Abstract
Background:
There has been a tremendous amount of interest focused on the topic of concussions over the past few decades. Neurosurgeons are frequently consulted to manage patients with mild traumatic brain injuries (mTBI) that have radiographic evidence of cerebral injury. These injuries share significant overlap with concussions, injuries that typically do not reveal radiographic evidence of structural injury, in the realms of epidemiology, pathophysiology, outcomes, and management. Further, neurosurgeons often manage patients with extracranial injuries that have concomitant concussions. In these cases, neurosurgeons are often the only “concussion experts” that patients encounter.
Results:
The literature has been reviewed and data have been synthesized on the topic including sections on historical background, epidemiology, pathophysiology, diagnostic advances, clinical sequelae, and treatment suggestions, with neurosurgeons as the intended target audience.
Conclusions:
Neurosurgeons should have a fundamental knowledge of the scientific evidence that has developed regarding concussions and be prepared to guide patients with treatment plans.
Keywords: Concussion, management, mild traumatic brain injury, review
INTRODUCTION
The past few decades have witnessed an exponential increase in the attention given to mild traumatic brain injuries (mTBI) and concussions. Much of this interest has been driven by non-scientific media attention focused on emerging concerns surrounding brain injuries in contact sports and wartime injuries.[80,96,97] A literature search with the topic of concussion limited to the past 10 years generates a list of over 1500 publications. This volume of work reflects the tremendous effort in the basic science and clinical arenas to understand the various aspects of concussions. Much of the commentary and treatment suggestions for concussions are focused in general medicine, sports medicine, neuropsychology, and trauma journals. A neurosurgeon must also bear a fundamental knowledge of the scientific evidence that has developed regarding concussions and be prepared to guide patients with treatment plans. Neurosurgeons are frequently consulted to manage patients with mTBI that have radiographic evidence of cerebral injury. These injuries share significant overlap with concussions, injuries that typically do not reveal radiographic evidence of structural injury, in the realms of epidemiology, pathophysiology, outcomes, and management. Further, neurosurgeons often manage patients with extracranial injuries that have concomitant concussions. In these cases, neurosurgeons are often the only “concussion experts” that patients encounter. Of note, much of the concussion data that are available are targeted to sports-related injuries. Fortunately, much of this information can be extrapolated to the general concussion population. The goal of this article is to review the current scientific evidence surrounding concussions and propose treatment suggestions for neurosurgeons caring for adult and pediatric patients who may or may not be involved in athletics.
BACKGROUND
Historically, the definition of concussion has not been well defined. The word concussion is derived from the Latin word concutere meaning to strike together.[53] Defining the word concussion has been an ongoing process occurring over a century. In an effort to clarify the term concussion, the Committee on Head Injury Nomenclature of the Congress of Neurological Surgeons (CNS) proposed a “consensus” definition of concussion in 1966. The CNS definition stated that concussion is “a clinical syndrome characterised by the immediate and transient post-traumatic impairment of neural function such as alteration of consciousness, disturbance of vision or equilibrium due to mechanical forces.”[98]
The definition has continued to be refined. It should be noted that concussion experts have been careful to define concussion as a separate term from mTBI, although they are often used interchangeably. Traumatic brain injury (TBI) has been defined based on subsets including mild, moderate, and severe. These subsets, which were intended to help clinicians prognosticate based on the initial injury, were initially correlated with scores on the Glasgow Coma Scale. While most concussions fall within the mTBI category, the converse is not true and the terms should not be used interchangeably.[98]
In 2001, concussion experts met in Vienna, Austria, to discuss the current knowledge base surrounding concussions and to establish an updated, universally accepted definition of concussion. From this conference and more recent meetings including the 3rd International Conference on Concussion in Sport held in Zurich in November 2008, a more widely recognized definition of concussion has developed.[53] At the recent meeting in Zurich, concussion was defined as “a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.” The statement also added that concussions typically result in the rapid onset of neurologic dysfunction that resolves spontaneously, may result in pathologic changes producing a functional rather than a structural injury, typically result in a graded set of symptoms that may or may not include loss of consciousness, and do not involve structural abnormalities on neuroimaging studies.[53,100]
EPIDEMIOLOGY
It has been conservatively estimated that 1.7 million new cases of TBI occur in the United States each year, with more than 52,000 deaths and 70,000–90,000 patients developing long-term disability. These data indicate that the annual incidence of TBI exceeds the combined rates of other neurological diagnoses such as multiple sclerosis, Parkinson's disease, and Alzheimer's disease. TBI is a contributing factor to a third (30.5%) of all injury-related deaths in the United States. About 75% of TBIs that occur each year are concussions or other forms of mTBI.[23,73]
Children aged 0–4 years, older adolescents aged 15–19 years, and adults aged 65 years and older are most likely to sustain a TBI. Adults aged 75 years and older have the highest rates of TBI-related hospitalization and death. In every age group, TBI rates are higher for males than for females. Direct medical costs and indirect costs such as lost productivity of TBI totaled an estimated $60 billion in the United States in 2000.[23,73]
TBI has also been recognized as a major source of disability for soldiers returning from the wars in Iraq and Afghanistan.[22,44,114] An estimated 320,000 service members deployed between 2001 and 2007 screened positive for a probable TBI based on a population-based survey of service members and veterans who served in Afghanistan or Iraq. Blast exposure has been identified as the most common cause of TBI in service members.[91]
Sports epidemiology
Each year, an estimated 44 million children and adolescents participate in organized sports in the United States. In addition, 170 million adults participate in physical activities, including sports. With this large population participating in sports, a significant number of individuals sustain TBIs.[80] The US Centers for Disease Control and Prevention (CDC) have estimated that the number of concussions that occur in sports and recreational activities annually could be as high as 3.8 million. However, these figures are difficult to estimate given that many people never seek medical care.[33,78]
In the USA, the majority of sports-related head injuries are observed in American football (incidence: 0.7–9.4 concussions per 1000 player hours),[19,113] ice hockey (incidence: 1.5–6.0 per 1000 player hours),[7,51] and soccer (incidence: 0.4–0.7 per 1000 player hours).[13] Internationally, rugby has one of the highest reported rates of concussion among organized sports.[60,74,88]
Of all sports played in the United States, American football not only is the sport associated with the greatest number of TBIs but also has the largest number of participants. Between the 1982–1983 season and the 2007–2008 season, a total of 35,641,573 high school athletes and 1,929,069 collegiate athletes competed in football.[33]
In 2009, the National Federation of State High School Associations estimated that there were approximately 1,500,000 football players playing at the high school level and below. The National Collegiate Athletic Association (NCAA), the National Association of Intercollegiate Athletics, and the National Junior College Athletic Association estimate that there are currently 75,000 collegiate football participants. A total of 225,000 participants are estimated to compete in fully padded, organized, nonprofessional football (sandlot) and professional football. Combined, these figures indicate that approximately 1,800,000 total athletes participated in football in the United States during the 2009 football season.[33]
Among youth sports, the CDC reported that 65% of the 200,000 athletes treated annually for concussions are patients between the ages of 5 and 18 years. Of the concussions that occurred in patients aged 5–18, two-thirds were related to bicycling, playing football, playing basketball, and playground activity.[52] Other authors estimate that high school football alone may be responsible for up to 83% of the sports-related concussions annually.[54] Another study estimated that 5,252,721 children and adolescents 6–17 years old were treated in US emergency departments for football-related injuries from 1990 to 2007. The annual number of cases increased by 26.6% over the study period. The 12–17-year-old age group accounted for 77.8% of all injuries and had nearly twice the odds of sustaining a concussion.[108] Table 1 summarizes the key epidemiological data reviewed above.
Table 1.
Key epidemiology points

Recent literature has also sought to improve our understanding of injury patterns in American sports, particularly football. Schnebel et al used in-helmet accelerometers to compare the frequency and magnitude of head impacts between football players at a Division 1 university and at an American high school. College football players had a significantly increased frequency and higher magnitude of head acceleration after impact than high school players. Due to these more powerful impacts, college players were three times more likely to lose consciousness when sustaining a concussion. In this study, certain positions such as quarterbacks, running backs, receivers, and defensive secondary players were also more vulnerable to concussions.[123] These studies highlighted the advanced risk inherent with higher levels of competition.
DIAGNOSIS
Concussion is diagnosed when an individual presents with typical symptoms and signs after direct or indirect trauma. Standard imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI) scans, are typically normal, demonstrating that the injury is a functional problem more than a structural one. More sophisticated studies such as functional MRI (fMRI), diffusion tensor imaging (DTI), neurochemical biomarkers, and genetic markers are being investigated as potential tools in the diagnosis of concussion.[37,77,130,148]
Much of the research evaluating the diagnostic modalities has focused on concussed athletes, although this information is also applicable to patients sustaining injuries in a non-athletic environment. The first major step in diagnosing concussion is recognizing the injury. Ideally, athletes have had a baseline evaluation completed that includes a list of symptoms present prior to a concussion, a neurologic examination, a cognitive evaluation, and balance testing. This evaluation should be repeated and compared to the baseline assessment after an injury occurs that is suspicious for a concussion.[118]
Subjective symptom reporting remains an essential element in the diagnosis and evaluation of concussions. It is beneficial to have a standardized symptom checklist that a healthcare provider uses to quantify the severity and duration of concussion symptoms. These symptom checklists are helpful in athletic or other healthcare settings. One survey revealed that 85% of athletic trainers used these scales as part of a concussion assessment battery.[63] Many assessment tools or scales have been described in the literature, but a recent systemic review of the literature identified six core scales and their derivatives. Common scales include Pittsburgh Steelers Post-concussion Scale and its derivatives (e.g. Post-concussion Scale Revised, Head Injury Scale, McGill ACE Post-concussion Symptoms Scale), Concussion Resolution Index, Sports Concussion Assessment Tool (SCAT), Post Concussion Symptom Scale, and the Concussion Symptom Inventory. The most recent symptom checklist published as part of the 3rd International Consensus Conference on Concussion is called SCAT 2.[118] The SCAT 2 incorporates some cognitive testing.[118] Balance and cognitive testing which includes an assessment of mental status, orientation, and simple tests of memory should also be performed as part of the neurological exam on the sideline.
