Abstract
Background:
Microdissection testicular sperm extraction (micro-TESE) has replaced conventional testis biopsies as a method of choice for obtaining sperm for in vitro fertilization for men with nonobstructive azoospermia. A technical challenge of micro-TESE is that the low magnification inspection of the tubules with a surgical microscope is insufficient to definitively identify sperm-containing tubules, necessitating tissue removal and cytologic assessment. Full field optical coherence tomography (FFOCT) uses white light interference microscopy to generate quick high-resolution tomographic images of fresh (unprocessed and unstained) tissue. Furthermore, by using a nonlaser safe light source (150 W halogen lamp) for tissue illumination, it ensures that the sperm extracted for in vitro fertilization are not photo-damaged or mutagenized.
Materials and Methods:
A focal Sertoli-cell only rodent model was created with busulfan injection in adult rats. Ex vivo testicular tissues from both normal and busulfan-treated rats were imaged with a commercial modified FFOCT system, Light-CT™, and the images were correlated with gold standard hematoxylin and eosin staining.
Results:
Light-CT™ identified spermatogenesis within the seminiferous tubules in freshly excised testicular tissue, without the use of exogenous contrast or fixation. Normal adult rats exhibited tubules with uniform size and shape (diameter 328 ±11 μm). The busulfan-treated animals showed marked heterogeneity in tubular size and shape (diameter 178 ± 35 μm) and only 10% contained sperm within the lumen.
Conclusion:
FFOCT has the potential to facilitate real-time visualization of spermatogenesis in humans, and aid in micro-TESE for men with infertility.
Keywords: micro-TESE, rat model, sertoli cell only, testis
INTRODUCTION
Advances in sperm retrieval techniques, such as microdissection testicular sperm extraction (micro-TESE) coupled with intracytoplasmic sperm injection (ICSI), has made it possible for men with severely impaired sperm production to father their own children.[1] Nonobstructive azoospermia (NOA), the lack of sperm in the ejaculate, affects nearly 1% of all men and 10%--15% of infertile men.[2] Micro-TESE is a technically demanding procedure that uses an operating light microscope to direct testis biopsies.[3] Micro-TESE requires a significant learning curve and long operative times, often 4-5 h.[1,4]
An important technical challenge with micro-TESE has been the inability to definitively identify seminiferous tubules that contain sperm, without removing testicular tissue. In the micro-TESE procedure, after surgically opening the tunica albugenia and exposing the testicular tissue, the tubules are subjectively evaluated for the presence of sperm, based on tubular size and color. Tubules deemed to contain sperm are then removed, and a cytological preparation is made in the operating room to assess whether sperm indeed are present in the tissue removed. If no sperm are found, the process continues with more tubules being similarly assessed. With this technique, sperm have been retrieved in 40%--60% of men depending on the underlying cause of low sperm production[5,6] and the experience of the operating surgeon.
Infertile men show significantly lower serum testosterone levels at baseline due to impaired Leydig cell function.[7] The removal of tissue for assessment, as described above, inevitably causes the loss of Leydig cells, which can further reduce serum testosterone levels, resulting in serious long-term consequences such as osteoporosis, increased insulin resistance, and depression.[8] Sometimes, these procedures can unfortunately result in a permanent decrease in serum testosterone levels,[9,10] requiring long-term testosterone replacement therapy. Optimizing the ability to identify sperm-containing tubules before removing the tissue could reduce these potential risks and increase the benefit:risk ratio for men attempting fatherhood by this method.
Full-field optical coherence tomography (FFOCT) is a high-resolution “optical biopsy” technique that can render quick images of freshly excised (unprocessed and unstained) tissue.[11–13] This technique makes use of a simple tungsten halogen lamp and is based on the principle of white light interference microscopy.[14,15] FFOCT has recently been utilized in several areas of clinical research, including ophthalmology,[16] cardiology,[17] gastroenterology,[18] and pulmonology.[19]
The goal of this study was to evaluate the feasibility of a commercial FFOCT device (Light-CT™, LLTech SAS, Paris, France) to identify the presence of spermatogenesis within the seminiferous tubules of the testes in a rat model. Utilizing FFOCT to directly visualize tubules has the potential to improve success in micro-TESE procedures, while minimizing the removal of unnecessary biopsies and thus reducing operative time and associated potential complications. Additionally, FFOCT-guided testis biopsy could prevent loss of Leydig cells in interstitial testicular tissue, thereby potentially decreasing the risk of male hypogonadism.
