Abstract
Background:
Herbal medicine is widely used in the treatment of diseases like diabetes mellitus. We investigated the effects of guar gum in diabetic rats for the reduction of the risk of diabetes and cardiovascular disease. Dietary pattern emphasizing foods high in complex carbohydrates and fiber are associated with low blood glucose and cholesterol levels.
Materials and Methods:
Diet containing 0%, 5%, 10% and 20% (w/w) guar gum was fed to diabetic rats for 28 days. Blood serum glucose, triglycerides, cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol levels, atherogenic index levels, body weights and food intake were monitored at 0, 7.14 and 28 days after induction of diabetes.
Results:
In spite of the fact that diabetes elevated blood lipids in all rats after 14 days, the guar gum diet significantly decreased the serum concentration of cholesterol, triacylglicerols and LDL-C and atherogenic index. The most significant result in this study was the reduction of blood glucose in diabetic rats treated with the guar gum diet after 28 days versus non- and glibenclamide-treated rats. The gum promoted a general improvement in the condition of the diabetic rats in body weight and food intake in comparison with nontreated rats.
Conclusion:
The results of this research suggest that guar gum was significantly effective in comparison with glibenclamide in the treatment of hyperlipidemia and hyperglycemia in diabetes rats. Therefore, it may be suggested as a reliable fiber in diabetic regimes in diabetic patients.
Keywords: Dietary fiber, diabetes mellitus, guar gum, lipid profile, rat
INTRODUCTION
Diabetes mellitus is a metabolic disorder. About 4–5% of the world population suffers from diabetes.[1] Diabetes mellitus is accompanied with discerning conversions in the plasma lipids and lipoprotein profile, with an increased danger of immature atherosclerosis, coronary insufficiency and myocardial infarction.[2] It has been demonstrated that diabetes mellitus is associated with a three- to four-fold increase in the risk of coronary artery disease.[3] Modifications occurring in diabetic dyslipidemia comprise quantitative and qualitative changes. Quantitative changes include increases in very-low-density lipoprotein (VLDL) as contrasted to normal due to an increase in the availability of glucose for VLDL synthesis and decrease in the lipoprotein lipase activity accompanied with a decrease of VLDL removal from peripheral circulation, increase in low-density lipoprotein cholesterol (LDL-C) levels and decrease in VLDL clearance. Qualitative changes include increased extent of triglycerides, LDL-C and high-density lipoprotein cholesterol (HDL-C), nonenzymatic glycation of LDL and nonenzymatic glycation of HDL thus increasing the probability of heart disease.[4] Known risk factors in diabetes are hypertension, hyperglycemia, obesity, elevated total and VLDL-triglycerides, low HDL-C levels and less consistency and increased total and LDL-C levels.[5] Because people with diabetes have almost two- to four-fold increased risk of dying from cardiovascular disease, the control of high-glucose levels and other attending coronary heart disease risk factors implicating high-lipid profile expresses the most competent approach to inhibition.[6] Obesity is considered as one of the strongest risk factors for type 2 diabetes manifestation.[7] It has been demonstrated that weight loss leads to a better glycemic control in these patients.[8] The treatment of obesity is traditionally carried out via the ingestion of hypocaloric diets.[9]
There is an absolute relationship between fiber-defective diets and expedition in the development of assured chronic and degenerative disorders, which had been abounding in modern countries.[10] Dietary fiber intake should preferentially be provided by the ingestion of whole grains, fruits and vegetables, which, besides being good fiber sources, are also a natural source of nutrients such as vitamins and minerals and also have an antioxidant effect.[11] Therefore, the ingestion of fiber supplements may become a useful alternative.[12] Dietary fiber has been divided into two fractions; the insoluble fraction containing essentially cellulose, lignin and some hemicelluloses; the soluble fraction composed of mainly pectin, some hemicelluloses and gums.[13]
Guar, Cyamopsis tetragonoloba (L.) Taub. or cluster bean, is a member of the Leguminosae (Fabaceae) family and is a glactomannan storage polysaccharide, which is grown in India and the United States, and also is economically the most important of the four species in the genus.[14] Currently, the major use of the crop is for the galactomannan gum extracted from the endosperm of the seed. About 42% of the guar seed is endosperm, of which the predominant portion is mucilage or gum (guar gum). As a soluble dietary fiber (SDF), guar gum is part of the total dietary fiber (TDF) fraction of the seed. Approximately 80–85% of the gum is a galactomannan, which is comprised of a (1-4) β-D-mannopyranosyl backbone with branch-points from the six-position linked to single β-D-galactopyranosyl residues.[15] There are typically 1.5–2.0 mannose residues for every galactose. Guar gum galactomannans form water-dispersible hydrocolloids, which thicken when dissolved in water, leading to their use as emulsifying, thickening or stabilizing agents for a wide range of processed foods.[16] The crude fiber method of analysis measures a portion of the cellulose and a small amount of the hemicellulose and lignin in a sample.[17] In contrast, TDF by definition includes all the plant nonstarch polysaccharides, oligosaccharides, resistant starch and lignin. The polysaccharides can be in either the soluble fraction, i.e. SDF, as are the galactomannans, or the insoluble fraction, i.e. insoluble dietary fiber, as are cellulose and resistant starch, with lignin as part of the insoluble dietary fiber. In addition to its viscogenic properties, guar gum may, like other soluble fibers such as β-glucan found in cereals, be associated with specific beneficial effects in human health, i.e. may be associated with lowering of serum cholesterol and triglycerides, reducing postprandial glucose, improving bowel function and having bifidogenic effects. In contrast, the insoluble fraction of TDF is required for normal lower intestinal function.[18]
