Abstract
Background:
The micropropagation protocol for Phyllanthus amarus, an important medicinal herb used widely for the treatment of hepatitis in ethnomedicinal systems, was standardized with shoot tip and single node explants.
Materials and Methods:
The micropropagation was carried out for the hyperproducing ecotype (phyllanthin content 463.828 ppm; hypophyllanthin content: 75.469 ppm) collected from Aanaikatti, Coimbatore, and grown in mist chamber, CPMB, TNAU. For micropropagation studies, the leaves were trimmed off and the shoot tips (6 mm long) and nodal segments (single node) were used for initiation.
Results:
Shoot tips and single node explants gave a maximum of 6.00 and 7.00 multiple shoots per explant with Benzyl Amino Purine (BAP) (1.0mg/L mg/L). Upon subculturing, a shoot length of around 7 cm with an average of eight internodes per shoot was observed after 20 days in the elongation medium supplemented with BAP (0.2 mg/Lmg/L) and Indole Acetic Acid (IAA) (2.0 mg/L). Seven to ten adventitious roots developed when the elongated microshoots were cultured in half strength MS medium with Indole Butyric Acid (IBA) (2.0 mg/Lmg/L) and NAA (1.0 mg/L mg/L) in 15-20 days after transfer. The rooted shoots acclimatized successfully to field conditions.
Conclusion:
A method for successful micropropagation of the valuable medicinal plant was established which will provide a better source for continuous supply of plants for manufacturing drugs.
Keywords: Antiviral properties, ethnomedicine, micropropagation, Phyllanthus amarus
INTRODUCTION
Medicinal plants are the source of various alkaloids and other chemical substances essential for mankind. Use of indigenous drugs from plant origin forms a major part of complementary, alternative and traditional medicine, and the total global herbal drug market is estimated to be US$ 62 billion and is expected to grow up to US$ 5 trillion by the year 2050.[1] India has a great wealth of traditional knowledge and wisdom, and the value of medicinal plants related trade in India is estimated at 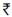 5000 crores per annum. As the demand for the plant-derived pharmaceutical compounds is increasing, possibilities for mass production need to be explored. Plant tissue culture techniques offer the rare opportunity to tailor the chemical profile of a phytochemical product by manipulation of the chemical or physical microenvironment, to produce a compound of potentially more value for human use.[2] Availability of the plant is subjected to seasonal variation, leading to uncertainty in stable supply throughout the year. Plant production under controlled conditions of in vitro system can eliminate these problems. Therefore, establishing a suitable micropropagation protocol for the high-yielding lines will have the potential of providing a better source for continuous supply of plants in the field of drug research as well as manufacturing of drugs.
5000 crores per annum. As the demand for the plant-derived pharmaceutical compounds is increasing, possibilities for mass production need to be explored. Plant tissue culture techniques offer the rare opportunity to tailor the chemical profile of a phytochemical product by manipulation of the chemical or physical microenvironment, to produce a compound of potentially more value for human use.[2] Availability of the plant is subjected to seasonal variation, leading to uncertainty in stable supply throughout the year. Plant production under controlled conditions of in vitro system can eliminate these problems. Therefore, establishing a suitable micropropagation protocol for the high-yielding lines will have the potential of providing a better source for continuous supply of plants in the field of drug research as well as manufacturing of drugs.