Following an acute assessment, there is more extensive neuropsychological (NP) testing that can be performed to provide information about the neurocognitive and neurobehavioral status of the examinee. These tests are currently only recommended for evaluation of complex concussions. Defined at the 2nd International Conference on Sports, complex concussions cause persistent symptoms (including persistent symptom recurrence with exertion), specific sequelae such as seizures, loss of consciousness greater than 1 minute, or prolonged cognitive impairment after the injury. Athletes who suffer multiple concussions over time or who sustain repeated concussions with progressively less impact are also considered to have complex concussions.[99] Specifically, the tests provide information about memory, processing time, reaction time, and post-concussive symptoms. The testing has become increasingly computer based to facilitate its widespread use. This testing is currently used by the National Football League (NFL), National Hockey League, Major League Baseball, and New Zealand Rugby Football Union. In addition to professional sports teams, approximately 350 universities and 2500 high schools are using computer-based NP batteries to assess athletes after concussions. Examples of available programs include ImPACT, CogSport, and Headminder.[70]
Ideally, a baseline NP assessment has been performed so that post-concussive testing can identify subtle cognitive deficits, and later, recovery from injury. These tests not only provide an objective measure of neurocognitive functioning, but also allow for an individualized approach to concussion management which will be discussed later. While interpreting these studies, it is important to remember that there are several factors including age, gender, and history of prior concussions that influence baseline performances.[29,32,70]
Several studies have explored the validity and utility of ImPACT, one of the more popular test batteries. Recent studies suggest that a baseline assessment with ImPACT should be updated every 2 years for high school athletes to maximize its clinical utility.[41,122] Numerous studies have illustrated ImPACT's ability to monitor recovery in athletes sustaining concussions.[43,65,79,142] The validity of ImPACT has also been established by comparing the testing to more traditional NP testing such as the Symbol Digit Modalities Test.[67] Concussion experts also stress that NP testing should be used as a part of a multifaceted approach to making management decisions.[15]
Exciting novel approaches are being developed to identify and assist in the management of concussed patients. fMRI may help identify residual functional deficits in recently concussed but asymptomatic individuals. Several studies have revealed that concussed athletes have shown increased recruitment of blood oxygen level-dependent (BOLD) activity in brain areas outside the working memory network when engaging in tasks that require increased working memory and selective attention compared to non-concussed counterparts.[25,68,112,130,132] Although these techniques are not yet readily available, they may help characterize the brain's compensatory response to structural microinjury and assist clinicians making management decisions.
Diffusion tensor imaging (DTI) is also being explored as a diagnostic modality to detect subtle, but clinically meaningful, changes following concussions. Two separate studies analyzing concussed pediatric patients have shown that alterations in fractional anisotropy in specific regions of the brain in concussed patients seemed to correlate with the severity of post-concussive symptoms.[148,149] Single photon emission computed tomography (SPECT) and proton magnetic resonance spectroscopy (PMRS) have also identified more subtle cerebral changes following concussions. Using PMRS, Vagnozzi et al have identified significant metabolic alterations that were present in 40 concussed athletes compared to his controls in the immediate post-concussive period.[141]
Multiple studies have assessed the relationship between brain-related proteins found in the serum at the time of injury and concussions. The biochemical markers with the most attention include S100 proteins, neuron-specific enolase (NSE), and cleaved Tau protein (CTP). Only S100B has consistently been shown to predict injury and outcome in adults with the most severe TBI.[5,77] More work needs to be done to identify clinically useful markers in concussed patients who have more moderate head injuries.
Preliminary work has also been done evaluating the relationship between post-concussive symptoms and abnormal electrophysiologic testing with quantitative EEG (QEEG) and motor evoked potentials (MEPs). These studies have identified electrophysiological changes indicative of focal cortical dysfunction that may help diagnose and define the pathogenesis of concussed patients in the future.[34,76,82,95] Table 2 includes a list of commonly used and experimental diagnostic modalities.
Table 2.
Diagnostic modalities for concussions

BIOMECHANICS OF CONCUSSION
Concussion results from traumatic biomechanical forces transmitted to the brain, resulting in a disturbance of brain function. Mechanical energy is transferred to the brain and vascular tissue at the tissue and molecular level. The brain is one of the softest biologic materials, demonstrates nonlinear behavior on a compliance curve, and alters its properties in response to the rate of tissue loading and deformation. Predicting the mechanical strains that occur in the brain resulting from linear and rotational forces is challenging given its complex anatomy and heterogeneous compartments.[104]
Special consideration must be given to the biomechanics of concussive injury between adult and pediatric patients. Factors that account for these differences include the relative size of the head compared to the rest of the body, brain water content, vasculature, degree of myelination, and shape of the skull. Younger patients typically have decreased cervical muscle strength and tone which can lead to an increased magnitude of force delivered to the cranial compartment compared to older counterparts. Berney et al investigated the effects of age on head injury and found that children younger than 3 years sustained head injuries and skull fractures at much lower energy mechanisms than older children and adolescents.[9]
Some researchers have attempted to identify an injury or impact threshold that predicts the likelihood of sustaining a concussion. Some have suggested that a force in the range of 80–90 g sustained over 4 ms may be a theoretical injury level in football players. Impact data has been collected using in-helmet accelerometers to examine impact accelerations and impact locations. To date, no defined set of variables has been established that predicts a concussive injury with sufficient reliability.[16,69,92]
PATHOPHYSIOLOGY OF CONCUSSIONS
Recent decades have seen significant advancement in neuroscientists’ understanding of the complex biochemical, molecular, and physiological events that follow concussions. Previous papers have provided excellent summaries, and this review will highlight some of the key events including glutamate excitotoxicity, changes in the membranes of CNS cells, microglial activation, inflammation, mitochondrial disruption, disruption of the blood–brain barrier (BBB), and cerebrovascular reactivity changes.[11]
Excitotoxicity is a process resulting in neuronal cell damage from excessive release of excitatory neurotransmitters, primarily glutamate. Glutamate is the most abundant neurotransmitter in the brain and is predominately released by astrocytes and microglia. It stimulates ionotropic glutamate receptors such as the N-methyl-D-aspartate (NMDA), α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), and kainate receptors that control numerous functions including calcium and sodium channels. The effect of glutamate on glutamate receptors may be potentiated by certain cytokines.[11]
The process of excitotoxicity involves generation of high levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS), lipid peroxidation products (LPPs), prostaglandins, and nitric oxide (NO). The process may also coincide with microtubule dysfunction, membrane injury, dendritic retraction, synaptic loss, mitochondrial dysfunction, calcium dysregulation, and apoptosis.[11]
Microglia, the major immune cells of the brain, not only are intimately involved with the process of excitotoxicity, but also act as mediators in an inflammatory cascade that accompanies brain trauma. They express receptors for many factors released following cranial trauma, including ATP, glutamate, growth factors, and cytokines.[83] In response to high levels of stimulation, microglia may become primed and subsequently activated. Activation of microglia has been identified as an early and key event following TBI.[12] When activated, microglia secrete a combination of anti- and pro-inflammatory cytokines, chemokines, NO, prostaglandins, trophic factors, free radicals, LPPs, and quinolinic acid.[2,119] Combinations of these factors, which may include cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), may be neurotoxic themselves or enhance the sensitivity of neurons to excitotoxicity. One study examined the effects of TNF-α and IL-1β on neuronal death caused by exposure of mouse hippocampal slice cultures to toxic concentrations of AMPA. High concentrations of the cytokines significantly enhanced excitotoxicity.[8]
Microglia are also capable of recruiting additional immune cells such as macrophages, B-cell and T-cell lymphocytes to the brain. Increased concentrations of immune cells may produce large quantities of ROS and RNS, which expose the cerebrovascular endothelium to increased oxidative stress. This is one explanation for the BBB disruption that occurs following TBI. When rodent cerebrovascular endothelium was exposed to ROS, the NMDA receptors were upregulated which exposed a susceptibility to glutamate-induced BBB disruption.[10] Free radicals themselves have also been shown to disrupt tight-junction proteins, degrade basement membranes, and lead to permeability of microvascular endothelial cells.[46]
With axons, some mechanical injury may occur with initial impact. Mechanical stretching of axonal cell membranes leads to ionic flux, depolarization, and mitochondrial swelling. These changes may interfere with axonal transport. However, evidence has suggested with mild TBI, that much of the axonal injury occurs due to secondary events such as local influxes of calcium, leading to activation of cysteine proteases.[20] Myelin and oligodendrocytes also possess glutamate receptors and are likely susceptible to injury with toxic glutamate levels. These mechanical and cellular changes have been shown to impair intrahemispheric cortical communication networks.[59]
Calcium dysregulation plays a major role in excitotoxicity and secondary injuries after TBI. It has been shown in an in vitro model that traumatized Purkinje cells can alter AMPA receptors which may lead to an influx of intracellular calcium and subsequent neuronal cell death.[6] Increased intracellular calcium may also lead to ROS generation and decreased mitochondrial respiratory capacity.[109] In an animal model, disruption of calcium homeostasis has been found to persist as long as 30 days after TBI.[134]
After brain injury, elevations in concentrations of NO occur. NO is released by numerous cells including microglia. With high concentrations of free radicals, NO combines with superoxide, producing a potent peroxynitrite radical that is particularly destructive for mitochondrial function. NO also competes with oxygen for cytochrome oxidase within mitochondria and can disrupt oxidative phosphorylation, resulting in neuronal dysfunction or death.[1,17,18]
A concussion has complex pathophysiological implications for the cerebrovascular system. Although cerebrovascular reactions with concussions are less severe than those occurring with moderate or severe injuries, even minor injuries can result in alterations in blood flow and autoregulation.[11] Most data regarding derangements in the cerebrovascular system come from studying severe TBI. Severe TBI may be followed by a triphasic response that initially includes hypoperfusion, followed by hyperemia, and then vasospasm. Although it has not been well studied, this triphasic response may occur to some degree in milder head injuries.[3]
