Abstract
Background:
Rutin is a bioflavonoid of strong immunostimulating activity from the Toonea Sinensis Folium, which has shown a significant ability to increase the survival rate of white shrimp with bacterial infection. However, no method for the quantitation of this active ingredient in the herb has been reported to date.
Materials and Methods:
A reversed phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) method was developed to quantify Rutin in the Toonea Sinensis Folium, with the HPLC conditions optimized, followed by validation for linearity, accuracy, precision, limit of detection (LOD), repeatability, and stability. Then, the established method was used to determine the content of Rutin in two samples.
Results:
The separation was performed on a Waters XBridge Shield RP18 column (150 mm × 4.6 mm, 5 μm) kept at 25°C, and acetonitrile and water containing 0.1% acetate acid (18:82, v / v)-composed mobile phase was constantly driven at 1.0 mL / minute during the analysis. Twenty microliters of sample solution or standard solution were injected into the HPLC system and 254 nm was selected to monitor the separation. A strong linear relationship between the peak area and concentration of Rutin was observed within the range of 0.01044 – 0.2610 mg / mL (r2 = 1.0000). The LOD was 0.03915 μg / mL, and recovery of Rutin was from 97.6 to 99.6%. In addition, the method was also validated to be repeatable, stable, precise, and accurate.
Conclusions:
An efficient and reliable RP-HPLC-DAD method was established, which could be used for routine analysis of Rutin in Toonea Sinensis Folium and to assist in the quality control of this herb.
Keywords: Aquaculture, high performance liquid chromatography, Rutin, toona sinensis, toonea sinensis folium
INTRODUCTION
Toonea Sinensis Folium are the dried leaves of Toona sinensis Roem (Meliaceae; T. sinensis), a kind of arbor widely distributed throughout Asia.[1] For a long time, this herb has been employed as an oriental medicine for the treatment of enteritis, dysentery and itchiness, with no irreversible side effects observed after treatment.[2,3] Due to its great effectiveness and the demand for a natural immunostimulant, some efforts have been put into the investigation of its bioactivity as well as the corresponding phytochemical constituents, in recent years.[4–7] Rutin (quercetin-3-rutinoside, Figure 1), a renowned bioflavonoid, was found to help maintain a healthy immune system among the phenolic compounds isolated from the Toonea Sinensis Folium, and it has been reported to exhibit clinically relevant functions, including antioxidant, antihypertensive, anti-inflammatory activities, and so on.[8] More than the application in clinics, this natural flavonoid has also shown a significant effect of enhancing the immunity of white shrimp under the Vibrio alginolyticus challenge, by increasing the phenoloxidase activity and O2- levels, and hence, can be used as an immunostimulant for improving survival in the course of aquaculture practice.[9] Previously, two analytical methods that revolved around liquid chromatography-mass spectrometry (LC-MS) had been studied to identify the antioxidant compounds in this herb.[2,10] However, it can be seen that the reported methods mainly focused on the qualitative analysis of gallic acid and its derivatives in the raw material, but not another important active ingredient namely Rutin. As such, it is necessary to establish a convenient method for the quantitation of Rutin, and hence, for the first time a reversed phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) method has been developed and validated in this study, for this purpose, and can be reliably used for routine analysis of Rutin in Toonea Sinensis Folium and to assist in the quality control of this herb, while using it either in clinics or aquaculture practice.
Figure 1.
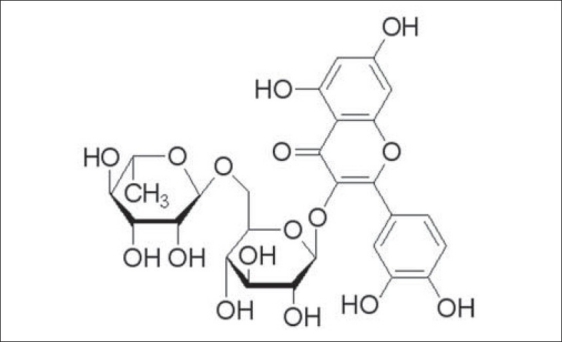
Chemical structure of Rutin
MATERIALS AND METHODS
Chemicals
Acetonitrile (ACN), methanol (MeOH) of gradient grade, glacial acetate acid (HAc) for HPLC analysis, and MeOH of analytical grade were all supplied by Sinopharm Chemical Reagent Co. Ltd., (Shanghai, China). Ultrapure water was prepared by using a Milli-Q Biocel system (Millipore, Molsheim, France). Standard Rutin of 98.0% purity (HPLC) was purchased from Nanjing ZeLang Medical Technology Co. Ltd. (Nanjing, China).