Antiviral properties of Phyllanthus amarus
Phyllanthus amarus, an important herbaceous medicinal plant, belongs to the large and complex genus Phyllanthus in the Euphorbiaceae family [Figure 1]. It is used in the traditional and folk medicines for the treatment of jaundice, asthma, hepatitis, tuberculosis, ulcer and urinary diseases. It is also used in stomach ailments like dyspepsia, colic, diarrhea, dysentery, dropsy, urinogenital problems and for external application in case of swelling and inflammation. P. amarus is distributed all over India, with varying medicinal quality. Hexane extract of P. amarus contains several hydrolyzable tannins and lignans, and the lignans such as niralin, nirtertralin and phyltetralin are strong inhibitors of protein kinase, responsible for its anticarcinogenic activity.[3] P. amarus was shown to be antihepatotoxic against carbon tetrachloride- and galactosamine-induced hepatotoxicity in primary cultured rat hepatocytes.[4] The antiviral activity of Phyllanthus species, particularly against hepatitis B virus (HBV) and Wood chunk Hepatitis Virus (WHV), was reported earlier.[5,6] A preliminary study reported that 59% of the HBV carriers treated for 30 days with the preparation of P. amarus lost HBV surface antigen.[7] The inhibition of hepatitis B surface antigen secretion by the down regulation of HBV mRNA transcription and replication by P. amarus was reported earlier.[8,9] A comparative study was made of P. amarus compound and interferon in the treatment of chronic viral hepatitis B and it was found that P. amarus compound had remarkable effect on the recovery of liver function and inhibition of the replication of HBV in chronic viral hepatitis B.[10] The crude extracts of P. amarus showed antigenotoxic action on tannery effluents treated Vicia faba was reported.[11] 10 lignans and a series of azalignans prepared from phyllanthin of P. amarus were structurally similar to two human immunodeficiency virus (HIV) reverse transcriptase inhibitors and showed antiviral activity against R5 pseudotype virus.[12] Antiviral activity of plants belonging to the Phyllanthus species against viruses other than HBV have also been reported. The organic and aqueous extracts of P. amarus, Phyllanthus urinaria, Phyllanthus arbiculatus and Phyllanthus pseudo-conami were tested for their plaque inhibition in mouse cell cultures of Sindbis virus (SV) and the herpesvirus murine cytomegalovirus (MCMV).[13] Both the types of extracts of all the four species inhibited MCMV when given prior to infection. In addition, the organic extracts showed effects post infection of SV. The extracts of less common species such as Phyllanthus mimicus and Phyllanthus odontadenius also were reported to have anti-DNAp activity.[14] The lignans phyllanthin and hypophyllanthin enhanced the cytotoxic responses with cultured multi-drug resistant cells.[15] The hydro-alcoholic extracts of five Ayurvedic medicinal plants, pericarp of Terminalia chebula, rhizome of Acorus calamus, stem bark of Bauhinia variegate, whole plant of P. amarus, and root of Glycyrrhiza glabra, were evaluated for their antiproliferative activity on 14 cancer cell lines. These plant extracts when tested by sulforhodamine-B (SRB) assay were found to be active against prostrate cancer cell line (DU145), except G. glabra.[16]
Figure 1.

Phyllanthus amarus – A hepatoprotective herb
MATERIALS AND METHODS
Preparation of nutrient media
MS nutrient medium[17] was used for micropropagation studies. The macro, minor and micronutrients, vitamins and myo-inositol were taken from stock solutions according to the concentration of the basal medium and mixed to known quantity of distilled water. Sucrose 30 g/L was also added and mixed well. The growth regulators at required concentration were added and homogenized. The volume was made up to 1 L after the pH of the medium was adjusted to 5.6–5.8 using 0.1 N HCI or 0.1 N NaOH. The agar 8 g/L was dissolved at the time of boiling with constant stirring. The medium was then distributed to culture tubes of 250 × 150-mm size or into bottles of 250 mL capacity, which were already sterilized. The culture tubes were plugged with non-absorbent cotton and the bottles with plastic autoclavable caps. Then, they were sterilized in an autoclave at a temperature of 121°C at a pressure of 15 pounds per inch for 20 min.[18]
Collection of plant material
The explants were collected from plants grown in net house. For micropropagation studies, the leaves were trimmed off and the shoot tips (6 mm long) and nodal segments (single node) were used for initiation. The explants were first washed in running tap water followed by treatment with Tween 20 emulsifier solution (two to three drops in 100 mL distilled water) for 5 min. After the distilled water wash for two to three times, the explants were taken to the laminar airflow chamber for further sterilization. Shoot tips and nodal segments were initially sterilized in ethyl alcohol (70%) for 30 seconds followed by sterilization in 0.1% mercuric chloride for 5 min. The treated explants were then washed four to five times with sterile distilled water to make them free from sterilants.
Inoculation of explants
The working table of the laminar airflow chamber was first surface sterilized with absolute alcohol. The Petri dishes and tools (forceps, blade) used for preparation and inoculation of the explants were sterilized in autoclave at 15 pounds per inch at 121°C for 20 min and kept in the laminar airflow chamber. The ultraviolet light was switched on for 20 min before inoculation. The ultraviolet light was switched off before inoculation and the laminar flow was put on for 10 min. Hands were sterilized with 70% alcohol. The tools were sterilized in the glass bead sterilizer, cooled off and used for inoculation. The explants were inoculated after giving a cut on both sides of the exposed surfaces. All these cultures were incubated at 25 ± 2°C at a relative humidity of 60–70% with a light intensity of 2000 lux using white fluorescent lamps. A photoperiod of 16 hours light and 8 hours darkness was maintained.