The mechanism for this vasospasm has been postulated to include chronic depolarization of smooth muscle tissue due to reduced potassium channel activity, release of endothelin and reduced availability of NO, depletion of cyclic GMP of smooth muscle, potentiation of prostaglandin-induced vasoconstriction, or free radical formation.[146] There is also growing evidence, mainly experimental, that intercellular adhesion molecules, chemokines, and cytokines including IL-1 and TNF-α contribute to the vascular response that leads to cerebral ischemia.[45,116]
It is also postulated that the brain's cerebral autoregulation and reactivity to vasomotor agents is impaired following even mild TBI.[38,146] These disturbances coupled with the brain's need for additional oxygen and glucose following TBI may explain the brain's susceptibility to injury following an initial trauma. This tenuous equilibrium between brain healing and the need for increased blood flow and delivery of nutrients has been termed “concussion penumbra.” This concept is similar to the ischemic penumbra that occurs following an ischemic stroke.[53] Evidence also suggests that TBI leads to disruption of neuroautonomic cardiovascular regulation. This impairment may affect organ systems throughout the body.[36,80]
Data from animal models suggest that the timing and degree of metabolic disruption that occurs following mild head trauma differs as a function of the brain's developmental age.[24] The pathophysiological response to TBI also differs between mature and developing human brains. Although the exact mechanisms are not known, diffuse brain swelling following TBI is more common in pediatric patients. The etiology may be related to differences in expression of aquaporin 4 by microglia, brain water content, or oxidative vulnerability.[106] Animal models have also suggested that younger patients may have a different pathophysiological response to brain trauma because of incomplete maturation of the BBB, differences in the makeup of glutamate receptor subunits, and immaturity of glial and neuronal enzyme systems. In a rat model, researchers have shown increased cerebral edema and BBB permeability in infant rats after brain trauma compared to adult rats.[24] Researchers have also identified developmental changes in the mRNA expression of neuropeptides and glutamate receptors in neonates and adult rats.[40]
Within a few days, depending on the severity of injury, the brain's physiology may begin to return to normal. However, some derangements such as intracellular calcium concentrations have been demonstrated to remain altered for up to a month or longer.[134,151]
An individual's unique genetic makeup also likely plays a role in the response to head injury. Patients with specific apolipoprotein E subtypes have been associated with increased risks of post-concussive symptoms.[71,131,139] Additionally, basic science research has demonstrated in an in vitro animal model that remarkable differential gene expression occurs with different levels of severity of trauma.[37] Not only is one individual likely to respond in a unique way to different brain injuries, but also one can expect different individuals to have unique molecular responses to brain injuries. The pathophysiological sequelae of concussions are summarized in Table 3.
Table 3.
Key pathological sequelae of concussion

COMPLICATIONS
Most authors agree that symptoms following a concussive injury are typically transient and resolve spontaneously within 2 weeks of injury. However, some concussed patients experience a much more protracted and difficult course. A significant body of data has emerged exploring complications in different populations that sustain concussions. Again, much of this data has focused on sports-related populations.
Following a concussion, patients can develop a post-concussive syndrome which is a constellation of symptoms that can persist from days to weeks following the injury. Statistical analysis of a large group of concussed athletes who completed a Post-concussion Symptom Scale revealed that symptoms tended to cluster into one of the three following groups: physical/somatic/sleep-related difficulties, cognitive, and affective.[70] Somatic complaints include headaches, dizziness, nausea, fatigue, sleep disturbances, blurred vision, tinnitus, and hypersensitivity to light or noise. Cognitive symptoms include memory difficulties, decreased concentration, and decreased processing speed. Affective symptoms include irritability, depression, and anxiety.[91]
Several studies have focused on symptoms in concussed high school football players and have followed the symptoms longitudinally. One study found that although reaction time and processing speed returned to baseline levels on approximately day 6 post injury, memory impairments often lasted up until day 10 post injury.[127] Another large study demonstrated that players usually reported a resolution of symptoms by day 7 post injury, but reaction time was significantly decreased up to 14 days post injury.[30]
Studies focusing on college-aged athletes have revealed similar patterns of recovery following concussions. One prospective cohort study of 1631 concussed football players from 15 US colleges found that typically symptoms resolved by day 7. Balance deficits usually dissipated first within 3–5 days, followed by cognitive processing and verbal memory by day 7. There were no significant differences in symptoms or functional impairments in the concussion and control groups, 90 days after concussion.[94]
While these symptoms typically resolve over short periods, one study reported that some symptoms such as fatigue persisted 6 months in one large adult sample of mTBI patients.[133] Some have proposed that memory deficits may be the most sensitive indicators of brain compromise or severity of injury,[42,137] while others have suggested that athletes experiencing headaches accompanied by typical migraine symptoms are most likely to have the most severe and long-lasting symptoms.[70] Migraines have been shown to be accompanied by elevations in cerebral glutamate,[144] and the post-concussive migraines may reflect persistent excitotoxicity from the initial injury.
There has been increased interest in understanding the cumulative effects of concussions. A prospective cohort study of 2905 football players from 25 US colleges revealed that there was an association between reported number of previous concussions and likelihood of an additional concussion. Players reporting a history of three or more previous concussions were 3 times more likely to have an additional concussion than players with no concussion history. They reported that concussions were most likely to occur in games, as opposed to practice, and offensive linemen, linebackers, and defensive backs were the positions most likely to sustain concussions. The authors suggested there may be a cumulative effect from repetitive concussions leading to increased neuronal vulnerability for subsequent injuries.[57] Another study reported that in one season, professional football players in a Canadian league were over 5 times more likely to sustain a second concussion compared to players who had never had a concussion.[81] Those athletes who suffer repeat concussion are more likely to experience severe symptoms on the field, such as amnesia and confusion.[27]
There is also evidence that athletes who suffer multiple concussions require longer periods to recover from their deficits. One study looked at NP evaluations in 223 high school athletes and noticed that there seemed to be subtle, yet significant prolonged NP effects in youth athletes with a history of two or more previous concussions.[107] Others have also reported significantly greater declines in memory performance rates in amateur athletes with a history of multiple concussions compared to athletes with a single concussion.[66] Another study also found that visual–kinesthetic integration in collegiate athletes with a history of multiple concussions demonstrated significantly slower rates of recovery.[129] In those athletes with greater than three previous concussions, symptoms were significantly more likely to linger beyond 1 week.[57]
Several authors have suggested there is likely a critical window that increases the likelihood of a repeat concussion in humans within the first 7–10 days post injury.[57,93] Rodent models have demonstrated that when the animals were subjected to a second mTBI within 5 days of the initial injury, the axonal injury, astrocytic activity, and memory impairment were significantly worse than the animals with only a single injury. In one of the models, no cognitive deficits were observed when the inter-concussion interval was ext ended to 7 days, which supported the notion that there is a critical period of increased vulnerability to concussions.[84,117]
In humans, the idea that a devastating head injury may occur after repetitive head traumas within a short temporal window was reported as early as in 1973. Richard Schneider reported that two young athletes died after sustaining a concussive head injury followed by a second minor head injury. Later, Saunders and Harbough coined the term second impact syndrome (SIS) in 1984 when they described a 19-year-old football player who sustained a concussion, returned to play, and died suddenly 4 days later. Post-mortem examination revealed no space-occupying hematoma or lesions, but only severe cerebral edema. Neuroscientists have hypothesized that this phenomenon may be related to cerebral autoregulation failure coupled with catecholamine surges that may result in fatal malignant brain swelling.[147] Glutamate surges are also likely a critical component driving this rapid cerebral edema, and evidence suggests that reducing the brain's concentration of glutamate can significantly reduce edema after TBI.[14] Why this physiological response only occurs in a minority of athletes sustaining two temporally related head injuries is unknown. Due to the rarity of this event, no prevalence or incidence data have been reported on second impact syndrome. It should also be noted that SIS has only been reported in youth athletes. For these reasons, there are many concussion experts who are suspicious that such a syndrome exists as a unique entity.[147] Nonetheless, this rare complication should be one of the many considerations for clinicians providing return to play recommendations.
Not only do athletes with a history of multiple concussions take longer to recover from the acute effects of a concussion, but significant long-term effects in athletes with repetitive head trauma has also been reported. Originally described by Corsellis in 1973 in a report on 15 retired boxers, chronic traumatic encephalopathy has been found to occur in other sports such as American football, hockey, soccer, professional wrestling, and even repetitive physical abuse.[48] Neurodegenerative changes including cerebral atrophy, cavum septi pellucid with fenestrations, mammillary body shrinkage, dense tau immunoreactive inclusions, and TDP-43 proteinopathy have been manifested in pathological brain specimens. Clinical changes including memory dysfunction, impaired executive functioning, behavioral and personality disturbances, Parkinsonism, and motor neuron disease may accompany these pathological changes.[48,55,56,103,110] Data suggest that cumulative concussions may also have long-term effects on gait patterns.[35,90]
Numerous studies have explored whether sex differences exist with respect to post-concussion symptoms and neurocognitive function in concussed athletes. Within a given sport, females tend to have higher concussion rates than males.[33,49] Concussed collegiate female athletes also are more likely to suffer cognitive impairment such as decrement in visual memory tasks than concussed male counterparts.[31,33] Female sex is associated with significantly higher odds of poor outcome after mTBI, as measured by post concussion symptom (PCS) score.[4] Differences between sexes have also been shown in the type of symptoms reported in collegiate and high school athletes. Concussed men in collegiate sports were significantly more likely than concussed women to report post-concussion symptoms of vomiting and sadness.[31] Among high school athletes, males reported more amnesia, confusion, and disorientation than females, whereas females reported more drowsiness and sensitivity to noise. In this same population, no differences were observed for symptom resolution time or return-to-play time between sexes.[47] Key sequelae that may follow concussions and points of consideration are listed in Table 4.