Plant material
The leaves of T. sinensis were harvested on 9 May, 2011 in the City of Baoding (Hebei Province, China; Sample 1) and on 18 May, 2011 in the City of Zhenjiang (Jiangsu Province, China; Sample 2), respectively. The fresh leaves were dried under shade in a well-ventilated place, and then the dried leaves were individually ground and passed through a 40-mesh sieve. Only fine powder was collected and stored in a dry cabinet (RH ≤ 50%) at room temperature throughout the analysis. Voucher specimens (20110509 and 20110518) were deposited at the Pharmacognosy Research Facility, School of Pharmacy, Jiangsu University (China).
High performance liquid chromatography instrumentations and conditions
A Prominence HPLC instrument (Shimadzu, Japan) was equipped with a DGU-20A degasser, a LC-20AT pump, a CTO-10AC VP column oven, and an SPD-20A UV / Vis Detector. The data were acquired and then processed using the N2000 SP1 software for chromatographic analysis (Zhejiang University, China).
The chromatographic experiments were performed on an XBridge Shield RP18 column (150 mm × 4.6 mm, 5 μm) obtained from the Waters Corporation (USA) under isocratic elution at 25°C. The mobile phase used was composed of ACN and water containing 0.1% HAc (18:82, v / v). The separation could be completed within 15 minutes and the column was then flushed with ACN for 10 minutes, followed by equilibration, with an initial composition for another five minutes prior to the next injection. Flow rate was constant at 1.0 mL / min. Injection volume was 20 μL and the wavelength for UV detection was set to 254 nm.
Preparation of standard and sample solutions
Rutin of 26.10 mg was dissolved in MeOH and scaled to 50 mL using MeOH as stock solution (0.5220 mg / mL). A series of dilutions were subsequently carried out to obtain 1 / 2, 1 / 4, 1 / 8, 1 / 10, 1 / 25, and 1 / 50 of the original concentration for the preparation of standard solutions.
The powder of Toonea Sinensis Folium, 0.500 g, was precisely weighed into a 50 mL capped conical flask; 20 mL of MeOH was then added to the flask and the total weight was recorded immediately. The suspension was swirled briefly prior to the ultrasonic extraction for 30 minutes. After cooling down to ambient temperature, the weight was taken again and a few drops of MeOH were added, to make up the loss of the weight.
Prior to injection, all the solutions were filtered through 0.45 mm PTFE membrane syringe filters (Thermo Fisher Scientific, USA), respectively.
Development and validation of the reversed phase-high performance liquid chromatography-diode array detector method
High performance liquid chromatography conditions, including analytical column, composition of mobile phase, and flow rate were optimized, followed by validation for linearity, accuracy, precision, limit of detection (LOD), repeatability, and stability.
RESULTS
Method development
According to the λmax of Rutin, 254 nm was used for monitoring the separation. Furthermore, as a common practice, for the achievement of the efficient separation of the analyte, the HPLC conditions were optimized in terms of resolution, peak shape, and elution time by preliminary assays, in which three crucial factors including column, mobile phase, and flow rate were evaluated. In detail, four available chromatographic columns in our laboratory, namely, the XBridge Shield RP18 column (4.6 mm × 150 mm, 5 μm; Waters, USA), Zorbax Eclipse XDB-C8 column (150 mm × 4.6 mm, 5 μm; Agilent, USA), Chromolith Performance RP-18e (100 mm × 4.6 mm, 5 μm; Merck, Germany), and the ODS Hypersil column (150 mm × 4.6 mm, 5 μm; Thermo, USA), were tested, and consequently the XBridge Shield RP18 column proved to be the best one in this application. In addition, both MeOH-water and ACN-water were tried as the mobile phase to elute the sample solution, however, the desired separation was not achieved. Accordingly, different concentrations of HAc (0.05, 0.1, and 0.5%) were used, to replace the water in the mobile phase, to enhance the resolution and minimize peak tailing. It was found that both 0.1 and 0.5% HAc showed good performance, but the mixture of ACN and water containing 0.1% HAc (18:82, v / v) was eventually chosen, as less acid was preferred, without compromising on the efficiency. After comparison among the experimented flow rates at 0.8, 1.0, and 1.2 mL / minute, 1.0 mL / minute was chosen to obtain satisfactory separation and rational analytical time. Figure 2 shows the typical HPLC chromatograms obtained from the standard (a) and the two tested samples (b and c). It was seen that a satisfying separation of Rutin was achieved within 15 minutes under the optimized HPLC conditions, as aforementioned.