RESULTS
Effect of cytokinin (Benzyl Amino Purine) on multiple shoot induction using single node cuttings
Bud induction was seen after 2–3 weeks of inoculation. Maximum number of multiple shoots (7.00) was induced when the single node cuttings were inoculated in MS medium supplemented with Benzyl Amino Purine (BAP) (1.0 mg/L) [Table 1]. Dense clumps of multiple shoots were obtained in the medium containing optimum concentrations of BAP 1.0 mg/L [Table 1; Figure 2].
Table 1.
Effect of Benzyl Amino Purine on multiple shoot induction using single node and shoot tip explants
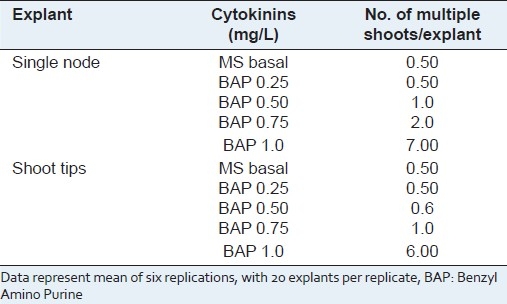
Figure 2.
Micropropagation of P. amarus using shoot tips and nodal explants
Effect of Benzyl Amino Purine on multiple shoot induction using shoot tip explants
Maximum numbers of multiple shoots were induced (6.00) when the shoot tips were inoculated in MS medium supplemented with BAP (1.0 mg/L) [Table 1; Figure 2]. There was callusing at the base when BAP was used continuously during further subculturing.
Multiplication of shoots
Two different explants were used, shoot tip and the single node explants, for initiating the aseptic cultures. For multiple shoot induction, MS medium with the BAP 1.0 mg/L was used. Thirty days after inoculation, the multiple shoots initiated were separated. Single node cuttings (after trimming of the leaves) were again inoculated into fresh medium with the same concentration of growth regulators as that of the induction medium for further multiple shoots induction.
Effect of Benzyl Amino Purine and Indole Acetic Acid on shoot elongation
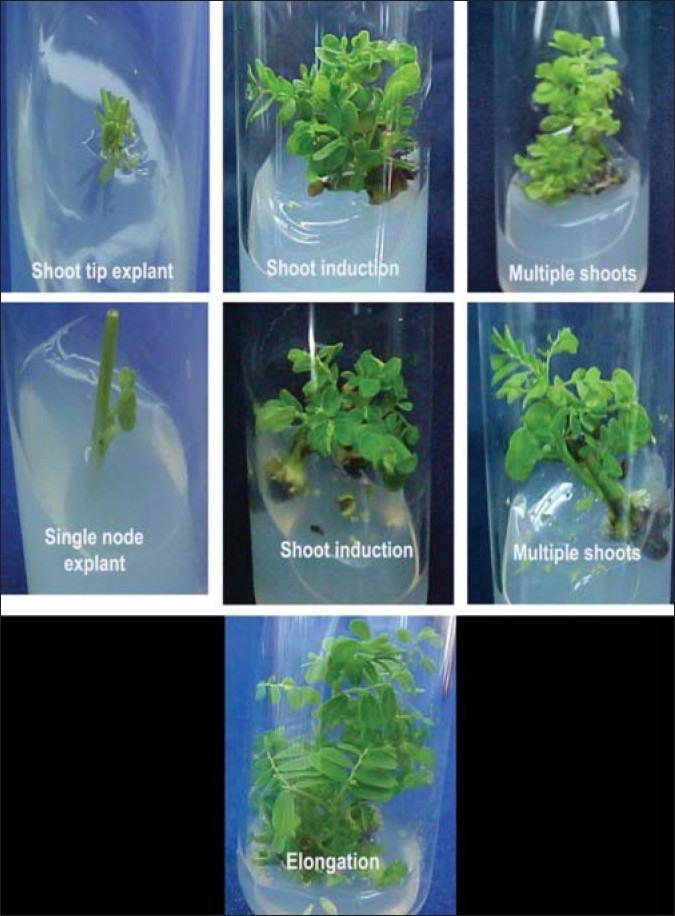
For shoot elongation, the individual shoots were excised and inoculated in MS medium supplemented with either BAP alone (0.2 mg/L) or BAP (0.2 mg/L) and Indole Acetic Acid (IAA) (2.0 mg/L). After a week, basal callus was seen in all the cultures. Shoot length of around 7 cm was recorded after 20 days of subculturing with an average of eight internodes in the medium supplemented with BAP and IAA. Shoot length of only 5 cm with six internodes was observed in medium with BAP [Figure 3].