Table 4.
Clinical sequelae of concussions

MANAGEMENT
When considering management guidelines for athletes who have sustained concussions, many healthcare professionals refer to the most recent consensus statement published from the 3rd International Conference on Concussion in Sport held in Zurich in November 2008. This document represents the most up-to-date, comprehensive review of the topic and provides suggestions from the world's concussion experts. This document is available for healthcare providers and salient points from it are incorporated into this discussion.
As emphasized earlier, the most important step in the initial management of a concussion is diagnosis. Simple phrases which seemed benign for years may actually be the first clue to concussion diagnosis. These phrases include: “dinged,” “stunned,” “having my bell rung,” and “seeing stars,” to name a few. Healthcare providers must be hypervigilant to evaluate players who make such comments or exhibit behavior consistent with a concussion, as many athletes drastically underreport post-concussive symptoms.[58] Coaches and athletic training staff are also valuable resources to help identify players who may have sustained a concussion. Over the last decade, renewed media interest, along with programs like the CDC's “Heads Up” initiative have helped facilitate education for those athletes at highest risk.[121]
When a player shows any signs of a concussion, he or she must be removed from play and evaluated with a standard emergency protocol with particular attention to exclude a cervical spine injury. Once the first aid issues are resolved, an assessment of the concussive injury should be completed using the SCAT2 or a similar assessment tool. Except in unique circumstances which will be discussed below, a player diagnosed with a concussion should not be allowed to return to play on the day of injury. All of these players need close monitoring for 24–48 hours and any subsequent deterioration should prompt immediate evaluation by a physician.[101]
In some cases, a player is first evaluated in the emergency department or a physician's office. This medical assessment should include a comprehensive history, a detailed neurological examination including cognitive and mental status assessment, and gait and balance testing. It should be determined whether the clinical status has improved or deteriorated. The physician must decide whether neuroimaging is indicated to exclude a more serious injury such as a mass lesion or structural injury. Other than CT scans or an MRI in some instances, no imaging technology can be recommended at this time outside of a research setting.[101]
As per the Zurich panel, NP testing has sufficient validation to be used as an adjunctive tool in making return to play decisions. As cognitive impairment often follows clinical symptom resolution, NP testing can aid in assessing recovery, particularly in patients with complex concussions, which were defined earlier. NP testing is best interpreted by a trained neuropsychologist.[101] It should be emphasized that NP testing should not be used as a unilateral decision-making tool.
The critical component of concussion management is to ensure that an athlete has physical and cognitive rest while recovering. While symptomatic, the athlete should minimize physical activity and cognitive tasks such as scholastic work that may exacerbate symptoms and delay recovery. Once symptoms have resolved and cognitive testing is normalized, a graduated return to play protocol should be followed. This protocol may involve a graduated increase in aerobic activity, followed by sport-specific, non-contact drills, and finally, full return to play. Although this protocol must be modified on an individual basis, 1 week may be adequate to complete this protocol.[101] Physicians must emphasize the importance of not returning to a sport too quickly as evidence has shown that up to 15% of US high school athletes returned to practice prematurely.[150] Future education initiatives should help reverse this trend in child and adolescent athletes. A management algorithm for non-elite athletes sustaining concussions is provided in Figure 1.
Figure 1.
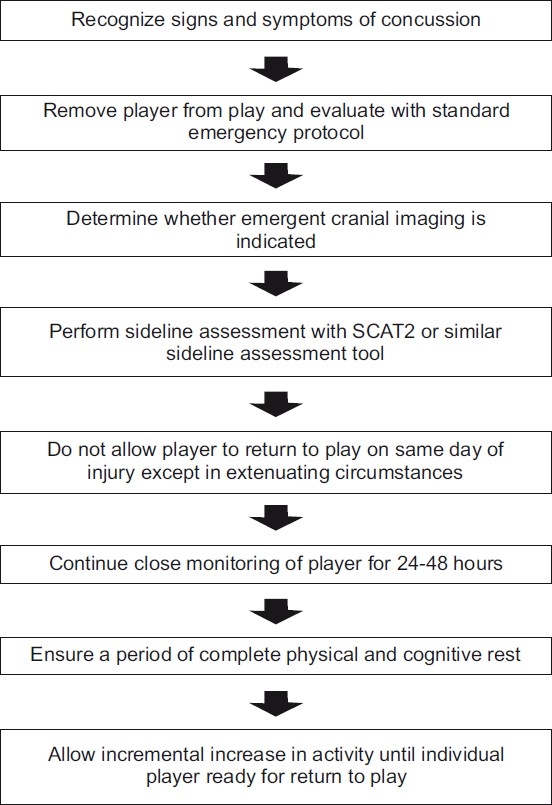
Management algorithm for non-elite athlete sustaining concussion
The panel in Zurich acknowledged that their recommendations could not be applied to children below the age of 10 years.[101] Many authors have concurred that pediatric patients constitute a unique population, and the pediatric brain is more vulnerable to traumatic injury.[53,75,85,106] Although it is not clear at what age this vulnerability is maximal, management in this population should be conservative. Education for children, parents, coaches, and training staff should be optimized to ensure heightened awareness about concussions and compliance with return to play guidelines. There should be little hesitation in children and adolescents to seek neuroimaging with even the smallest clinical concern. For these patients, the return to play protocol should be prolonged, and NP testing, when available, should be used liberally in cases with unusual or protracted courses of recovery. Academic accommodations that incorporate the student's parents, teachers, school nurse, and guidance counselor should also be utilized.[102]
As discussed earlier, evidence has shown that younger athletes who sustain concussions may be more prone to additional concussions and have longer recovery periods. Some authors have stressed that placing school-aged athletes back into environments where they may sustain repetitive concussion may dramatically affect the athlete's ability to perform on and off the field. These students, who are undergoing constant cognitive assessment, may have devastating socioeconomic consequences from decreased college acceptance, scholarship awards, and career achievements.[106]
For professional football players, management has become much more conservative in recent years, and stricter guidelines now dictate when players can return to the field. The 2009 statement by the NFL advises that a player who suffers a concussion should not return to play or practice on the same day if he shows any signs or symptoms of a concussion.[115] It further states:
“Once removed for the duration of a practice or game, the player should not be considered for return-to-football activities until he is fully asymptomatic, both at rest and after exertion, has a normal neurological examination, normal NP testing, and has been cleared to return by both his team physician(s) and the independent neurological consultant. A critical element of managing concussions is candid reporting by players of their symptoms following an injury. Accordingly, players are to be encouraged to be candid with team medical staffs and fully disclose any signs or symptoms that may be associated with a concussion.”[115]
As previously discussed, less literature discusses the management of concussions in patients who sustain the injury outside of athletics. The same management principles should apply. These patients should be evaluated by a healthcare provider. Immediate first aid should be provided. Once these issues are addressed, a comprehensive history and neurological exam should be obtained. Symptoms should be assessed, and the need for immediate neuroimaging should be determined. Education about concussions should be provided, and serial follow-up should be continued until symptoms and cognitive deficits resolve. Accommodations should be arranged to provide cognitive and physical rest. Infrequently, more elaborate NP testing may be useful to evaluate atypical courses of recovery.
In patients who experience a prolonged course of recovery with impairment in their quality of life, some healthcare providers have used pharmacological therapy. Of note, no medication has been shown to expedite recovery from TBI, although medications have been used to ameliorate symptoms. Sleep aids such as melatonin have been used to treat sleep disturbances after concussions. Antidepressants, β-blockers, calcium channel blockers, valproic acid, topiramate, triptans, dihydroergotamine, and gabapentin, have all been discussed as potential medical therapies for persistent headaches after concussion. After concussions, antidepressants such as sertraline have been used to treat depression and emotional disturbances. Also, other agents such as methyphenidate and amantadine have been used to treat neurobehavioral disturbances after TBI. No standard pharmacological approaches exist, and these medications should only be used in extenuating circumstances.[105]
THE FUTURE: DRUGS THAT MAY PROVIDE NEUROPROTECTION
Since the 1980s, numerous studies have attempted to identify neuroprotective agents. Because of the complexity of the biochemical and cellular cascades that follow TBI, there are many potential therapeutic targets that may help support individuals who have suffered TBI. These efforts have included magnesium administration, hormone supplementation, calcium channel blockade, bradykinin inhibition, use of anti-inflammatory drugs, blockade of immune receptors, as well as use of numerous other vitamins, minerals, and antioxidant agents. These agents often work by multiple mechanisms that may include limiting glutamate excitotoxicity, limiting of free radical and lipid peroxidation damage, or minimizing BBB disruption. Unfortunately, none of these completed trials have demonstrated significant clinical benefit in humans.[124,145] Many large clinical trials are, however, currently underway. Of note, most of the investigation that is underway involves treatment of more severe TBI. These agents will hopefully have clinical utility for concussions, TBIs that share many pathological features of more severe TBI. The discussion below reviews some of the promising agents being investigated.