The specific polysaccharide component of guar gum is guaran. In guaran, about one-half of the D-mannopyranosyl main chain units contain a D galactopyranosyl side chain.[19] A great deal of work has been articled since then, showing the valuable effects of guar gum against a variety of diseases like colon cancer, heart disease and gall stones.[20] In a short period, dietary fiber like guar gum exerts a physical action on the intestine, stimulating peristaltive movements. These actions tend to avoid constipation and reduce diverticulitis and prevalence of colon cancer.[21] The long-term effects of guar gum have not yet been examined, but some observant studies in healthy subjects and noninsulin-dependent diabetic patients showed that guar gum reduced the postprandial rise in blood glucose and insulin concentrations.[22] It is not known whether intimate mixing of guar gum with food or meal in diabetic patients is important in optimizing its blood glucose and lipid profile-lowering effect. Therefore, the aim of the present study was to evaluate the effects of diets containing 0, 5, 10 and 20% guar gum on the lipidemic and glycimic metabolic control and also to determine the effects of these diets versus the effects of glibenclamide on the food intake, body weight and atherosclerosis index in diabetic rats.
MATERIALS AND METHODS
Materials
Streptozotocin from Sigma (St. Louis, MO) and total cholesterol, triglyceride, HDL-C and LDL-C kits were purchased from Zistshimi (Tehran, Iran). Commercial guar gum was obtained from India (AliBaba Co. Ltd., Haryana, India), and Cyamopsis tetragonaloba (guar plant) and guar were identified by the herbal medicinal specialist at Ferdowsi University. The guar was powdered and all powdered samples were then pooled for processing in order to obtain different regimes in different percentages of guar gum.
Animals
Forty-two male Wistar rats weighing 245 ± 35 kg were purchased from Razi Institute (Mashhad, Iran) and kept in their own cages at constant room temperature (22 ± 1°) under a normal 12-h light:12-h dark regime with free access to food (as follows) and water. The animals were housed according to the regulations of the welfare of experimented animals. All treatments and diets were formally approved by the Mashhad Medical University Animal Ethics Committee.
Induction of experimental diabetes and experimental procedure
Diabetes was induced by a single intraperitoneal injection of 60 mg/kg streptozotocin, diluted in 0.1 M sodium citrate buffer (pH = 4.5). Streptozotocin-treated rats received 5% glucose instead of water for 24 h after diabetes induction in order to reduce death due to hypoglycemic shock. Blood samples were taken from the tail vein 72 h after streptozotocin injection to measure the glucose levels with a portable glucometer. Only animals with 12-h fasting blood glucose (over 300 mg/dl) were considered diabetic and used for the present study. Diabetes development was then checked every week by determination of glucose concentration in the blood. During the experiment, blood glucose and lipid profile levels were verified four times at 0, 7, 14 and 28 days after the beginning of the treatment. All the diabetic rats were randomly assigned to five dietary groups of nine rats each for 28 days based on the chow contained [0% (diabetic control), 5%, 10%, 20% guar gum] and glibenclamide (2 mg/kg). During the treatment period, the rats were subjected to measure, body weight and food intake and also to collection of blood samples. Blood was collected from the retroorbital vein puncture using the microcapillary technique on days 0, 7, 14 and 28 from the day of being diabetic. Before blood sampling, all rats were fasted for 12 h. The serum obtained after centrifugation was used to estimate the blood glucose levels, triglyceride, total cholesterol, HDL and LDL-C.
Biochemical analysis
Plasma lipid parameters such as total cholesterol, triglyceride, glucose, HDL-cholesterol and LDL-C were determined by enzymatic colorimetric methods using commercial kits from Pars Azmoon (Tehran, Iran). Briefly, blood samples were transferred directly into centrifuge tubes, allowed to clot at room temperature for 20 min and centrifuged for 20 min at 2000 rpm. The supernatant obtained was transferred into test tubes for lipid analysis. The CHOD-PAP method (Boehringer Mannheim) was used to determine serum cholesterol and HDL. Then, absorbance was read at 546 nm in an Auto analyzer (Microlab100, E. Merck, Germany) and compared with the standard curve. LDL-C fraction was determined according to the Friedewald equation,[23] LDL-C (in mg/100 ml) = total cholesterol - HDL-C-1/5 triglycerides. Blood glucose was determined by the GOD-POD method,[24] serum total cholesterol was determined by the CHOD-PAP method[21] and serum triglyceride concentration was determined by the GPO-PAP method.[22] Serum HDL-C and LDL-C concentrations were determined by the method described by Patel, et al.[25] The individual body weight and food intake were recorded weekly during the study. The LDL-C fraction and athrosclerosis index (AI) were determined by the Friedeward equations
LDL-C (in mg/400ml) = total cholesterol - (HDL-C/triglyceride)
AI = (TC-(HDL-C/HDL-C)
Statistical analysis
The data were analyzed using statistical package program stat view software. Data were exposed as mean ± SEM; statistical significance between glucose, lipid profiles, body weight and food intake in each group and during treatment period was determined with one-way ANOVA followed by Turkey's test. P-values less than 0.05 were considered statistically significant, such that we used different letters indicating statistically different results (# = P < 0.01, * = P < 0.001).