Figure 3.
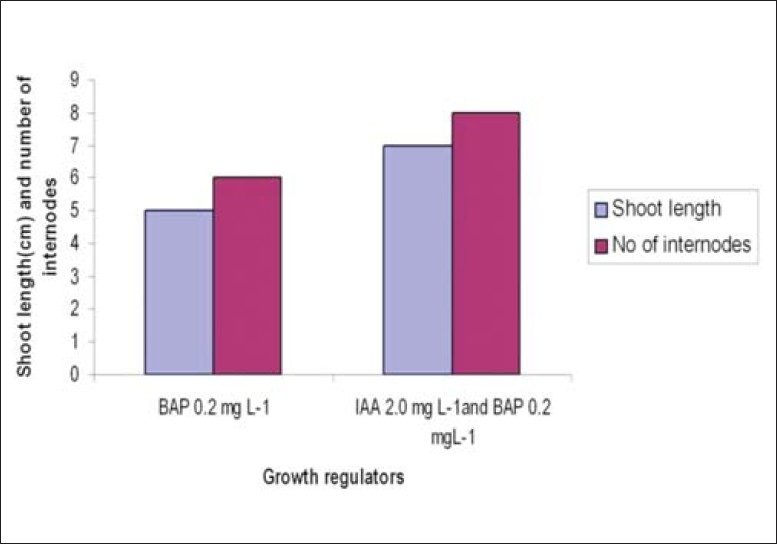
Effect of Benzyl Amino Purine and Indole Acetic Acid on shoot elongation
In vitro rooting
Twenty days after subculturing in the shoot elongation media, the elongated microshoots were transferred to half strength MS medium supplemented with Indole Butyric Acid (IBA) (2.0 mg/L) and NAA (1.0 mg/L). Seven to ten adventitious roots initiated from the cut ends of the microshoots in 15–20 days.
DISCUSSION
The exploitation of tissue culture techniques in medicinal plants is indeed desirable for their in vitro propagation and extraction for important chemical compounds.[19] In recent times, the demand of P. amarus plant has increased owing to its antiviral property, which has triggered the indiscriminate harvesting from the natural flora. Moreover, the availability of the plant is subject to seasonal variation, leading to uncertainty in stable supply throughout the year.[20] Hence, it has become imperative to establish a suitable protocol for its micropropagation. In view of the growing demand and for continuous supply of plants to be used for extraction of the pure compounds, the micropropagation was carried out for the hyperproducing ecotype (phyllanthin content: 463.828 ppm; hypophyllanthin content: 75.469 ppm)[21] collected from Aanaikatti, Coimbatore, and grown in mist chamber, CPMB, TNAU.