One agent that deserves discussion is magnesium. Magnesium has demonstrated neuroprotective properties in numerous TBI models. Magnesium has been shown to have many beneficial effects including noncompetitive NMDA receptor blockade, inhibition of presynaptic excitatory neurotransmitter release, blockade of voltage-gated calcium channels, and reduction in inflammatory cascades.[21] Researchers have demonstrated that animals pretreated with magnesium were less susceptible to neuronal cell death from induced ischemia than sham animals.[72] Magnesium has also been shown to have antidepressant properties in animals and humans.[136] Despite robust experimental evidence supporting magnesium's neuroprotective effects, Temkin et al reported that a 5-day continuous administration of magnesium to patients with severe TBI did not demonstrate a neuroprotective effect. There was even a higher mortality among patients treated with higher doses of magnesium.[138] There has been some concern that the availability of magnesium in cerebral extracellular fluid, in contrast to serum levels, may not have been adequate. Additional studies should help clarify the clinical utility of magnesium supplementation following TBI.[87,124]
Progesterone is an agent that appears to provide neuroprotection by multiple mechanisms. After TBI, progesterone has been shown to decrease oxidative stress by reducing membrane lipid peroxidation, reduce BBB disruption, and ameliorate the brain's inflammatory response. A phase III clinical trial, the Progesterone for the Treatment of Traumatic Brain Injury (ProTECT™ III), is currently underway to further assess the clinical efficacy of progesterone.[145]
The hormone erythropoietin has been identified as a neuroprotective agent that appears to ameliorate TBI by multiple avenues. The Erythropoietin in Traumatic Brain Injury (EPO-TBI) trial is currently underway to assess whether erythropoietin can improve neurological outcome in severe TBI patients.[145]
N-type calcium channel antagonist SNX-111 or Ziconotide showed promise in animal models of TBI at reducing calcium accumulation in the cortex and white matter structures.[61,120] The agents have also been shown to partially restore mitochondrial function after TBI. Unfortunately, a clinical trial with SNX-111 was terminated prematurely because of increased mortality in the treatment group.[124] More selective N-type calcium channel antagonists, like SNX-185, with better bioavailability are being investigated as alternative, neuroprotective agents.[126]
Kinins, which include substance P and neurokinin A, are a group of protein mediators that have many functions including a proinflammatory one. The NK1 receptor, the receptor for substance P, has been a therapeutic target to suppress the inflammation following TBI. In animal TBI models, administration of NK1 receptor antagonists has reduced vascular permeability and edema formation and has improved both motor and cognitive neurologic outcomes.[39] Future clinical studies in TBI patients may reveal a greater clinical role for this agent.
The antibiotic minocycline has been extensively studied in mice and has been shown to be an effective antioxidant and attenuator of the inflammatory sequelae of TBI. The study, A Safety and Feasibility of Minocycline in the Treatment of Traumatic Brain Injury, is currently underway which will likely lead to additional clinical investigation of this drug.[124]
Cyclosporin is another multifactorial compound that has neuroprotective effects including improving mitochondrial functioning, blocking free radical production, and inhibiting calcium accumulation. The animal data have been compelling that cyclosporin A (CsA) has therapeutic benefits in TBI models. However, the variability of study designs from the clinical studies has made the outcome data difficult to interpret. The safety of CsA use in TBI has been demonstrated, but additional investigation is needed to clarify the efficacy of the drug.[86]
Toll-like receptors (TLRs) are a key component of the brain's innate immune system and activate intracellular signaling pathways that propagate the inflammatory cascade. There is considerable experimental interest in manipulating these receptors to mitigate the neuroinflammation following TBI.[62]
Numerous other vitamins, minerals, and antioxidant agents are being investigated as potential therapeutic modalities. These include nicotinamide, a soluble B-group vitamin. Animal TBI models have demonstrated beneficial effects including reduced cortical damage, inflammation, and behavioral disruption in animals receiving infusions.[50,135] Cytoflavin, a drug which contains nicotinamide, has shown to improve behavioral and cognitive symptoms in a small group of humans, following mild TBI.[128] Omega-3 essential fatty acids (EFAs) have been shown in experimental TBI models to improve the blood flow, reduce the toxic effects of glutamate, and stabilize membranes. Animal models have shown that specific fatty acids can be supplemented in the diet and provide a protective function for the brain, especially in regard to seizures.[111] Although no long-term phase III trials have been completed with EFAs and no optimal dosing regimen has been defined, some authors have recommended supplementation with high-dose fish oil in the 2- to 4-g/day range for patients suffering from PCS.[89] Similarly, Vitamin E has been shown to have neuroprotective effects in a rodent TBI model by reducing lipid peroxidation levels.[28]
An antioxidant, α-lipoic acid, has also shown neuroprotective effects in an animal model by reducing inflammatory markers, preserving BBB permeability, and reducing brain edema.[140] Another agent called Resveratrol, which is a polyphenol, also has antioxidant properties and has been shown to improve behavioral outcome in a rat TBI model.[89] The α-phenyl-N-tert-butyl nitrone (PBN) is a spin trap agent that has antioxidant effect and has shown to reduce brain inflammation in animal TBI models.[125] Zinc protoporphyrin has also been shown to attenuate brain edema and BBB permeability in an animal model.[143]
Micronutrients such as zinc and magnesium are critical for optimal functioning of many organ systems in addition to the CNS. All athletes or individuals at risk of TBI should consume balanced diets to ensure adequate levels of these nutrients. One study has demonstrated that plasma levels of magnesium and zinc in athletes can effectively be elevated by oral supplementation during a 4-week period of intense athletic activity.[26] Determining whether athletes at risk of TBI should be evaluated for nutritional deficiencies and supplemented with nutrients that provide neuroprotection warrants further investigation.
Treating brain injuries with neuroprotective drugs has been a challenging and, at times, a frustrating process. The complexity of the brain's pathophysiological response to injury and the heterogeneity of these injuries make it difficult to identify single agents that improve outcome in the human TBI population. Numerous agents listed above are receiving additional investigation and may provide good therapeutic options. One key to treating patients with TBI may be by using multiple agents. A recent trial demonstrated significant reduction in brain edema and BBB permeability in a rodent model of TBI by using magnesium and MK-801, an NMDA receptor antagonist, compared to monotherapy.[64] Additionally, as more is learned about the pathophysiology of TBI, the designs of clinical trials may be refined which will help elucidate the utility of these therapeutic agents.
CONCLUSIONS
In recent decades, concussions have received unprecedented attention from the scientific community. The definition of the term has become more refined, and epidemiological data have revealed how remarkably common the injury is. Common diagnostic tools include neurological exams and subjective symptom reporting. CT and MRI may be used to rule out more severe injuries. In cases with complex concussions, neuropsychiatric testing is available to document more subtle deficits and recovery from concussions. In the future, more sophisticated diagnostic modalities such as fMRI will likely be used more frequently in the diagnosis and management of concussions. As the pathophysiological picture that accompanies concussions becomes clearer, clinicians will be able to understand patients’ complex outcomes and refine treatment plans.
Neurosurgeons may not become involved in the care of a concussion patient until late in their treatment. This does not mean that neurosurgeons do not have a lot to offer this patient population. A neurosurgeon's intimate knowledge of various head injuries from similar mechanisms gives a distinct advantage in the ability to educate athletes about the possible consequences of their injury. This education is paramount to prevent subsequent concussions and to improve reporting of these injuries in the future by athletes at all levels. Neurosurgical involvement in concussion management has been less than that of trainers, sports medicine physicians, or other primary care providers. This is mostly attributable to the limited supply of neurosurgeons and the mostly non-surgical pathology of concussions.
A neurosurgeon should be familiar with the current diagnostic and management guidelines for concussions, paying particular attention to return to play protocols. Neurosurgeons are limited in number and will likely never have the numbers to provide “frontline” care for concussion injuries. Neurosurgeons should, however, provide a solid foundation for those that do provide “frontline” care and be willing to provide assistance and insight whenever consulted.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/16/92930
Contributor Information
Matthew T. Neal, Email: mneal@wfubmc.edu.
Jonathan L. Wilson, Email: jlwilson@wfubmc.edu.
Wesley Hsu, Email: whsu@wfubmc.edu.
Alexander K. Powers, Email: apowers@wfubmc.edu.