One specific advantage of FFOCT over other competing optical biopsy techniques, most of which use powerful lasers as the illumination source, is the fact that this technology uses very safe incident light, coming from a 150 W halogen lamp. Since the sperm isolated during this procedure will be used for ICSI, it is imperative that they not be photo-damaged---even at subclinical levels---by exposure to intense and potentially mutagenic light. The use of this safe illumination makes FFOCT especially suitable for the application under investigation in this report.
MATERIALS AND METHODS
Animals and Treatment
Adult male Sprague-Dawley rats were obtained from Charles River Laboratories, Wilmington, MA. The rats were housed at a constant temperature (20–23°C), illumination (12-h light/12-h dark cycle, light on at 08:00 h) with food and water ad libitum. Since it is insoluble in both water and oil, busulfan (ICN Biochemicals, USA) was freshly dissolved in dimethyl sulfoxide (DMSO) at a concentration of 2% w/v, immediately prior to use. Four adult male rats (250–300 g) were injected intraperitoneally with two doses of busulfan (10 mg/kg body weight) 24 days apart, as described previously.[20] At low doses, busulfan destroys the spermatogenic epithelium but not the spermatogenic stem cells, which leaves the animal temporarily sterile.[20] Finally, 45 days following the second busulfan injection, the animals were sacrificed, and the testes were imaged with Light-CT™ (as described below). Then the testes were fixed in Bouin's solution and subsequently stored in 70% ethanol until they were embedded in paraffin, sectioned, and mounted onto slides for hematoxylin and eosin (HandE) staining. Four control animals, housed under the same conditions, were sacrificed with the experimental group. Their testes were imaged and processed in the manner identical to that described for the experimental group.
Sample Preparation
The tunica albuginea of the testes (~0.5 mm thick) were cut in order to expose the seminiferous tubules. The exposed testicular tissue was immersed in an isotonic solution of phosphate buffered saline (PBS; 2.7 mM potassium chloride and 137 mM sodium chloride; pH 7.4) and placed in a sample holder (provided with the Light-CT™ system). A clean silica cover-slip was placed on top of the sample. The holder cover was closed by gently moving the base of the holder so that the sample was slightly flattened, in order to provide an even imaging surface. Precaution was taken to avoid any air bubbles in the sample. A thick layer of silicone oil was applied on the silica cover-slip as the immersion medium, and the specimen was imaged through a 10x/0.3 NA water immersion objective (Olympus America, Center Valley, PA).
FFOCT Instrumentation
Light-CT™ is a modified FFOCT system that allows real time corrections and calculations, so that visually meaningful contrast-enhanced images are displayed within seconds to minutes after the acquisition of the tomographic images. A 150 W white halogen lamp is used as the illumination source. The system consists of an upright microscope with an object and a reference arm in Linnik interferometric configuration.[11] Two 10×/0.3 numerical aperture (NA) water immersion objectives are used---one in each arm. The field of view of a single acquisition frame is 1 mm2, with 2, and 1 μm lateral and axial resolution, respectively. The frame rate for the system for tomographic acquisitions is 69 Hz. Given that ~60 images are typically averaged to obtain good-quality data, the frame rate for single frame acquisitions is roughly 1 Hz (i.e., one frame/sec).
Image Acquisition
The objective lens was focused on the sample through a motorized adjustment of the whole interferometer. To secure the virtual thinness of the slice and to optimize signal to noise ratio, the instrument was calibrated every day prior to imaging.
Large Surface Acquisition
The large field image function was used when a broad area on a sample needed to be scanned. The surface area to be imaged was defined by setting a reference point as zero and the XY coordinates were defined by moving a joystick. A final stitched image was generated by the integrated image processing module.
Stack Acquisition
The stack function was used to obtain depth resolved images from the samples. The number of stacks and the distance between each stack was user defined. For this experiment the specimens were imaged from the surface until 60 μm deep, at 10 μm intervals.
