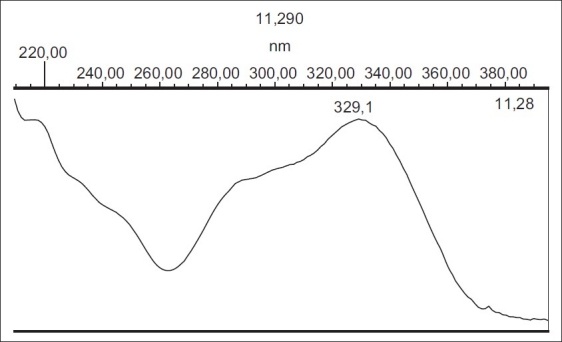
UV spectrum of rosmarinic acid obtained from PDA detector at 11.16 min
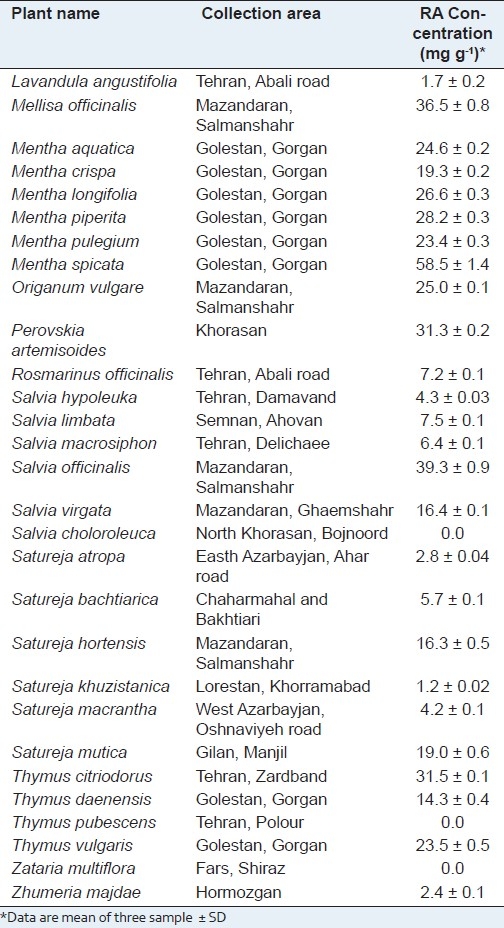
The results showed that among analyzed plants belong to different genus of Labiatae family, the most RA contents were found in Mentha species. As it is observed in Table 2, all Mentha species contain RA in considerable concentration (19.3--58.5 mg g-1) and M. spicata showed the highest amount of RA. Rosemary has been considered as a main source of RA in many countries[25] but the results demonstrated that the plant growing in Iran contains low RA concentration (7.2 mg g-1) compare to other investigated plants. Therefore, other plants such as Salvia officinalis, Melissa officinalis, Thymus citriodorous, Perovskia artemisoides and especially Mentha spicata which is widespread in Iran and very easy to access can be used as a source of RA in pharmaceutical, food and cosmetic industries. As it has been shown in Table 2, no rosmarinic acid was detected in Thymus pubescens, Salvia choloroleuca and Zataria multiflora. Several investigations have been carried out in order to find new RA resources among plants. Achamlale et al.,[25] showed that RA contents in Zostera noltii and Z. marina samples varied from 2.2 to 18.0 mg g-1 and 1.3--11.2 mg g-1, respectively. They believed that the high RA content of these two sea-grasses is of interest for both cosmetic and herbal industries. Similar study has been performed on Melissa officinalis during different harvesting time. It has been shown that M. officinalis contained 39.1 mg g-1 of RA during full flowering stage[28] which is almost similar to RA content of M. officinalis from Iran (36.5 mg g-1). Another investigation on rosemary, sage, thyme, spearmint and lavender has proved that the plants contained 10.3, 10.4, 6.6, 10.7, and 2.0 mg g-1 of RA, respectively.[1] RA contents of rosemary and lavender obtained in our study were almost similar to the previous study. Therefore, rosemary can not be considered as RA source in all countries. Since, RA content of a plant is known to depend considerably on extrinsic and intrinsic factors including soil and climatic conditions, plant ontogenesis phases, harvest and plant storage,[29–32] therefore, it is necessary to analyze the plants which are growing in each country for finding the best source of rosmarinic acid.