REFERENCES
- 1.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–91. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banati RB. Neuropathological imaging: In vivo detection of glial activation as a measure of disease and adaptive change in the brain. Br Med Bull. 2003;65:121–31. doi: 10.1093/bmb/65.1.121. [DOI] [PubMed] [Google Scholar]
- 3.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma. 2010;27:527–39. doi: 10.1089/neu.2009.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begaz T, Kyriacou DN, Segal J, Bazarian JJ. Serum biochemical markers for post-concussion syndrome in patients with mild traumatic brain injury. J Neurotrauma. 2006;23:1201–10. doi: 10.1089/neu.2006.23.1201. [DOI] [PubMed] [Google Scholar]
- 6.Bell JD, Ai J, Chen Y, Baker AJ. Mild in vitro trauma induces rapid Glur2 endocytosis, robustly augments calcium permeability and enhances susceptibility to secondary excitotoxic insult in cultured Purkinje cells. Brain. 2007;130:2528–42. doi: 10.1093/brain/awm164. [DOI] [PubMed] [Google Scholar]
- 7.Benson BW, Mohtadi NG, Rose MS, Meeuwisse WH. Head and neck injuries among ice hockey players wearing full face shields vs half face shields. JAMA. 1999;282:2328–32. doi: 10.1001/jama.282.24.2328. [DOI] [PubMed] [Google Scholar]
- 8.Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, Oliveira CR, et al. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci. 2005;25:6734–44. doi: 10.1523/JNEUROSCI.1510-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berney J, Froidevaux AC, Favier J. Paediatric head trauma: Influence of age and sex. II. Biomechanical and anatomo-clinical correlations. Childs Nerv Syst. 1994;10:517–23. doi: 10.1007/BF00335074. [DOI] [PubMed] [Google Scholar]
- 10.Betzen C, White R, Zehendner CM, Pietrowski E, Bender B, Luhmann HJ, et al. Oxidative stress upregulates the NMDA receptor on cerebrovascular endothelium. Free Radic Biol Med. 2009;47:1212–20. doi: 10.1016/j.freeradbiomed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surg Neurol Int. 2011;2:107. doi: 10.4103/2152-7806.83391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 13.Boden BP, Kirkendall DT, Garrett WE., Jr Concussion incidence in elite college soccer players. Am J Sports Med. 1998;26:238–41. doi: 10.1177/03635465980260021301. [DOI] [PubMed] [Google Scholar]
- 14.Boyko M, Zlotnik A, Gruenbaum BF, Gruenbaum SE, Ohayon S, Kuts R, et al. Pyruvate's blood glutamate scavenging activity contributes to the spectrum of its neuroprotective mechanisms in a rat model of stroke. Eur J Neurosci. 2011;34:1432–41. doi: 10.1111/j.1460-9568.2011.07864.x. [DOI] [PubMed] [Google Scholar]
- 15.Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050–7. doi: 10.1227/01.NEU.0000255479.90999.C0. discussion 1057-8. [DOI] [PubMed] [Google Scholar]
- 16.Broglio SP, Schnebel B, Sosnoff JJ, Shin S, Fend X, He X, et al. Biomechanical properties of concussions in high school football. Med Sci Sports Exerc. 2010;42:2064–71. doi: 10.1249/MSS.0b013e3181dd9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–21. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- 18.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–7. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 19.Buckley WE. Concussions in college football. A multivariate analysis. Am J Sports Med. 1988;16:51–6. doi: 10.1177/036354658801600109. [DOI] [PubMed] [Google Scholar]
- 20.Buki A, Povlishock JT. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta Neurochir (Wien) 2006;148:181–93. doi: 10.1007/s00701-005-0674-4. discussion 193-184. [DOI] [PubMed] [Google Scholar]
- 21.Burd I, Breen K, Friedman A, Chai J, Elovitz MA. Magnesium sulfate reduces inflammation-associated brain injury in fetal mice. Am J Obstet Gynecol. 2010;202(292):e1–9. doi: 10.1016/j.ajog.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldroney RD, Radike J. Experience with mild traumatic brain injuries and postconcussion syndrome at Kandahar, Afghanistan. US Army Med Dep J. 2010:22–30. [PubMed] [Google Scholar]
- 23.Report to Congress on mild traumatic brain injury in the United States: Steps to prevent a serious public health problem. Atlanta (GA): Centers for Disease Control and Prevention; 2003. Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control. [Google Scholar]
- 24.Cernak I, Chang T, Ahmed FA, Cruz MI, Vink R, Stoica B, et al. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci. 2010;32:442–53. doi: 10.1159/000320085. [DOI] [PubMed] [Google Scholar]
- 25.Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Cinar V, Mogulkoc R, Baltaci AK, Nizamlioglu M. Effect of magnesium supplementation on some plasma elements in athletes at rest and exhaustion. Biol Trace Elem Res. 2007;119:97–102. doi: 10.1007/s12011-007-0024-x. [DOI] [PubMed] [Google Scholar]
- 27.Collins MW, Lovell MR, Iverson GL, Cantu RC, Maroon JC, Field M. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51:1175–9. doi: 10.1097/00006123-200211000-00011. discussion 1180-71. [DOI] [PubMed] [Google Scholar]
- 28.Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, et al. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004;90:758–64. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- 29.Covassin T, Elbin R, Kontos A, Larson E. Investigating baseline neurocognitive performance between male and female athletes with a history of multiple concussion. J Neurol Neurosurg Psychiatry. 2010;81:597–601. doi: 10.1136/jnnp.2009.193797. [DOI] [PubMed] [Google Scholar]
- 30.Covassin T, Elbin RJ, Nakayama Y. Tracking neurocognitive performance following concussion in high school athletes. Phys Sportsmed. 2010;38:87–93. doi: 10.3810/psm.2010.12.1830. [DOI] [PubMed] [Google Scholar]
- 31.Covassin T, Schatz P, Swanik CB. Sex differences in neuropsychological function and post-concussion symptoms of concussed collegiate athletes. Neurosurgery. 2007;61:345–50. doi: 10.1227/01.NEU.0000279972.95060.CB. [DOI] [PubMed] [Google Scholar]
- 32.Covassin T, Swanik CB, Sachs M, Kendrick Z, Schatz P, Zillmer E, et al. Sex differences in baseline neuropsychological function and concussion symptoms of collegiate athletes. Br J Sports Med. 2006;40:923–7. doi: 10.1136/bjsm.2006.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daneshvar DH, Nowinski CJ, McKee AC, Cantu RC. The epidemiology of sport-related concussion. Clin Sports Med. 2011;30:1–17. doi: 10.1016/j.csm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Beaumont L, Brisson B, Lassonde M, Jolicoeur P. Long-term electrophysiological changes in athletes with a history of multiple concussions. Brain Inj. 2007;21:631–44. doi: 10.1080/02699050701426931. [DOI] [PubMed] [Google Scholar]
- 35.De Beaumont L, Lassonde M, Leclerc S, Theoret H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery. 2007;61:329–36. doi: 10.1227/01.NEU.0000280000.03578.B6. [DOI] [PubMed] [Google Scholar]
- 36.DeWitt DS, Prough DS. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- 37.Di Pietro V, Amin D, Pernagallo S, Lazzarino G, Tavazzi B, Vagnozzi R, et al. Transcriptomics of traumatic brain injury: gene expression and molecular pathways of different grades of insult in a rat organotypic hippocampal culture model. J Neurotrauma. 2010;27:349–59. doi: 10.1089/neu.2009.1095. [DOI] [PubMed] [Google Scholar]
- 38.Dicheskul ML, Kulikov VP. [Arterial and venous brain reactivity in the acute period of brain concussion] Zh Nevrol Psikhiatr Im S S Korsakova. 2009;109:65–8. [PubMed] [Google Scholar]
- 39.Donkin JJ, Nimmo AJ, Cernak I, Blumbergs PC, Vink R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1388–98. doi: 10.1038/jcbfm.2009.63. [DOI] [PubMed] [Google Scholar]
- 40.El-Rawas R, Saade NE, Thiriet N, Atweh S, Jaber M, Al-Amin HA. Developmental changes in the mRNA expression of neuropeptides and dopamine and glutamate receptors in neonates and adult rats after ventral hippocampal lesion. Schizophr Res. 2009;113:298–307. doi: 10.1016/j.schres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Elbin RJ, Schatz P, Covassin T. One-Year Test-Retest Reliability of the Online Version of ImPACT in High School Athletes. Am J Sports Med. 2011;39:2319–24. doi: 10.1177/0363546511417173. [DOI] [PubMed] [Google Scholar]
- 42.Erlanger D, Kaushik T, Cantu R, Barth JT, Broshek DK, Freeman JR, et al. Symptom-based assessment of the severity of a concussion. J Neurosurg. 2003;98:477–84. doi: 10.3171/jns.2003.98.3.0477. [DOI] [PubMed] [Google Scholar]
- 43.Fazio VC, Lovell MR, Pardini JE, Collins MW. The relation between post concussion symptoms and neurocognitive performance in concussed athletes. NeuroRehabilitation. 2007;22:207–16. [PubMed] [Google Scholar]
- 44.Felber ES. Combat-related posttraumatic headache: diagnosis, mechanisms of injury, and challenges to treatment. J Am Osteopath Assoc. 2010;110:737–8. [PubMed] [Google Scholar]
- 45.Feuerstein GZ, Wang X, Barone FC. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation. 1998;5:143–59. doi: 10.1159/000026331. [DOI] [PubMed] [Google Scholar]
- 46.Fraser PA. The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med. 2011;51:967–77. doi: 10.1016/j.freeradbiomed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Frommer LJ, Gurka KK, Cross KM, Ingersoll CD, Comstock RD, Saliba SA. Sex differences in concussion symptoms of high school athletes. J Athl Train. 2011;46:76–84. doi: 10.4085/1062-6050-46.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30:179–88. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gessel LM, Fields SK, Collins CL, Dick RW, Comstock RD. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42:495–503. [PMC free article] [PubMed] [Google Scholar]
- 50.Goffus AM, Anderson GD, Hoane M. Sustained delivery of nicotinamide limits cortical injury and improves functional recovery following traumatic brain injury. Oxid Med Cell Longev. 2010;3:145–52. doi: 10.4161/oxim.3.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodman D, Gaetz M, Meichenbaum D. Concussions in hockey: there is cause for concern. Med Sci Sports Exerc. 2001;33:2004–9. doi: 10.1097/00005768-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Gottschalk AW, Andrish JT. Epidemiology of sports injury in pediatric athletes. Sports Med Arthrosc. 2011;19:2–6. doi: 10.1097/JSA.0b013e31820b95fc. [DOI] [PubMed] [Google Scholar]
- 53.Grady MF. Concussion in the adolescent athlete. Curr Probl Pediatr Adolesc Health Care. 2010;40:154–69. doi: 10.1016/j.cppeds.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Grindel SH. Epidemiology and pathophysiology of minor traumatic brain injury. Curr Sports Med Rep. 2003;2:18–23. doi: 10.1249/00149619-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–26. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 56.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903–9. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 57.Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, et al. Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. JAMA. 2003;290:2549–55. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- 58.Halstead ME, Walter KD. American Academy of Pediatrics. Clinical report--sport-related concussion in children and adolescents. Pediatrics. 2010;126:597–615. doi: 10.1542/peds.2010-2005. [DOI] [PubMed] [Google Scholar]