In the present study, maximum number of multiple shoots (7.00 and 6.00) was induced when single node explants and the shoot tips were inoculated in MS medium supplemented with BAP (1.0 mg/L, respectively). Shoot tips are the best source of explants for multiple shoot induction in case of P. amarus, Vanda coerulea and Catalpa ovate.[22–24] Similar observations were made during shoot multiplication in Phyllanthus fraternus with BAP 1.0 mg/L.[25] But the frequency of the multiple shoots was more when BAP was used at 0.5 mg/L with 15% coconut milk (CM).[26]
In the present study, the in vitro developed shoots elongated better in a medium supplemented with BAP with IAA than in a medium with BAP alone. Lowering of BAP was needed to prevent further induction of multiple shoots. Shoots grew to a length of around 7 cm with an average of eight internodes after 20 days of subculturing in the medium supplemented with BAP and IAA, whereas shoot length of around 5 cm with approximately six internodes was noticed in the medium with BAP alone. Higher concentrations of BAP were observed to inhibit the formation of shoots, and the shoots so formed were thick and short. Other workers have also reported similar thick, rosette type of shoot formation in higher concentrations of BAP in the case of Melissa officinalis and Hedeoma multifolium.[27,28]
Rooting occurred spontaneously in Phyllanthus species without the addition of any growth regulators.[29] The shoot elongation was accompanied by spontaneous rooting during the multiplication stage in the medium supplemented with IAA and kinetin or IAA and BAP.[21] But in the present study, profuse rooting (7–10 roots/plantlet) was induced in 15–20 days when the elongated shoots were transferred to a medium with IBA (2.0 mg/L) and NAA (1.0 mg/L). The role of IBA in favoring the conjugation between endogenous IAA and amino acid that leads to the synthesis of the specific proteins necessary for the formation of root initiation was already reported.[30] The promoting effect of IBA in root formation was also reported in the case of Centella asiatica[31] and Acacia sinuate.[32] The rooting frequency, number of roots per explant and the root length were more in the medium containing 0.5 mg/L IBA or NAA.[22] The rooted shoots were successfully transplanted (70%) to plastic cups containing garden soil, and the humidity was maintained at approximately 80%. The plants were watered twice every 7 days, and after 3 months they were transferred to larger pots for acclimatization.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Kalpana J, Chavan P, Warude D, Patwardhan B. Molecular markers in herbal drug technology. Curr Sci. 2004;87:159–65. [Google Scholar]
- 2.Trigiano RN, Grey DJ. Plant tissue culture concepts and laboratory exercises. London: CRC press; 2000. [Google Scholar]
- 3.Anjaneyulu AS, Jaganmohan R, Row LR, Subrahmanyam C. Crystalline constituents of Euphorbiaceae. xii. isolation and structural elucidation of three new lignans from the leaves of Phyllanthus niruri. Tetrahedron. 1973;29:1291. [Google Scholar]
- 4.Syamasunder KV, Singh B, Thakur RS, Hussain A, Kiso Y, Hkino H. Antihepatotoxic principles of Phyllanthus niruri herbs. J Ethnopharmacol. 1989;14:41–4. doi: 10.1016/0378-8741(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 5.Venkateswaran PS, Millman I, Blumberg BS. Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: In vivo and in vitro studies. Proc Natl Acad Sci U S A. 1987;84:274–8. doi: 10.1073/pnas.84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg BS, Milman I, Venkateswaran PS, Thangarajan SP. Hepatitis B virus and hepatocellular carcinoma- treatment of HBV carriers with Phyllanthus amarus. Cancer Detect Prev. 1989;14:195–201. [PubMed] [Google Scholar]
- 7.Thyagarajan SP, Subramanian S, Thirunalasundari T, Venkateswaran PS, Blumberg BS. Preliminary study: The effect of Phyllanthus amarus on chronic carriers of Hepatitis B virus. Lancet. 1988;2:764–6. doi: 10.1016/s0140-6736(88)92416-6. [DOI] [PubMed] [Google Scholar]
- 8.Jayaram S, Thyagarajan SP. Inhibition of HBsAg secretion from Alexander cell line by Phyllanthus amarus. Indian J Pathol Microbiol. 1996;39:211–5. [PubMed] [Google Scholar]
- 9.Lee CD, Ott M, Thyagarajan SP, Shafritz DA, Burk RD, Gupta S. Phyllanthus amarus down-regulates hepatitis B virus mRNA transcription and replication. Eur J Clin Invest. 1996;26:1069–76. doi: 10.1046/j.1365-2362.1996.410595.x. [DOI] [PubMed] [Google Scholar]