RESULTS
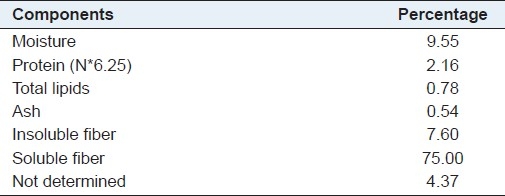
The approximate percent composition of the guar gum used in the study is illustrated in Table 1. As expected, the main consistent of the guar gum appears as soluble fiber (75%), followed by insoluble fiber (7.6%). Total fiber accounted for 82% of the material.
Table 1.
Percent composition of guar gum
Hypoglycemic effects of guar gum
The profiles of blood glucose for diabetic rats during the 28-day feeding period are shown in Figure 1. Streptozocin-diabetic rats were found to exhibit significant (P < 0.01) hyperglycemia as compared with control rats. The basic value of 350 mg/dl remained constant for the diabetic rats fed the control diet with 0% guar gum for almost the whole treatment period. However, the results of the 28-day diabetic study clearly indicated that guar gum (20%) exhibited significant hypoglycemic activity in streptozotocin-diabetic rats versus diabetic controls, 0% guar gum (P < 0.01). No significant difference was detected between the glucose level of rats fed with 5%, 10% and 20% guar gum during the 28 days. There was no significant difference at serum glucose levels between standard drug glibenclamide and diabetic control (0% guar gum) but, interestingly, there was a significant drop (P < 0.01) of glucose level for the rats fed the diet with 20% guar gum compared with rats receiving glibenclamide (2 mg/kg) after 28 days of treatment. At the end of 28 days of treatment, there was a 52% decrease of glucose level of the rats that were fed 20% guar gum [Figure 1].
Figure 1.
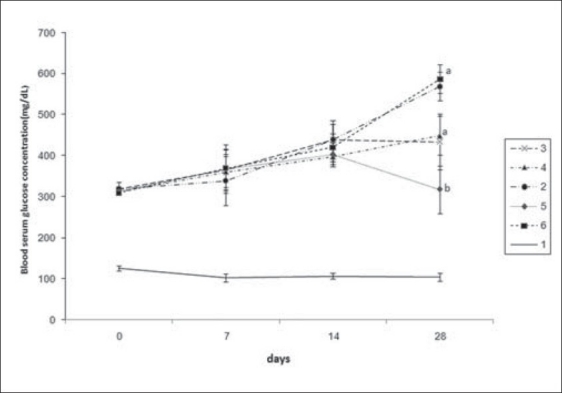
Blood serum glucose (mg/dl) concentration of male diabetic Wistar rats fed variant diets after 0, 7, 14 and 28 days feeding periods. Values are given as mean ± SEM in nine rats in each group. (a, b) Different letters indicate statistically different results. (P < 0.01), 1 = normal, 2 = diabetic + 0% guar gum, 3 = diabetic + 5% guar gum, 4 = diabetic + 10% guar gum, 5 = diabetic + 20% guar gum, 6 = diabetic + glibenclamide
Hypolipidemic effect of guar gum
The influence of guar gum (0, 5, 10 and 20%) and glibenclamide on total cholesterol, triglyceride, HDL- and LDL-C of diabetic rats for a period of 28 days is shown in Figure 2. Blood concentrations were determined on Days 7(A), 14 (B) and 28 (C) of the assay. It is noticeable that there was, in general, an increase in blood concentrations of the various components in the diabetic control compared with the normal rats. Although there was no significant difference in total cholesterol and triglyceride, HDL-C and LDL-C in the diabetic rats fed with 0% guar gum during 7 days of induction of diabetes compared with normal rats [Figure 2a], after 14 days, however, there was a significant increase in total cholesterol and triglyceride levels compared with normal rats (P < 0.05) [Figure 2b]. On the other hand, there was no significant difference in LDL and HDL-C of 14 days of diabetes[Figure 2b], but only LDL-C significantly increased after 28 days of treatment and, in rats fed with 10 and 20% guar gum, the elevated LDL-C in comparison with diabetic controls was reduced [Figure 2c].
Figure 2.