- 59.Hammond-Tooke GD, Goei J, du Plessis LJ, Franz EA. Concussion causes transient dysfunction in cortical inhibitory networks but not the corpus callosum. J Clin Neurosci. 2010;17:315–9. doi: 10.1016/j.jocn.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 60.Hinton-Bayre AD, Geffen G, Friis P. Presentation and mechanisms of concussion in professional Rugby League Football. J Sci Med Sport. 2004;7:400–4. doi: 10.1016/s1440-2440(04)80035-5. [DOI] [PubMed] [Google Scholar]
- 61.Hovda DA, Fu K, Badie H, Samii A, Pinanong P, Becker DP. Administration of an omega-conopeptide one hour following traumatic brain injury reduces 45calcium accumulation. Acta Neurochir Suppl (Wien) 1994;60:521–3. doi: 10.1007/978-3-7091-9334-1_143. [DOI] [PubMed] [Google Scholar]
- 62.Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, et al. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. J Neuroinflammation. 2011;8:42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunt T, Asplund C. Concussion assessment and management. Clin Sports Med. 2010;29:5–17. doi: 10.1016/j.csm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Imer M, Omay B, Uzunkol A, Erdem T, Sabanci PA, Karasu A, et al. Effect of magnesium, MK-801 and combination of magnesium and MK-801 on blood-brain barrier permeability and brain edema after experimental traumatic diffuse brain injury. Neurol Res. 2009;31:977–81. doi: 10.1179/174313209X385617. [DOI] [PubMed] [Google Scholar]
- 65.Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. Brain Inj. 2006;20:245–52. doi: 10.1080/02699050500487910. [DOI] [PubMed] [Google Scholar]
- 66.Iverson GL, Gaetz M, Lovell MR, Collins MW. Cumulative effects of concussion in amateur athletes. Brain Inj. 2004;18:433–43. doi: 10.1080/02699050310001617352. [DOI] [PubMed] [Google Scholar]
- 67.Iverson GL, Lovell MR, Collins MW. Validity of ImPACT for measuring processing speed following sports-related concussion. J Clin Exp Neuropsychol. 2005;27:683–9. doi: 10.1081/13803390490918435. [DOI] [PubMed] [Google Scholar]
- 68.Jantzen KJ, Anderson B, Steinberg FL, Kelso JA. A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR Am J Neuroradiol. 2004;25:738–45. [PMC free article] [PubMed] [Google Scholar]
- 69.Jaworski CA. Latest clinical research published by ACSM. Curr Sports Med Rep. 2011;10:5–6. doi: 10.1249/JSR.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 70.Johnson EW, Kegel NE, Collins MW. Neuropsychological assessment of sport-related concussion. Clin Sports Med. 2011;30:73–88. doi: 10.1016/j.csm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Jordan BD. Chronic traumatic brain injury associated with boxing. Semin Neurol. 2000;20:179–85. doi: 10.1055/s-2000-9826. [DOI] [PubMed] [Google Scholar]
- 72.Kang SW, Choi SK, Park E, Chae SJ, Choi S, Jin Joo H, et al. Neuroprotective effects of magnesium-sulfate on ischemic injury mediated by modulating the release of glutamate and reduced of hyperreperfusion. Brain Res. 2011;1371:121–8. doi: 10.1016/j.brainres.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 73.Kelly JP. Traumatic brain injury and concussion in sports. JAMA. 1999;282:989–91. doi: 10.1001/jama.282.10.989. [DOI] [PubMed] [Google Scholar]
- 74.Kemp SP, Hudson Z, Brooks JH, Fuller CW. The epidemiology of head injuries in English professional rugby union. Clin J Sport Med. 2008;18:227–34. doi: 10.1097/JSM.0b013e31816a1c9a. [DOI] [PubMed] [Google Scholar]
- 75.Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006;117:1359–71. doi: 10.1542/peds.2005-0994. [DOI] [PubMed] [Google Scholar]
- 76.Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J Clin Neurophysiol. 2005;22:1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- 77.Kovesdi E, Luckl J, Bukovics P, Farkas O, Pal J, Czeiter E, et al. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir (Wien) 2010;152:1–17. doi: 10.1007/s00701-009-0463-6. [DOI] [PubMed] [Google Scholar]
- 78.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19:216–21. doi: 10.1097/JSM.0b013e31819d6edb. [DOI] [PubMed] [Google Scholar]
- 80.Len TK, Neary JP. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging. 2011;31:85–93. doi: 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 81.Levy ML, Ozgur BM, Berry C, Aryan HE, Apuzzo ML. Analysis and evolution of head injury in football. Neurosurgery. 2004;55:649–55. doi: 10.1227/01.neu.0000134598.06114.89. [DOI] [PubMed] [Google Scholar]
- 82.Livingston SC, Saliba EN, Goodkin HP, Barth JT, Hertel JN, Ingersoll CD. A preliminary investigation of motor evoked potential abnormalities following sport-related concussion. Brain Inj. 2010;24:904–13. doi: 10.3109/02699051003789245. [DOI] [PubMed] [Google Scholar]
- 83.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Longhi L, Saatman KE, Fujimoto S, Raghupathi R, Meaney DF, Davis J, et al. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56:364–74. doi: 10.1227/01.neu.0000149008.73513.44. [DOI] [PubMed] [Google Scholar]
- 85.Lovell MR, Fazio V. Concussion management in the child and adolescent athlete. Curr Sports Med Rep. 2008;7:12–5. doi: 10.1097/01.CSMR.0000308671.45558.e2. [DOI] [PubMed] [Google Scholar]
- 86.Lulic D, Burns J, Bae EC, van Loveren H, Borlongan CV. A review of laboratory and clinical data supporting the safety and efficacy of cyclosporin A in traumatic brain injury. Neurosurgery. 2011;68(5):1172–1185. doi: 10.1227/NEU.0b013e31820c6cdc. [DOI] [PubMed] [Google Scholar]
- 87.Maas AI, Murray GD. Magnesium for neuroprotection after traumatic brain injury. Lancet Neurol. 2007;6:20–1. doi: 10.1016/S1474-4422(06)70668-8. [DOI] [PubMed] [Google Scholar]
- 88.Makdissi M, McCrory P, Ugoni A, Darby D, Brukner P. A prospective study of postconcussive outcomes after return to play in Australian football. Am J Sports Med. 2009;37:877–83. doi: 10.1177/0363546508328118. [DOI] [PubMed] [Google Scholar]
- 89.Maroon JC, Bost J. Concussion management at the NFL, college, high school, and youth sports levels. Clin Neurosurg. 2011;58:51–56. doi: 10.1227/neu.0b013e3182269efe. [DOI] [PubMed] [Google Scholar]
- 90.Martini DN, Sabin MJ, DePesa SA, Leal EW, Negrete TN, Sosnoff JJ, et al. The chronic effects of concussion on gait. Arch Phys Med Rehabil. 2011;92:585–9. doi: 10.1016/j.apmr.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 91.Maruta J, Lee SW, Jacobs EF, Ghajar J. A unified science of concussion. Ann N Y Acad Sci. 2010;1208:58–66. doi: 10.1111/j.1749-6632.2010.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCaffrey MA, Mihalik JP, Crowell DH, Shields EW, Guskiewicz KM. Measurement of head impacts in collegiate football players: clinical measures of concussion after high- and low-magnitude impacts. Neurosurgery. 2007;61:1236–43. doi: 10.1227/01.neu.0000306102.91506.8b. [DOI] [PubMed] [Google Scholar]
- 93.McCrea M, Guskiewicz K, Randolph C, Barr WB, Hammeke TA, Marshall SW, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876–82. doi: 10.1227/01.NEU.0000350155.89800.00. [DOI] [PubMed] [Google Scholar]
- 94.McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: The NCAA Concussion Study. JAMA. 2003;290:2556–63. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 95.McCrea M, Prichep L, Powell MR, Chabot R, Barr WB. Acute effects and recovery after sport-related concussion: a neurocognitive and quantitative brain electrical activity study. J Head Trauma Rehabil. 2010;25:283–92. doi: 10.1097/HTR.0b013e3181e67923. [DOI] [PubMed] [Google Scholar]
- 96.McCrory P. Future advances and areas of future focus in the treatment of sport-related concussion. Clin Sports Med. 2011;30:201–8. doi: 10.1016/j.csm.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 97.McCrory P. Sports concussion and the risk of chronic neurological impairment. Clin J Sport Med. 2011;21:6–12. doi: 10.1097/JSM.0b013e318204db50. [DOI] [PubMed] [Google Scholar]
- 98.McCrory P. What's in a name? Br J Sports Med. 2001;35:285–6. doi: 10.1136/bjsm.35.5.285-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCrory P, Johnston K, Meeuwisse W, Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Br J Sports Med. 2005;39:196–204. doi: 10.1136/bjsm.2005.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport--the 3rd International Conference on concussion in sport, held in Zurich, November 2008. J Clin Neurosci. 2009;16:755–63. doi: 10.1016/j.jocn.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 101.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport - the Third International Conference on Concussion in Sport held in Zurich, November 2008. Phys Sportsmed. 2009;37:141–59. doi: 10.3810/psm.2009.06.1721. [DOI] [PubMed] [Google Scholar]
- 102.McGrath N. Supporting the student-athlete's return to the classroom after a sport-related concussion. J Athl Train. 2010;45:492–8. doi: 10.4085/1062-6050-45.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–35. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meaney DF, Smith DH. Biomechanics of concussion. Clin Sports Med. 2011;30:19–31. doi: 10.1016/j.csm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meehan WP., 3rd Medical therapies for concussion. Clin Sports Med. 2011;30:115–24. doi: 10.1016/j.csm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meehan WP, 3rd, Taylor AM, Proctor M. The pediatric athlete: Younger athletes with sport-related concussion. Clin Sports Med. 2011;30:133–44. doi: 10.1016/j.csm.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moser RS, Schatz P, Jordan BD. Prolonged effects of concussion in high school athletes. Neurosurgery. 2005;57:300–6. doi: 10.1227/01.neu.0000166663.98616.e4. [DOI] [PubMed] [Google Scholar]
- 108.Nation AD, Nelson NG, Yard EE, Comstock RD, McKenzie LB. Football-related injuries among 6- to 17-year-olds treated in US emergency departments, 1990-2007. Clin Pediatr (Phila) 2011;50:200–7. doi: 10.1177/0009922810388511. [DOI] [PubMed] [Google Scholar]
- 109.Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Ann N Y Acad Sci. 2008;1147:53–60. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- 110.Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions. J Forensic Nurs. 2010;6:40–6. doi: 10.1111/j.1939-3938.2009.01064.x. [DOI] [PubMed] [Google Scholar]
- 111.Pages N, Maurois P, Delplanque B, Bac P, Martin JC, Du Q, et al. Brain protection by rapeseed oil in magnesium-deficient mice. Prostaglandins, leukotrienes, and essential fatty acids. 2011;85:53–60. doi: 10.1016/j.plefa.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pardini JE, Pardini DA, Becker JT, Dunfee KL, Eddy WF, Lovell MR, et al. Postconcussive symptoms are associated with compensatory cortical recruitment during a working memory task. Neurosurgery. 2010;67:1020–7. doi: 10.1227/NEU.0b013e3181ee33e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pellman EJ, Powell JW, Viano DC, Casson IR, Tucker AM, Feuer H, et al. Concussion in professional football: epidemiological features of game injuries and review of the literature--part 3. Neurosurgery. 2004;54:81–94. doi: 10.1227/01.neu.0000097267.54786.54. [DOI] [PubMed] [Google Scholar]
- 114.Polusny MA, Kehle SM, Nelson NW, Erbes CR, Arbisi PA, Thuras P. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Arch Gen Psychiatry. 2011;68:79–89. doi: 10.1001/archgenpsychiatry.2010.172. [DOI] [PubMed] [Google Scholar]
- 115.Press A. Goodell issues memo changing return-to-play rules for concussions. [Internet article] 2009. [Last updated on 2009 Dec 2]. Available from: http://www.nfl.com/news/story?confirm=trueandid=09000d5d814a9ecdandtemplate=with-video-with-comments .