Figure 2.
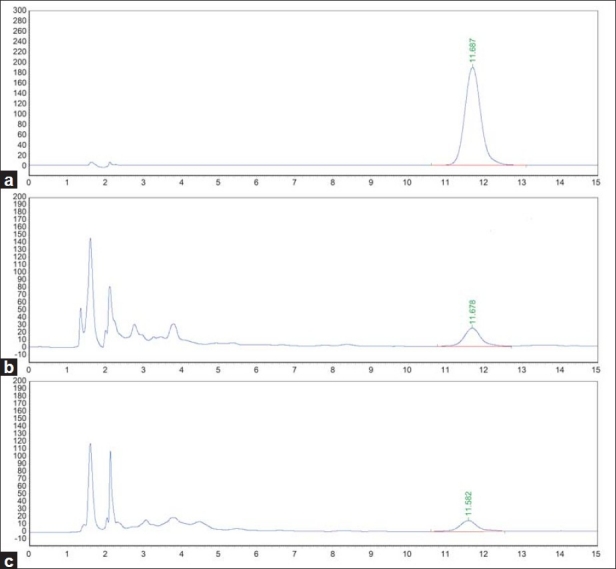
Typical chromatograms of standard solution and sample solutions (a: standard solution of Rutin; b: solution of sample 1; c: solution of sample 2)
Validation of the developed method
Linearity was tested using six different concentrations of the analyte. The solutions corresponding to each concentration level were injected in triplicate and the linear regression analysis of Rutin, peak area (y) versus concentration (x), was carried out. A high correlation coefficient (r2 = 1.0000) of the calibration curve (y = 43300656 x - 6476) was obtained, indicating that there was a strong linear relationship between the peak area and the concentration of the analyte in the range of 0.01044 – 0.2610 mg / mL.
The limit of detection, defined as the concentration of a compound needed to produce a signal approximately thrice the noise, was determined under the present chromatographic conditions to be 0.03915 μg / mL.
Intra-day precision of the developed method was evaluated by repeating six determinations of a standard solution (1 / 8 concentration of the stock solution) and a sample solution on the same day. Moreover, inter-day precision was determined by analyzing the standard solution on three consecutive days. The RSDs of the peak areas were calculated and found to be 0.62, 0.96, and 0.87%, respectively.
In addition, six samples were extracted and analyzed using the proposed method to evaluate the repeatability. The RSD of the content of Rutin was less than 1.63%.
A freshly prepared sample solution was injected immediately and then tested after storage at room temperature under shade for 6 hours, 12 hours, 18 hours, and 24 hours. The RSD of the peak areas was then calculated. The result (RSD = 1.49%) revealed that the prepared sample solution was stable over this period.
The recovery test was conducted to evaluate the accuracy of the developed method. The standards dissolved in MeOH were added to known amounts of the samples, with a proper volume of MeOH, making it up to 20 mL. The sample solutions were prepared and tested, and experiments were performed at each level, in triplicate. The percentage of the recoveries was calculated based on the following formula: (Detected amount - Original amount) / Spiked amount × 100%. The results are presented in Table 1 and the recoveries of Rutin were within the range of 97.6 – 99.6%, with the RSD values ranging from 3.13 to 4.02%, very well verifying the high accuracy of this developed method.
Table 1.
Recoveries of the analyte Rutin (n = 3)
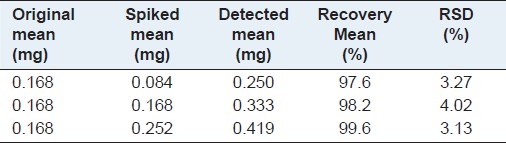
Determination of Rutin in the samples
The content of Rutin in two samples was determined thrice under optimized HPLC conditions, as mentioned earlier. The chromatograms of these are illustrated in Figure 2 (b and c), and the average contents in the samples are 0.673 ± 0.010 mg·g-1 (Sample 1) and 0.498 ± 0.005 mg·g-1 (Sample 2), respectively.