Table 2.
Collection areas and concentration of rosmarinic acid in plants of Labiatae family
CONCLUSION
RA is found in most of Labiatae plants growing in Iran and its concentration in some of species such as Salvia officinalis, Melissa officinalis, Thymus citriodorous, Perovskia artemisoides and Mentha spicata is considerable. These plants especially Mentha spicata can be used as RA resources in food, cosmetic and pharmaceutical industries instead of rosemary which contains low concentration of RA compare to other studied plants.
ACKNOWLEDGMENTS
This research has been supported by Tehran University of Medical Sciences and Health Services (Grant No. 87-04-56-7508).
Footnotes
Source of Support: Tehran University of Medical Sciences and Health Services (Grant No. 87-04-56-7508).
Conflict of Interest: None declared.
REFERENCES
- 1.Wang H, Provan GJ, Helliwell K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004;87:307–11. [Google Scholar]
- 2.Vieira A. A comparison of traditional anti-inflammation and anti-infection medicinal plants with current evidence from biomedical research: Results from a regional study. Pharmacogn Res. 2010;2:293–5. doi: 10.4103/0974-8490.72326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajimehdipoor H, Shekarchi M, Khanavi M, Adib N, Amri M. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharmacogn Mag. 2010;6:154–8. doi: 10.4103/0973-1296.66927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–70. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 5.Al-Sereiti MR, Abu-Amer KM, Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol. 1999;37:124–30. [PubMed] [Google Scholar]
- 6.Ito H, Miyazaki T, Ono M, Sakurai H. Antiallergic activities of Rabdosiin and its related compounds: Chemical and biochemical evaluations. Bioorg Med Chem. 1998;6:1051–6. doi: 10.1016/s0968-0896(98)00063-7. [DOI] [PubMed] [Google Scholar]
- 7.Takeda H, Tsuji M, Matsumiya T, Kubo M. Identification of rosmarinic acid as a novel antidepressive substance in the leaves of Perilla frutescens Britton var. acuta Kudo (Perillae Herba) Nihon Shinkei Seishin Yakurigaku Zasshi. 2002;22:15–22. [PubMed] [Google Scholar]
- 8.Kumar PM, Sasmal D, Mazumder PM. The antihyperglycemic effect of aerial parts of Salvia splendens (scarlet sage) in streptozotocin-induced diabetic-rat. Pharmacogn Res. 2010;2:190–4. doi: 10.4103/0974-8490.65520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nascimento EM, Rodrigues FF, Campos AR, Costa JG. Phytochemical prospection, toxicity and antimicrobial activity of Mentha arvensis (Labiatae) from northeast of Brazil. J Young Pharm. 2009;1:210–2. [Google Scholar]
- 10.Jain R, Kosta S, Tiwari A. Ayurveda and urinary tract infection. J Young Pharm. 2010;2:337. doi: 10.4103/0975-1483.66811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zomorodian K, Saharkhiz MJ, Rahimi MJ, Bandegi A, Shekarkhar G, Bandegani A, et al. Chemical composition and antimicrobial activities of the essential oils from three ecotypes of Zataria multiflora. Pharmacogn Mag. 2011;7:53–9. doi: 10.4103/0973-1296.75902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson M, Simmonds MS. Rosmarinic acid. Phytochemistry. 2003;62:121–5. doi: 10.1016/s0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin YL, Chang Y, Kuo YH, Shiao MS. Anti-lipid-peroxidative principles from Tournefortia sarmentosa. J Nat Prod. 2002;65:745–7. doi: 10.1021/np010538y. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Foo LY. Polyphenolics of Salvia- A review. Phytochemistry. 2002;75:197–202. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 15.Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanes encephalitis. Antimicrob Agents Chemother. 2007;51:3367–70. doi: 10.1128/AAC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooker CW, Lott WB, Harrich D. Inhibitors of human immunodeficiency virus type 1 reverse transcriptase target distinct phases of early reverse transcription. J Virol. 2001;75:3095–104. doi: 10.1128/JVI.75.7.3095-3104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang SS, Zheng RL. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006;239:271–80. doi: 10.1016/j.canlet.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Parnham MJ, Kesselring K. Rosmarinic acid. Drugs Future. 1985;10:756–7. [Google Scholar]
- 19.Makino T, Furuta A, Fujii H, Nakagawa T, Wakushima H, Saito K, et al. Effect of oral treatment of Perilla frutescens and its constituents on type-I allergy in mice. Biol Pharm Bull. 2001;24:1206–9. doi: 10.1248/bpb.24.1206. [DOI] [PubMed] [Google Scholar]
- 20.Sanbongi C, Takano H, Osakabe N, Sasa N, Natsume M, Yanagizawa K, et al. Rosmarinic acid in Perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin Exp Allergy. 2004;34:971–7. doi: 10.1111/j.1365-2222.2004.01979.x. [DOI] [PubMed] [Google Scholar]
- 21.Zou ZW, Xu LN, Tian JY. Antithrombotic and antiplatelet effects of rosmarinic acid, a water-soluble component isolated from radix Salviae miltiorrhizae (danshen) Yao Xue Xue Bao. 1993;28:241–5. [PubMed] [Google Scholar]
- 22.Lee J, Kim YS, Park D. Rosmarinic acid induces melanogenesis through protein kinase A activation signalling. Biochem Pharmacol. 2007;74:960–8. doi: 10.1016/j.bcp.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: Anti-carcinogenetic effects of Perilla frutescens extract in the murine two-stage skin mode. Carcinogenesis. 2004;25:549–57. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- 24.Liu GT, Zhang TM, Wang BE, Wang YW. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem Pharmacol. 1992;43:147–52. doi: 10.1016/0006-2952(92)90271-j. [DOI] [PubMed] [Google Scholar]
- 25.Achamlale S, Rezzonico B, Grignon-Dubois M. Rosmarinic acid from beach waste: Isolation and HPLC quantification in Zostera detritus from Arcachon lagoon. Food Chem. 2009;113:878–83. [Google Scholar]
- 26.Holzmannová V. Rosmarinic acid and its biological activity. Chem List. 1996;90:486–96. [Google Scholar]
- 27.Orhan I, Aslan S, Kartal M, Şener B, Başer KH. Inhibitory effects of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008;108:663–8. doi: 10.1016/j.foodchem.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Tóth J, Mrlianová M, Teke’ová D, Koreňová M. Rosmarinic acid- an important phenolic active compound of lemon balm (Melissa officinalis L.) Acta Fac Pharm Univ Comenianae. 2003;50:139–46. [Google Scholar]
- 29.Adzet T, Ponz R, Wolf E, Schulte E. Genetic variability of essential oil content of Melissa officinalis. Planta Med. 1992;58:558–61. doi: 10.1055/s-2006-961550. [DOI] [PubMed] [Google Scholar]
- 30.Adzet T, Ponz R, Wolf E, Schulte E. Content and composition of Melissa officinalis oil in relation to leaf position and harvest time. Planta Med. 1992;58:562–4. doi: 10.1055/s-2006-961551. [DOI] [PubMed] [Google Scholar]
- 31.Hose S, Zänglein A, Van Den Berg T, Schultze W, Kubeczka KH, Czygan FC. Ontogenetic variation of the essential leaf oil of Melissa officinalis L. Pharmazie. 1997;52:247–53. [Google Scholar]
- 32.Mrlianová M, Teke’ová D, Felklová M, Reinöhl V, Tóth J. The influence of the harvest cut height on the quality of the herbal drugs Melissa folium and Melissa herba. Planta Med. 2002;68:178–80. doi: 10.1055/s-2002-20247. [DOI] [PubMed] [Google Scholar]