- 116.Price CJ, Warburton EA, Menon DK. Human cellular inflammation in the pathology of acute cerebral ischaemia. J Neurol Neurosurg Psychiatry. 2003;74:1476–84. doi: 10.1136/jnnp.74.11.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prins ML, Hales A, Reger M, Giza CC, Hovda DA. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci. 2010;32:510–8. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Putukian M. The acute symptoms of sport-related concussion: Diagnosis and on-field management. Clin Sports Med. 2011;30:49–61. doi: 10.1016/j.csm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 119.Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 120.Samii A, Badie H, Fu K, Luther RR, Hovda DA. Effects of an N-type calcium channel antagonist (SNX 111; Ziconotide) on calcium-45 accumulation following fluid-percussion injury. J Neurotrauma. 1999;16:879–92. doi: 10.1089/neu.1999.16.879. [DOI] [PubMed] [Google Scholar]
- 121.Sarmiento K, Mitchko J, Klein C, Wong S. Evaluation of the Centers for Disease Control and Prevention's concussion initiative for high school coaches: “Heads Up: Concussion in High School Sports”. J Sch Health. 2010;80:112–8. doi: 10.1111/j.1746-1561.2010.00491.x. [DOI] [PubMed] [Google Scholar]
- 122.Schatz P. Long-term test-retest reliability of baseline cognitive assessments using ImPACT. Am J Sports Med. 2010;38:47–53. doi: 10.1177/0363546509343805. [DOI] [PubMed] [Google Scholar]
- 123.Schnebel B, Gwin JT, Anderson S, Gatlin R. In vivo study of head impacts in football: A comparison of National Collegiate Athletic Association Division I versus high school impacts. Neurosurgery. 2007;60:490–5. doi: 10.1227/01.NEU.0000249286.92255.7F. [DOI] [PubMed] [Google Scholar]
- 124.Schouten JW. Neuroprotection in traumatic brain injury: A complex struggle against the biology of nature. Curr Opin Crit Care. 2007;13:134–42. doi: 10.1097/MCC.0b013e3280895d5c. [DOI] [PubMed] [Google Scholar]
- 125.Sen S, Goldman H, Morehead M, Murphy S, Phillis JW. Alpha-Phenyl-tert-butyl-nitrone inhibits free radical release in brain concussion. Free Radic Biol Med. 1994;16:685–91. doi: 10.1016/0891-5849(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 126.Shahlaie K, Lyeth BG, Gurkoff GG, Muizelaar JP, Berman RF. Neuroprotective effects of selective N-type VGCC blockade on stretch-injury-induced calcium dynamics in cortical neurons. J Neurotrauma. 2010;27:175–187. doi: 10.1089/neu.2009.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sim A, Terryberry-Spohr L, Wilson KR. Prolonged recovery of memory functioning after mild traumatic brain injury in adolescent athletes. J Neurosurg. 2008;108:511–6. doi: 10.3171/JNS/2008/108/3/0511. [DOI] [PubMed] [Google Scholar]
- 128.Skoromets AA, Pugacheva EL. [Efficacy of the complex drug cytoflavin in the treatment of consequences of mild brain injury] Zh Nevrol Psikhiatr Im S S Korsakova. 2010;110:31–6. [PubMed] [Google Scholar]
- 129.Slobounov S, Slobounov E, Sebastianelli W, Cao C, Newell K. Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery. 2007;61:338–44. doi: 10.1227/01.NEU.0000280001.03578.FF. [DOI] [PubMed] [Google Scholar]
- 130.Slobounov SM, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: A functional MRI study. Exp Brain Res. 2010;202:341–54. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith C, Graham DI, Murray LS, Stewart J, Nicoll JA. Association of APOE e4 and cerebrovascular pathology in traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77:363–6. doi: 10.1136/jnnp.2005.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smits M, Dippel DW, Houston GC, Wielopolski PA, Koudstaal PJ, Hunink MG, et al. Postconcussion syndrome after minor head injury: Brain activation of working memory and attention. Hum Brain Mapp. 2009;30:2789–803. doi: 10.1002/hbm.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stulemeijer M, van der Werf S, Bleijenberg G, Biert J, Brauer J, Vos PE. Recovery from mild traumatic brain injury: a focus on fatigue. J Neurol. 2006;253:1041–7. doi: 10.1007/s00415-006-0156-5. [DOI] [PubMed] [Google Scholar]
- 134.Sun DA, Deshpande LS, Sombati S, Baranova A, Wilson MS, Hamm RJ, et al. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. The Eur J Neurosci. 2008;27:1659–72. doi: 10.1111/j.1460-9568.2008.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swan AA, Chandrashekar R, Beare J, Hoane MR. Preclinical efficacy testing in middle-aged rats: nicotinamide, a novel neuroprotectant, demonstrates diminished preclinical efficacy after controlled cortical impact. J Neurotrauma. 2011;28:431–40. doi: 10.1089/neu.2010.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Szewczyk B, Poleszak E, Sowa-Kucma M, Siwek M, Dudek D, Ryszewska-Pokrasniewicz B, et al. Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacol Rep. 2008;60:588–9. [PubMed] [Google Scholar]
- 137.Tay SY, Ang BT, Lau XY, Meyyappan A, Collinson SL. Chronic impairment of prospective memory after mild traumatic brain injury. J Neurotrauma. 2010;27:77–83. doi: 10.1089/neu.2009.1074. [DOI] [PubMed] [Google Scholar]
- 138.Temkin NR, Anderson GD, Winn HR, Ellenbogen RG, Britz GW, Schuster J, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: A randomised controlled trial. Lancet Neurol. 2007;6:29–38. doi: 10.1016/S1474-4422(06)70630-5. [DOI] [PubMed] [Google Scholar]
- 139.Tierney RT, Mansell JL, Higgins M, McDevitt JK, Toone N, Gaughan JP, et al. Apolipoprotein E genotype and concussion in college athletes. Clin J Sport Med. 2010;20:464–8. doi: 10.1097/JSM.0b013e3181fc0a81. [DOI] [PubMed] [Google Scholar]
- 140.Toklu HZ, Hakan T, Biber N, Solakoglu S, Ogunc AV, Sener G. The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radic Res. 2009;43:658–67. doi: 10.1080/10715760902988843. [DOI] [PubMed] [Google Scholar]
- 141.Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: A multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–42. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 142.Van Kampen DA, Lovell MR, Pardini JE, Collins MW, Fu FH. The “value added” of neurocognitive testing after sports-related concussion. Am J Sports Med. 2006;34:1630–5. doi: 10.1177/0363546506288677. [DOI] [PubMed] [Google Scholar]
- 143.Vannemreddy P, Ray AK, Patnaik R, Patnaik S, Mohanty S, Sharma HS. Zinc protoporphyrin IX attenuates closed head injury-induced edema formation, blood-brain barrier disruption, and serotonin levels in the rat. Acta Neurochirurgica. 2006;96:151–6. doi: 10.1007/3-211-30714-1_34. [DOI] [PubMed] [Google Scholar]
- 144.Vikelis M, Mitsikostas DD. The role of glutamate and its receptors in migraine. CNS Neurol Disord Drug Targets. 2007;6:251–7. doi: 10.2174/187152707781387279. [DOI] [PubMed] [Google Scholar]
- 145.Vink R, Nimmo AJ. Multifunctional drugs for head injury. Neurotherapeutics. 2009;6:28–42. doi: 10.1016/j.nurt.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 147.Wetjen NM, Pichelmann MA, Atkinson JL. Second impact syndrome: concussion and second injury brain complications. J Am Coll Surg. 2010;211:553–7. doi: 10.1016/j.jamcollsurg.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 148.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–55. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 149.Wozniak JR, Krach L, Ward E, Mueller BA, Muetzel R, Schnoebelen S, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22:555–68. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yard EE, Comstock RD. Compliance with return to play guidelines following concussion in US high school athletes, 2005-2008. Brain Inj. 2009;23:888–98. doi: 10.1080/02699050903283171. [DOI] [PubMed] [Google Scholar]
- 151.Zafonte R. Diagnosis and management of sports-related concussion: A 15-year-old athlete with a concussion. JAMA. 2011;306:79–86. doi: 10.1001/jama.2011.819. [DOI] [PubMed] [Google Scholar]