DISCUSSION
Rutin is an important immunostimulant in Toonea Sinensis Folium, and hence, an RP-HPLC-DAD method for the quantitation of Rutin in the herb has been developed in this study, with an expectation of assisting in its quality assessment. By using the developed method, the whole analysis including column wash and equilibration can be completed within one hour. Moreover, the method has been validated to be repeatable, precise, and accurate, and the prepared sample solution has been found to be stable for 24 hours. However, some polyphenols corresponding to the antioxidant activity of this herb, such as gallic acid and methyl gallate, are yet to be determined.
It can be seen that the contents of Rutin differ greatly from each other in two production sites, which may be due to the difference in the age of the arbor, harvest time, environmental conditions, including climate, water source, soil, atmosphere and altitude, and endophytes as well. It will be interesting to further investigate how these causes result in such a differentiation, and it is worth ascertaining the regular pattern of the content of Rutin in the herb during one year, so that the harvest of the leaves would be more reasonable.
In addition, different parts on this medicinal arbor, such as the bark, stem, flower, fruit, and root, may have different bioactivities, therefore, the pharmacological features can be characterized and then its applications in enhancing a human being's health or aquaculture practice can be extended.
CONCLUSION
It has been demonstrated that the newly proposed RP-HPLC-DAD method in this study is a reliable method for the determination of Rutin in the herb Toonea Sinensis Folium. Furthermore, this validated method can also be applied for the purpose of quality control of this herb.
ACKNOWLEDGEMENT
The work was financially supported by the Fund for Talented Teacher of Jiangsu University (China) 11JDG073, the Fund for Undergraduate's Scientific Research of Jiangsu University (China) 10A239 the Fund for the Talents in Traditional Chinese Medicine of Jiangsu Province (China) and SATCM key Lab of New Drug Delivery System of Chinese Materia Medica (China, 2011NDDCM01002).
Footnotes
Source of Support: Fund for Talented Teacher of Jiangsu University (China) 11JDG073, the Fund for Undergraduate's Scientific Research of Jiangsu University (China) 10A239 the Fund for the Talents in Traditional Chinese Medicine of Jiangsu Province (China) and SATCM key Lab of New Drug Delivery System of Chinese Materia Medica (China, 2011NDDCM01002)
Conflict of Interest: None declared.
REFERENCES
- 1.Hseu YC, Chang WH, Chen CS, Liao JW, Huang CJ, Lu FJ, et al. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food Chem Toxicol. 2008;46:105–14. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Wang J, Xing ZE, Dai YQ, Chen M. Identification of phenolics in Chinese toon and analysis of their content changes during storage. Food Chem. 2011 [In Press] [Google Scholar]
- 3.Edmonds JM, Staniforth M. Toona sinensis. Botanical Mag. 1998;15:186–96. [Google Scholar]
- 4.Wang KJ, Yang CR, Zhang YJ. Phenolic antioxidants from Chinese toon (fresh young leaves and shoots of Toona sinensis) Food Chem. 2007;101:365–71. [Google Scholar]
- 5.Poon SL, Leu SF, Hsu HK, Liu MY, Huang BM. Regulatory mechanism of Toona sinensis on mouse leydig cell steroidogenesis. Life Sci. 2005;76:1473–87. doi: 10.1016/j.lfs.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Hseu YC, Chen SC, Lin WH, Hung DZ, Lin MK, Kuo YH, et al. Toona sinensis (leaf extracts) inhibit vascular endothelial growth factor (VEGF)-induced angiogenesis in vascular endothelial cells. J Ethnopharmcol. 2011;134:111–21. doi: 10.1016/j.jep.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 7.Yang HL, Chang WH, Chia YC, Huang CJ, Lu FJ, Hsu HK, et al. Toona sinensis extracts induces apoptosis via reactive oxygen species in human premyelocytic leukemia cells. Food Chem Toxicol. 2006;44:1978–88. doi: 10.1016/j.fct.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–80. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh TJ, Wang JC, Hu CY, Li CT, Kuo CM, Hsieh SL. Effects of rutin from Toona sinensis on the immune and physiological responses of white shrimp (Litopenaeus vannamei) under Vibrio alginolyticus challenge. Fish Shellfish Immun. 2008;25:581–8. doi: 10.1016/j.fsi.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Cheng KW, Yang RY, Tsou SC, Lo CS, Ho CT, Lee TC, et al. Analysis of antioxidant activity and antioxidant constituents of Chinese toon. J Func Foods. 2009;1:253–9. [Google Scholar]