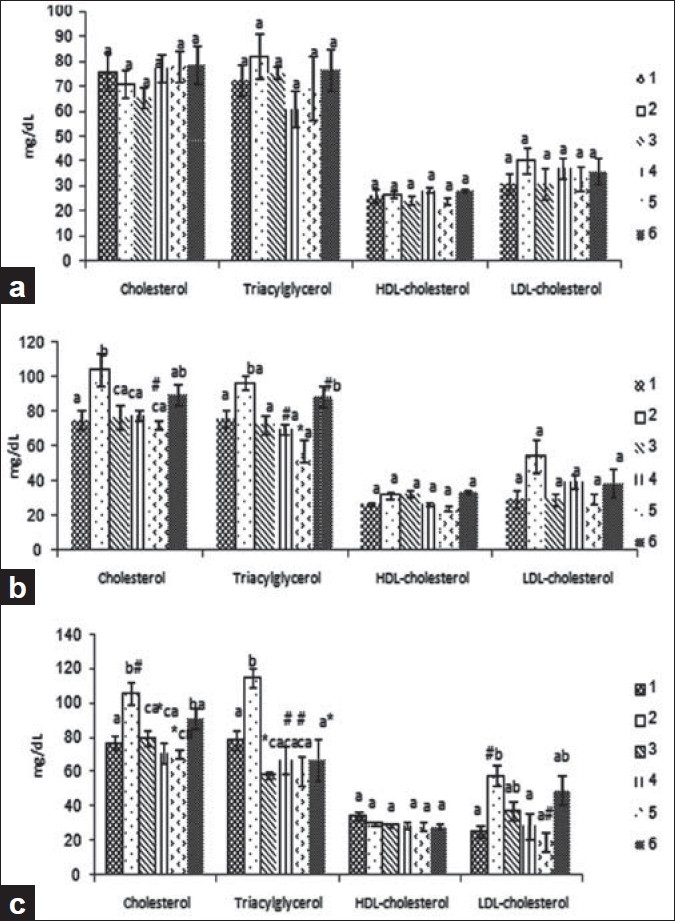
Blood serum cholesterol, triglycerides, HDL- and LDLcholesterol concentration (mg/dl) of male diabetic rats fed variant diet of the 7, 14 and 28 (a, b and c, respectively) days feeding periods. Values are given as mean ± SEM in nine rats in each group. (a–c) Different letters indicate statistically different results. 1 = normal, 2 = diabetic + 0% guar gum, 3 = diabetic + 5% guar gum, 4 = diabetic + 10% guar gum, 5 = diabetic + 20% guar gum, 6 = diabetic + glibenclamide. (# = P < 0.01, * = P < 0.001)
These data emphasize the strong hypocholestromic and hyperglycemic effects of diabetes after at least 2 weeks of induction of diabetes. Our data showed that after 14 days, animals fed diet containing 5, 10 and 20% guar gum had significantly lower levels of total cholesterol and triglycerides compared with diabetic controls (0% guar gum) (P < 0.01 and P < 0.001, respectively) but at both total cholesterol and triglyceride levels, there was no significant difference between the rats receiving glibenclamide and the diabetic controls [Figure 2b]. After 28 days of treatment with 5, 10 and 20% guar gum diets, the elevated total cholesterol and triglyceride levels in treated rats (P < 0.001 and P < 0.01, respectively) were significantly reduced. In the present study, treatment with guar gum diminished hyperlipidemia from 58.14 to 19.71 mg/dl, by 66.09% (P < 0.01). In relation with triglycerides, guar gum decreased the basal hypertriglyceridemia from 115.43 to 60.71 mg/dl, exerting a therapeutic effect of 49.74% (P < 0.001).
Effect of guar gum on body weight and food intake
The influence of various guar gum concentrations and glibenclamide in the diet on body weight and food intake of diabetic rats is presented in Tables 2 and 3, respectively. Streptozocin-diabetic rats were found to exhibit significant (P < 0.05) reduced body weight after 28 days compared with the first day of induction of diabetes. But, treatment with different doses of guar gum concentrations and glibenclamide elevated the reduced body weight so that there was no significant decrease in the body weight during the treatment period in rats fed the control diet [Table 2]. Diabetic controls and normal rats and the rats fed with glibenclamide showed significantly increased food intake after 28 days compared with the day of induction of diabetes. However, there was no significant increase in food intake in the rats treated with 5, 10 and 20% guar gum during the treatment period [Table 3].
Table 2.
Effect of different guar gum concentrations on body weight in different diabetic groups during 28 days. Values are mean (g) ± SEM (n = 9)

Table 3.
Effect of different guar gum concentrations on food intake in different diabetic groups during 28 days. Values are mean (g) ± SEM (n = 9)

As shown in Figure 3, the AI value was significantly elevated (35.6%) in diabetic rats versus normal rats (A). Administration of guar gum for a period of 28 days to diabetic rats showed that there was no significant difference in the value of AI in treated rats versus nontreated groups at 7 days of induction of diabetes; however, after 14 and 28 days of treatment of guar gum, the AI value was significantly decreased (62.5%) in all treated groups (5%, 10%, 20%) compared with the nontreated diabetic group (0% guar gum) (P < 0.001). Treatment with glibenclamide after 14 days of induction of diabetes showed no significant difference in comparison with the nontreated diabetic group but, after 28 days of induction of diabetes, administration of glibenclamide significantly reduced the AI value when compared with the nontreated diabetic groups even after 28 days of diabetes (P < 0.01).