Image Processing
The images were processed in real time with a DICOM (Digital Imaging and Communication in Medicine) viewer and saved. They were read and further processed with the Image J software (National Institutes of Health, Bethesda, MD), if necessary. Speckle noise was minimized using Gaussian filtering in Adobe Photoshop CS5 (San Jose, CA).
RESULTS
FFOCT was able to visualize the structures within the seminiferous tubules from a normal Sprague-Dawley rat. The tubules were relatively uniform in size and shape (diameter 328 ± 11 μm). Mature tubules showed a well-defined dark lumen with bright white hair-like structures indicating spermatogenesis [Figure 1a]. We also present a movie [Video 1], showing the optical sections through an ex vivo mouse testis. Please note that as we near the center of a tubule, we observe the dark lumen, containing bright hair-like sperm. This uniformity and the presence of intra-luminal sperm was clearly observed in the corresponding HandE stained histological section [Figure 1b].
Figure 1.
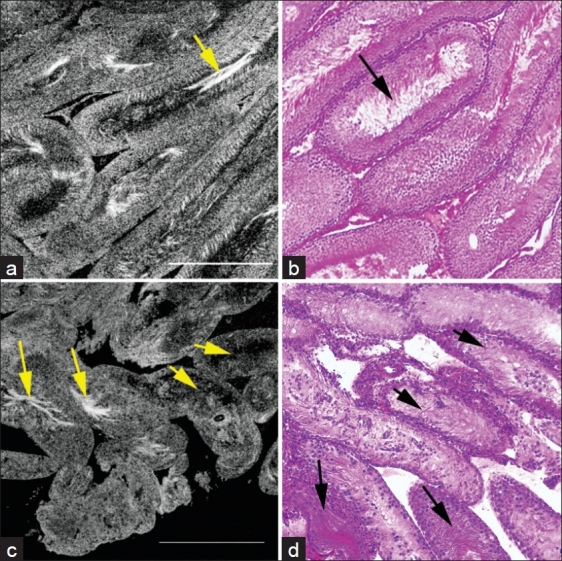
Comparative FFOCT and HandE-stained histology images from the testes of normal adult Sprague-Dawley rats, and rats exhibiting a sertoli-cell only phenotype. (a) Seminiferous tubules in the testis of a normal rat. The tubules are relatively uniform in size and shape (diameter 328 ± 11 μm). (b) Same specimen processed and stained for conventional (HandE) histology. Arrows point to the sperm within the tubule lumen. (c) Seminiferous tubules in the testis of a rat treated with busulfan. Tubules, on average are thinner, and show a greater degree of heterogeneity in size and shape (diameter 178 ± 35 μm). Only ~10% of the tubules show normal spermatogenesis as identified by presence of sperm tails (bright white hair-like structures within the lumen; long arrows). The remainder of the tubules showed no sperm within the lumen (short arrows). (d) HandE staining of the same specimen confirms the observations. Long and short arrows point to tubules with and without spermatogenesis, respectively. Field of view in each panel: 1 mm2
Following busulfan treatment, testicular size decreased by ~50% compared to the untreated animal. Imaging with FFOCT demonstrated marked heterogeneity in size and shape of the tubules (diameter 178 ± 35 μm). Only ~ 10% of the tubules showed normal spermatogenesis as identified by presence of sperm tails (bright white hair-like structures) within the lumen. The remainder of the tubules showed no sperm within the lumen [Figure 1c]. This finding was correlated with the HandE staining of the same specimen [Figure 1d].
CONCLUSIONS
We evaluated the feasibility of Light-CT™ to reliably identify tubules containing sperm by imaging testicular specimens from a busulfan-treated rodent model. Ability of Light-CT™ to reliably recapitulate histology of seminiferous tubules could potentially make FFOCT an ideal tool in identifying sperm within the tubules. In the busulfan-treated testis, Light-CT™ was able to distinguish between tubules with and without spermatogenesis, based on what was visualized in the lumen.
Tubules with mature sperm contained bright signal within the lumen (sperm tails), similar to those observed in the normal adult rats. The bright signal emanates from the unique structure of sperm tails, which are composed of microtubules surrounded by a dense fibrous sheath.[21] In our follow up studies, we propose to image human testicular biopsy specimens with FFOCT, in order to assess whether similar characteristics as those found here with rat testes can be used to identify tubules with spermatogenesis. Specifically for human biopsies, we propose to assess the ability to FFOCT to utilize the thickness of the lining epithelium of the seminiferous tubules as a variable to determine the presence of spermatogenesis.