- 10.Xin-Hua W, Chang-Qing L, Xing-Bo G, Lin-Chun F. A comparative study of Phyllanthus amarus compound and interferon in the treatment of chronic viral hepatitis B. Southeast Asian J Trop Med Public Health. 2001;32:140–2. [PubMed] [Google Scholar]
- 11.Gowrishanker B, Vivekanandan OS. In vivo studies of a crude extract of Phyllanthus amarus L. in modifying the genotoxicity induced in Vicia faba L by tannery effluents. Mutat Res. 1994;322:185–92. doi: 10.1016/0165-1218(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Sagar KS, Chang CC, Wang WK, Lin JY, Lee SS. Preparation and anti-HIV activities of retrojusticidin B analogs and azalignans. Bioorg Med Chem. 2004;12:4045–54. doi: 10.1016/j.bmc.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Macrae WD, Hudson JB, Towers GH. Studies on pharmacological activities of Amazonian Euphorbiaceae. J Ethnapharmacol. 1988;22:143–72. doi: 10.1016/0378-8741(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 14.Unander DW. Callus induction in Phyllanthus species and inhibition of viral polymerase and reverse transcriptase by callus extracts. Plant Cell Rep. 1991;10:461–6. doi: 10.1007/BF00233815. [DOI] [PubMed] [Google Scholar]
- 15.Somanabandhu A, Nitayangkura S, Mahidol C, Ruchirawat S, Likhitwitayawuid K, Shieh L, et al. 1H and 13C-NMR assignments of phyllanthin and hypophyllnathin: Lignans that enhance cytotoxic responses with cultured multidrug- resistant cells. J Nat Prod. 1993;56:233–9. doi: 10.1021/np50092a008. [DOI] [PubMed] [Google Scholar]
- 16.Gaidhani SN, Lavekar GS, Juvekar AS, Sen S, Singh A, Kumari S. In-vitro anticancer activity of standard extracts used in ayurveda. Pharmacogn Mag. 2009;5:425–9. [Google Scholar]
- 17.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–9. [Google Scholar]
- 18.Dodds JH, Roberts LW. Experiments in tissue culture. London: Cambridge University Press; 1982. p. 278. [Google Scholar]
- 19.Tabata M. Recent advances in the production of medicinal substances by plant cell cultures. Plant tissue culture and its Bio-Technological Application. Proceedings of the First International Congress on Medicinal Plant Research. 1976 Sep 6-10, 3-16, 1976; [Google Scholar]
- 20.Rajasubramaniam S, Paradhasaradhi P. Rapid multiplication of Phyllanthus fraternus: A plant with anti- hepatitis viral activity. Ind Crops Prod. 1997;6:35. [Google Scholar]
- 21.Raj Janifer X. 2006. Coimbatore-3: MSc Thesis, CPMB, Tamil Nadu Agricultural University; Micropropagation of Phyllanthus amarus with high Phyllanthin and Hypophyllanthin and molecular profiling of Phyllanthus species. [Google Scholar]
- 22.Bhattacharyya R, Bhattacharya S. High frequency in vitro propagation of Phyllanthus amarus by shoot tip culture. Indian J Exp Biol. 2001;39:1184–7. [PubMed] [Google Scholar]
- 23.Katarzyna L, Halina W. In vitro propagation of Cataipa ovata. Plant Cell Tissue Organ Cult. 2000;60:171–6. [Google Scholar]
- 24.Seeni S, Latha PG. In vitro multiplication and ecohabituation of endangered blue vanda (Vanda coerulea) Plant Cell Tissue Organ Cult. 2001;6:1–8. [Google Scholar]
- 25.Saradhi PP, Islamia JM. Phyllanthus fraternus- A plant with antihepatitis viral activity. Ind Crops Prod. 1997;6:35–40. [Google Scholar]
- 26.Ghanti KS, Govindaraju B, Venugopal RB, Ramgopal Rao S, Kaviraj CP, Jabeen FT, et al. High frequency regeneration from Phyllanthus amarus Schum. and Thonn. Indian J Biotechnol. 2004;3:103–7. [Google Scholar]
- 27.Tavare AC. Micropropagation of Melissa officinalis through proliferation of axillary shoots. Plant Cell Rep. 1996;15:441. doi: 10.1007/BF00232072. [DOI] [PubMed] [Google Scholar]
- 28.Koroch AR. Micropropagation and acclimatization of Hedeoma multifolium. Plant Cell Tissue Organ Cult. 1997;48:213. [Google Scholar]
- 29.Catapan E, Luis M, da Silvia B, Moreno FN, Viana AM. Micropropagation, callus and root culture of Phyllanthus urinaria. Plant Cell Tissue Organ Cult. 2002;70:301–9. [Google Scholar]
- 30.Ryugo TC, Breen PJ. IAA metabolism in cutting of plum (Prunus cerasifura, X.P. Munsoniane cv. Mariana 2624) Proc Am Soc Hortic Sci. 1994;99:247. [Google Scholar]
- 31.Tiwari K. Micropropagation of Centella asiatica a valuable medicinal herb. Plant Cell Tissue Organ Cult. 2000;63:23–8. [Google Scholar]
- 32.Vangadesan G. In vitro organogenesis and plant formation in Acacia sinuata. Plant Cell Tissue Organ Cult. 2000;61:23–8. [Google Scholar]