Figure 3.
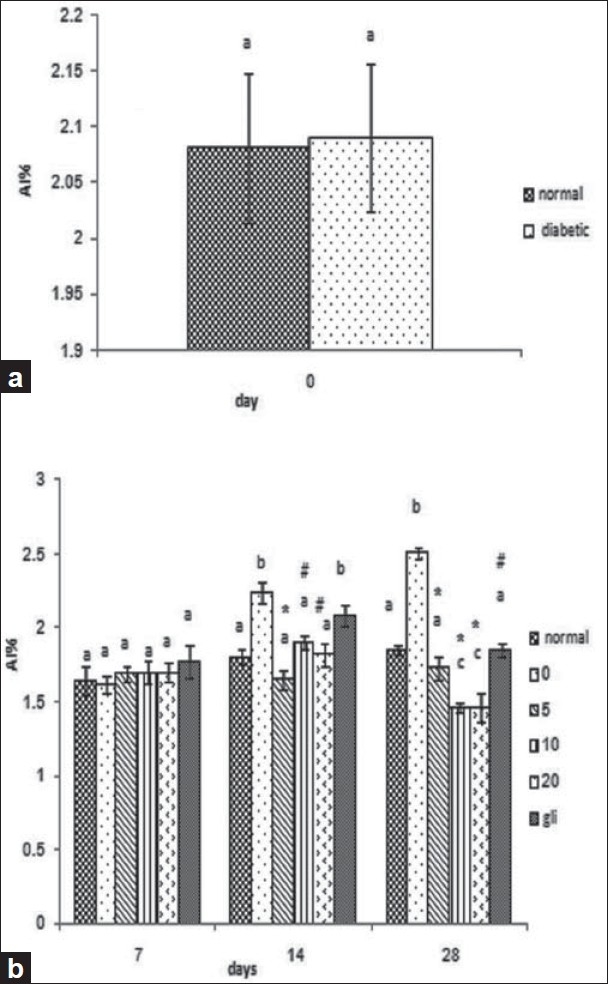
Effect of different guar gum concentrations on the atherogenic index (AI) level. (a) 0th day of induction of diabetes. (b) Results of the 7th, 14th and 28th day of treatment (treated group) with 5%, 10% and 20% are compared with diabetic controls. Each bar represents the mean ± SEM from nine rats. (a–c) Different letters indicate statistically different results (# = P < 0.01, * = P < 0.001). 1 = normal, 2 = diabetic + 0% guar gum, 3 = diabetic + 5% guar gum, 4 = diabetic + 10% guar gum, 5 = diabetic + 20% guar gum, 6 = diabetic + glibenclamide
DISCUSSION
In the present research, we found that streptozotocin produced characteristics of diabetes, like polyphagia, polyuria, heperglycemia and dyslipidemia. These results are consistent with those reported earlier.[25] Guar gum at three concentrations after end of treatment significantly (P < 0.05) decreased the diet intake compared with the starting level (zero time). Chronic feeding diabetic rats with different concentrations of guar gum prevent the loss of body weight, polyphagia and polyuria. In this study, in spite of an increase of other lipidemic parameters induced by diabetes, the rats fed guar gum diets showed lower serum cholesterol, triglycerides and LDL-C concentrations compared with diabetic controls (rats fed 0% guar gum diet). The mechanisms involved in the circulating and tissue cholesterol reduction are not yet clearly established. A few theories try to explain the involvement of fibers in the mechanism. Soluble fibers are very viscous and can decrease food intake due essentially to its effects on the gastric emptying time. The increased viscosity of the gastric content produced by the hydrophilic character of some gums slows the gastric emptying rate, increasing the satiety and, consequently, reducing the food intake.[26] Induction of diabetes by streptozotocin leads to loss of body weight due to increased muscle wasting and loss of tissue proteins.[27] The results achieved with the guar gum treatment in chronic diabetic models further defined the antidiabetic effects of the guar gum. After 28 days of guar gum treatment, gain in body weight was recognized in diabetic rats, and the results were comparable with that of the standard drug, glibenclamide. Intraperitoneal injection of depletes DNA ultimately produces fragmentation of the DNA of β-cells of the pancreas, which stimulates poly (ADP-ribose) and depletes DNA, ultimately leading to disrupting of β-cells that is confirmed by clinical symptoms of hyperglycemia and hypoinsulinemia.[28] In the present study, streptozotocin produced a significant increase in the glucose levels associated with a decrease in the insulin levels in type 1 diabetic rats. The administration of 20% guar gum in the daily diet reduced the fasting blood glucose by 44.2% by the forth week. The glycemic control produced by guar gum in this study was significantly efficient in comparison with the effect produced by glibenclamide. The results addressed in this paper on the effect of guar gum on blood serum glucose concentration agree with those of others who have also determined a reduction in the postprandial glycemia in diabetic individuals receiving fiber-like pectin in the diet.[29] However, there are studies on guar gum wherein no decrease was detected.[30] These conflicting results among researchers can probably be defined by the different fiber concentrations applied or by the different methods of fiber administration in the experiment. On the other hand, it has been demonstrated that fiber needs to be well mixed with the food that is to be ingested to allow its maximum capability.[31] Therefore, the effect of guar gum on lipid profile is controversial. Wilson showed that guar gum reduced the cholesterol level without reducing HDL,[32] but our data for the first time showed that dietary fiber (guar gum) reduces triglycerides, LDL-C and cholesterol and also increases HDL-C in diabetic rats. Therefore, the use of dietary fiber has become a medically recommended attitude. However, there is a wide variety of commercially available fibers and gums. The present work for the first time showed the effectiveness of insoluble fiber (guar gum) in all aspect of atherosclerosis diseases. Our data showed that guar gum not only decreases the lipid profile but also decreases the body weight, food intake and AI. In our data, we also focused on comparing the effect of guar gum and glibenclamide, a first-line option for treating type 2 diabetes, on the lipid profile in diabetic rats. Our data showed that guar gum (20%) for long-period treatment induced a hypoglycemic effect. In our knowledge, this is the first report showing that guar gum significantly induced hypoglycemic in diabetic rats versus those receiving the ordinary diabetic drug, glibenclamide.