Some advantages of Light-CT™ are the speed (approximately 1 frame/s) and the ease with which tomographic images can be obtained from relatively large areas of tissue. The use of a halogen lamp as the light source ensures that the technique is safe for use in the clinical setting, especially since the sperm retrieved from this procedure are intended for use in ICSI. The incident power used for Light-CT™, approximately 1 mW/mm2 centered around 750 nm, is significantly lower than the safety limits for near-infrared wavelengths recommended both by the European and the American standards. While confocal microscopy[22] can provide similar resolution images as FFOCT, these devices have several drawbacks, including the use of relatively high powered lasers as their light source, utilization of high numerical aperture objectives resulting in smaller fields of view, use of visible light for illumination, thereby limiting the depth of imaging in tissue, and a longer time to generate images of comparable dimensions, since the images are generated by raster scanning of a focused laser beam, rather than a whole frame capture with a CCD camera.
The major limitations of the FFOCT device used in this study, Light-CT™, are the absence of cellular details, limited depth of imaging below the specimen surface, and the fact that this system can only image ex vivo specimens. In the future, many of these limitations are expected to be overcome. Laboratory-based systems have already been described with much higher resolution and ability to image at greater depths.[23] Furthermore, very recently, a rigid needle-like FFOCT probe was described,[24] which achieves an axial and transverse resolution in tissue of 1.8 μm and 3.5 μm, respectively, for a sensitivity of –80 dB. The report[24] presents preliminary ex vivo images of human breast tissue, and in vivo images of different areas of human skin, which reveal cellular-level structures. It is expected that further improvement in both the optical design of this prototype, and its packaging into a stand-alone handheld tool, will allow the translation of FFOCT technology to the operating room, to guide micro-TESE surgeries, potentially reducing operative times, and both intrasurgical and post-surgical complications.
In summary, this study provides, to our knowledge, the first evidence for the use of FFOCT to evaluate spermatogenesis within seminiferous tubules. In this rodent model, seminiferous tubular size and content can be reliably determined. FFOCT-guided testis biopsy could potentially aid sperm retrieval in men undergoing micro-TESE. The unique advantage of this technique is the rapid acquisition of images of fresh tissue without the need for any extrinsic labeling agent. In addition, the use of a halogen light bulb, already used in all operating rooms, eliminates the chance of physical and/or genetic damage to sperm. The results of this unblinded feasibility study suggest that FFOCT can be used to detect sperm within seminiferous tubules. Further studies need to be carried out to determine whether FFOCT has the same efficacy for identification of spermatogenesis in human testicular tissue.
Video 1 available on www.jpathinformatics.org
ACKNOWLEDGMENTS
We acknowledge a grant support from LLTech, Inc. that placed a prototype Light-CT™ instrument at the Weill Cornell Medical College. We also acknowledge Prof. Claude Boccara, for helpful discussions about FFOCT principles.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2012/3/1/4/93401
REFERENCES
- 1.Tsujimura A, Matsumiya K, Miyagawa Y, Tohda A, Miura H, Nishimura K, et al. Conventional multiple or microdissection testicular sperm extraction: a comparative study. Hum Reprod. 2002;17:2924–9. doi: 10.1093/humrep/17.11.2924. [DOI] [PubMed] [Google Scholar]
- 2.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 3.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa T, Nose R, Yamaguchi K, Chiba K, Fujisawa M. Learning curves of microdissection testicular sperm extraction for nonobstructive azoospermia. Fertil Steril. 2010;94:1008–11. doi: 10.1016/j.fertnstert.2009.03.108. [DOI] [PubMed] [Google Scholar]