It is also potential that the activity of soluble polysaccharides in reducing postprandial hyperglycemia is due to its viscosity. The diets rich in soluble fiber accommodate an increase of intestinal content viscosity as these fibers are molecules that hold water and have the property of forming colloidal gels. This decreases the association of food with the intestinal mucosa and the enzymatic digestion rate, consequently decreasing the intestinal absorption of monosaccharides and disaccharides.[32] The guar gum diets used in this investigation promoted an important development in the physiological conditions of the diabetic rats, essentially because the hyperglycemia in these animals was controlled throughout the treatment period. This improvement extended to body weight gain. Although the body weight gain was not high, it could be considered important as the diabetes condition usually induces catabolic processes that lead to body weight reduction due to the accelerated catabolism of protein, carbohydrate and lipids. The guar gum minimized these catabolic processes, providing higher nitrogen retention. In our study, the lower protein utilization by the rats in the control diabetic group could be a reflex of a high-reserve energy mobilization induced by the diabetes condition, leading to the significant weight loss observed. Other applied parameters for calculating the metabolic control on diabetics are triglycerides and cholesterol[33] in diabetic patients hypertriglyceridemia and low HDL-C levels are conventional.[34] In the present study, serum cholesterol, triglycerides and LDL-C levels of diabetic rats were shown to be significantly decreased by treatment with guar gum.
These results are in accommodation with other researches[35] noted in humans and in experimental animals, where there is a reduction of cholesterol levels by ingestion of soluble fibers such as pectin. Thus, reduction of cholesterol, triglyceride and LDL-C levels by guar gum may develop valuable effects on streptozotocin-induced cardiovascular complications. However, other researchers articled an increase in blood triglycerides but a reduction in cholesterol by applying pectin.[36] Because a marked decrease in triglyceride, total cholesterol and LDL-C levels was observed while an increase in HDL-C has not been observed in guar gum-treated diabetic rats, it may be suggested that HDL-C is not tightly inversely related to the total body cholesterol [Figure 2]. Several clinical researches have shown that guar gum absorption reduces the plasma cholesterol concentrations mainly due to a reduction of plasma LDL-C concentration, without affecting the HDL-C levels in normal, diabetic rats and in patients with hyperlipidemia.[37] In our study, there was no significant change in the blood serum HDL-C level of diabetic versus normal rats.
We can conclude that guar gum possesses an affecting pharmacological effect on type 2 diabetics. It was able to significantly decrease the fasting blood glucose in comparison with diabetic controls, while the total cholesterol, triglyceride and LDL-C concentrations were diminished. Therefore, this may result in their potential ability to decrease macrovascular complications.[38] It also reduced the food absorption drastically, but increased body weight gain or maintenance. This study confirmed the beneficial effects of guar gum intake in improving the condition of rats with experimentally induced diabetes. All these results may show that the antihyperglycemic effect produced by guar gum at the high dose is higher than that produced by glibenclamide.
ACKNOWLEDGMENTS
The authors would like to thank the Research Affairs of Mashhad University of Medical Sciences for financially supporting this work. They would also wish to thank the Javaneh Khorasan Company for making different concentrations of guar gum food for the rats.
Footnotes
Source of Support: Research Affairs of Mashhad University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Wei M. Excessive weight gain and effects on lipids with intensive therapy of type 1 diabetes. JAMA. 1998;280:140–6. [PubMed] [Google Scholar]
- 2.Sikarwar MS, Patil MB, Kokate CK, Sharma S, Bhat V. Antidiabetic activity of Nerium indicum leaf extract in alloxan-induced diabetic rats. J Young Pharm. 2009;1:330–5. [Google Scholar]
- 3.Chackrewarthy S, Thabrew MI, Weerasuriya MK, Jayasekera S. Evaluation of the hypoglycemic and hypolipidemic effects of an ethylacetate fraction of Artocarpus heterophyllus (jak) leaves in streptozotocin-induced diabetic rats. Phcog Mag. 2010;6:186–90. doi: 10.4103/0973-1296.66933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel G, Marie-Claude V, Patrick C, Sital M, Gerald T, Patrice P, et al. Contribution of receptor negative versus receptor defective mutations in the LDL-receptor gene to angiographically assessed coronary artery disease among young (25-49 years) versus middle-aged (50-64 years) men. Atherosclerosis. 1999;143:153–61. doi: 10.1016/s0021-9150(98)00268-8. [DOI] [PubMed] [Google Scholar]
- 5.Zenobi PD, Holzmann P, Glatz Y, Riesen WF, Froesch ER. Improvement of lipid profile in Type 2 (non-insulin-dependent) diabetes mellitus by insulin-like growth factor I. Diabetologia. 1993;36:465–9. doi: 10.1007/BF00402285. [DOI] [PubMed] [Google Scholar]
- 6.Dinh W, Füth R, Lankisch M, Bansemir L, Nickl W, Scheffold T, et al. Cardiovascular autonomic neuropathy contributes to left ventricular diastolic dysfunction in subjects with Type 2 diabetes and impaired glucose tolerance undergoing coronary angiography. Diabet Med. 2011;28:311–8. doi: 10.1111/j.1464-5491.2010.03221.x. [DOI] [PubMed] [Google Scholar]
- 7.Gatenby VK, Kearney MT. The role of IGF-1 resistance in obesity and type 2 diabetes-mellitus-related insulin resistance and vascular disease. Expert Opin Ther Targets. 2010;14:1333–42. doi: 10.1517/14728222.2010.528930. [DOI] [PubMed] [Google Scholar]
- 8.Brown A, Desai M, Taneja D, Tannock LR. Managing highly insulin-resistant diabetes mellitus: Weight loss approaches and medical management. Postgrad Med. 2010;122:163–71. doi: 10.3810/pgm.2010.01.2110. [DOI] [PubMed] [Google Scholar]
- 9.Boling CL, Westman EC, Yancy WS., Jr Carbohydrate-restricted diets for obesity and related diseases: An update. Curr Atheroscler Rep. 2009;11:462–9. doi: 10.1007/s11883-009-0069-8. [DOI] [PubMed] [Google Scholar]
- 10.Parvathi KM, Ramesh CK, Krishna V, Paramesha M, Kuppast IJ. Hypolipidemic activity of gum ghatti of Anogeissus latifolia. Phcog Mag. 2009;5:11–4. [Google Scholar]
- 11.Ruottinen S, Lagström HK, Niinikoski H, Rönnemaa T, Saarinen M, Pahkala KA, et al. Dietary fiber does not displace energy but is associated with decreased serum cholesterol concentrations in healthy children. Am J Clin Nutr. 2010;91:651–61. doi: 10.3945/ajcn.2009.28461. [DOI] [PubMed] [Google Scholar]
- 12.Gemen R, de Vries JF, Slavin JL. Relationship between molecular structure of cereal dietary fiber and health effects: Focus on glucose/insulin response and gut health. Nutr Rev. 2011;69:22–33. doi: 10.1111/j.1753-4887.2010.00357.x. [DOI] [PubMed] [Google Scholar]
- 13.Frias ACD, Sgarbieri VC. Guar gum effects on food intake, blood serum lipids and glucose levels of Wistar rats. Plant Foods Hum Nutr. 1998;53:15–28. doi: 10.1023/a:1008052216477. [DOI] [PubMed] [Google Scholar]
- 14.Acartürk F, Celkan A. Comparison of guar gum from different sources for the preparation of prolonged-release or colon-specific dosage forms. Pharm Dev Technol. 2009;14:271–7. doi: 10.1080/10837450802572375. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Wang J, Zhang J, Zhao B, Yao J, Wang Y. Structure-antioxidant relationships of sulfated galactomannan from guar gum. Int J Biol Macromol. 2010;46:59–66. doi: 10.1016/j.ijbiomac.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Kurakake M, Sumida T, Masuda D, Oonishi S, Komaki T. Production of galacto-manno-oligosaccharides from guar gum by beta-mannanase from Penicillium oxalicum SO. J Agric Food Chem. 2006;54:7885–9. doi: 10.1021/jf061502k. [DOI] [PubMed] [Google Scholar]
- 17.Bhosle S, Kothekar V. Mutagenic efficiency and effectiveness in cluster bean (Cyamopsis tertagonoloba (L.) Taub.) J Phytolo. 2010;2:21–7. [Google Scholar]