- 5.Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65:1190–4. doi: 10.1016/j.urology.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 6.Okada H, Dobashi M, Yamazaki T, Hara I, Fujisawa M, Arakawa S, et al. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol. 2002;168:1063–7. doi: 10.1016/S0022-5347(05)64575-2. [DOI] [PubMed] [Google Scholar]
- 7.Andersson AM, Jørgensen N, Frydelund-Larsen L, Rajpert-De Meyts E, Skakkebaek NE. Impaired Leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab. 2004;89:3161–7. doi: 10.1210/jc.2003-031786. [DOI] [PubMed] [Google Scholar]
- 8.MacIndoe JH. The challenges of testosterone deficiency.Uncovering the problem, evaluating the role of therapy. (57-8, 61-2).Postgrad Med. 2003;114:51–3. doi: 10.3810/pgm.2003.10.1508. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa T, Yamaguchi K, Chiba K, Takenaka A, Fujisawa M. Serum hormones in patients with nonobstructive azoospermia after microdissection testicular sperm extraction. J Urol. 2009;182:1495–9. doi: 10.1016/j.juro.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Takada S, Tsujimura A, Ueda T, Matsuoka Y, Takao T, Miyagawa Y, et al. Androgen decline in patients with nonobstructive azoospemia after microdissection testicular sperm extraction. Urology. 2008;72:114–8. doi: 10.1016/j.urology.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Dubois A, Boccara C. Full-field OCT. Med Sci (Paris) 2006;22:859–64. doi: 10.1051/medsci/20062210859. [DOI] [PubMed] [Google Scholar]
- 12.Dubois A, Grieve K, Moneron G, Lecaque R, Vabre L, Boccara C. Ultrahigh-resolution full-field optical coherence tomography. Appl Opt. 2004;43:2874–83. doi: 10.1364/ao.43.002874. [DOI] [PubMed] [Google Scholar]
- 13.Jain M, Shukla N, Manzoor M, Nadolny S, Mukherjee S. Modified full-field optical coherence tomography: A novel tool for rapid histology of tissues. J Pathol Inform. 2011;2:28. doi: 10.4103/2153-3539.82053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois A, Vabre L, Boccara AC, Beaurepaire E. High-resolution full-field optical coherence tomography with a Linnik microscope. Appl Opt. 2002;41:805–12. doi: 10.1364/ao.41.000805. [DOI] [PubMed] [Google Scholar]
- 15.Vabre L, Dubois A, Boccara C. Thermal-light full-field optical coherence tomography. Opt Lett. 2002;27:530–2. doi: 10.1364/ol.27.000530. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda S, Kawana K, Yasuno Y, Oshika T. Wound architecture of clear corneal incision with or without stromal hydration observed with 3-dimensional optical coherence tomography. Am J Ophthalmol. 2011;151:413–9.e1. doi: 10.1016/j.ajo.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Guagliumi G, Musumeci G, Sirbu V, Bezerra HG, Suzuki N, Fiocca L, et al. Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc Interv. 2010;3:531–9. doi: 10.1016/j.jcin.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Hatta W, Uno K, Koike T, Yokosawa S, Iijima K, Imatani A, et al. Optical coherence tomography for the staging of tumor infiltration in superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2010;71:899–906. doi: 10.1016/j.gie.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 19.Coxson HO, Quiney B, Sin DD, Xing L, McWilliams AM, Mayo JR, et al. Airway wall thickness assessed using computed tomography and optical coherence tomography. Am J Respir Crit Care Med. 2008;177:1201–6. doi: 10.1164/rccm.200712-1776OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang FX. Behaviour of spermatogonia following recovery from busulfan treatment in the rat. Anat Embryol (Berl) 1998;198:53–61. doi: 10.1007/s004290050164. [DOI] [PubMed] [Google Scholar]
- 21.Fawcett DW. The Cell. In: Fawcett DW, editor. Sperm flagellum. Philadelphia, PA: W. B. Saunders; 1917. p. 604. [Google Scholar]
- 22.Kempe M, Rudolph W, Welsch E. Comparative study of confocal and heterodyne microscopy for imaging through scatteringmedia. J Opt Soc Am. 1996;A13:46–52. [Google Scholar]
- 23.Boccara CA, Gigon S, Roth M, Binding J. 0Optical coherence microscopy (OCM) and full field OCT (FFOCT) for wavefront correction in dense tissues, in Conference on Biomedical Optics, OSA Technical Digest, Optical Society of America. 2010 [Google Scholar]
- 24.Latrive A, Boccara A. In vivo and in situ cellular imaging full-field optical coherence tomography with a rigid endoscopic probe. Biomed Opt Express. 2011;2:2897–904. doi: 10.1364/BOE.2.002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