- 18.Pandit R, Phadke A, Jagtap A. Antidiabetic effect of Ficus religiosa extract in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2010;128:462–6. doi: 10.1016/j.jep.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Su HP, Lien CP, Lee TA, Ho JH. Development of low-fat mayonnaise containing polysaccharide gums as functional ingredients. J Sci Food Agric. 2010;90:806–12. doi: 10.1002/jsfa.3888. [DOI] [PubMed] [Google Scholar]
- 20.Roberfroid M. Dietary fiber, insulin and oligofructose: A review comparing their physiological effects. Crit Rev Food Sci Nutr. 1993;33:103–48. doi: 10.1080/10408399309527616. [DOI] [PubMed] [Google Scholar]
- 21.Brodribb JN. Dietary fiber in diverticular disease of the colon. In: Spiller GA, Kay RM, editors. Medical aspects of dietary fiber. New York: Plenum Press; 1980. [Google Scholar]
- 22.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 23.Rotimi SO, Omotosho OE, Rotimi OA. Persistence of acidosis in alloxan-induced diabetic rats treated with the juice of Asystasia gangetica leaves. Phcog Mag. 2011;7:25–30. doi: 10.4103/0973-1296.75887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinder P. Determination of glucose in blood using glucose oxidase with alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24. [Google Scholar]
- 25.Patel DK, Patel KA, Patel UK, Thounaojam MC, Jadeja RN, Ansarullah, et al. Assessment of lipid lowering effect of Sida rhomboidea: Roxb methanolic extract in experimentally induced hyperlipidemia. J Young Pharm. 2009;1:233–8. [Google Scholar]
- 26.Krotkiewski M. Effect of guar gum on body-weight hunger ratings and metabolism in obese subjects. Br J Nutr. 1984;52:97–105. doi: 10.1079/bjn19840075. [DOI] [PubMed] [Google Scholar]
- 27.Swanston-Flatt SK, Day C, Flatt PR, Gould BJ, Bailey CJ. Glycaemic effects of traditional European plant treatments for diabetes, Studies in normal and Streptozotocin diabetic mice. Diabetes Res. 1989;10:69–73. [PubMed] [Google Scholar]
- 28.Ray TK, Mansell KM, Knight LC, Malimud LS, Owen OE, Boden G. Long term effects of dietary fiber on glucose tolerance and gastric empting in non-insulin dependent diabetic patients. Am J Clin Nutr. 1983;37:376–81. doi: 10.1093/ajcn/37.3.376. [DOI] [PubMed] [Google Scholar]
- 29.Watters K, Blaisdell P. Reduction of glycemic and lipid levels in db/db diabetic mice by Psyllium plant fiber. Diabetes. 1989;38:1528–31. doi: 10.2337/diab.38.12.1528. [DOI] [PubMed] [Google Scholar]
- 30.Uusitupa M, Siitonen O, Savolainen K, Silvasti M, Penttila I, Parviainen M. Metabolic and nutritional effects of long term use of guar gum in the treatment of non-insulin-dependent diabetes of metabolic control. Am J Clin Nutr. 1989;49:345–1. doi: 10.1093/ajcn/49.2.345. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins DJ, Nineham R, Craddock C, Feely MC, Danaldson K, Snook J. Fiber and diabetes. Lancet. 1979;2:434–5. [Google Scholar]
- 32.Wilson TA, Behr SR, Nicolosi RJ. Addition of guar gum and soy protein increases the efficacy of the American Heart Association (AHA) step I cholesterol-lowering diet without reducing high density lipoprotein cholesterol levels in non-human primates. J Nutr. 1998;128:1429–33. doi: 10.1093/jn/128.9.1429. [DOI] [PubMed] [Google Scholar]
- 33.Thounaojam MC, Jadeja RN, Ansarullah B, Patel V, Devkar RV, Ramachandran AV. Potential of Sida rhomboidea. Roxb Leaf Extract in Controlling Hypertriglyceridemia in Experimental Models. Phcog Res. 2009;1:208–12. [Google Scholar]
- 34.Taskinen MR. Quantitative and qualitative lipoprotein abnormalities in diabetes mellitus. Diabetes. 1992;41:12–7. doi: 10.2337/diab.41.2.s12. [DOI] [PubMed] [Google Scholar]
- 35.Nishina PM, Schneeman BO, Freedland RA. Effects of dietary fibers on nonfasting plasma lipoprotein and apolipoprotein levels in rats. J Nutr. 1991;121:431–7. doi: 10.1093/jn/121.4.431. [DOI] [PubMed] [Google Scholar]
- 36.Judd PA, Truswell AS. Comparison of the effects of high- and low-methoxyl pectins on blood and faecal lipids in man. Bri J Nutr. 1985;48:451–8. doi: 10.1079/bjn19820130. [DOI] [PubMed] [Google Scholar]
- 37.Uberoi SK, Vadhera S, Soni GL. Role of dietary fiber from pulses and cereals as hypocholesterolemic and hypolipidemic agent. J Food Sci Technol. 1992;29:281–3. [Google Scholar]
- 38.Freie PM. Mechanism and clinical effects of pioglitazone as a new agent for the treatment of Type-2 diabetes. Arzneimittel Schung. 1999;49:835–42. doi: 10.1055/s-0031-1300511. [DOI] [PubMed] [Google Scholar]


