Abstract
Background. Kidney disease is a risk factor for mortality and cardiovascular disease in older adults, but the separate and combined effects of albuminuria and cystatin C, a novel marker of glomerular filtration, are not known.
Methods. We examined associations of these markers with mortality and cardiovascular outcomes during a median follow-up of 8.3 years in 3291 older adults in the Cardiovascular Health Study. Kidney disease was assessed using urinary albumin/creatinine ratio (ACR), cystatin C and Modification of Diet in Renal Disease estimated glomerular filtration rate (eGFR). We defined subgroups based on presence of microalbuminuria (MA, ACR > 30 mg/g) and categories of normal kidney function (cystatin C < 1.0 mg/L and eGFR > 60 mL/min/1.73 m2); preclinical kidney disease (cystatin C level > 1.0 mg/l but eGFR > 60 mL/min/1.73 m2); and chronic kidney disease (CKD) (eGFR < 60 mL/min/1.73 m2). Cox proportional hazards models were used to examine associations between these six subgroups and all-cause or cardiovascular mortality, myocardial infarction and heart failure.
Results. One thousand one hundred fifty (34.9%) had normal kidney function (12.2% with MA), 1518 (46.1%) had preclinical kidney disease (17.9% with MA) and 622 (18.9%) had CKD (47% with MA). After adjustment, the presence of either preclinical kidney disease or MA was associated with an over 50% increase in mortality risk; the presence of both was associated with a 2.4-fold mortality risk. Those with CKD and MA were at highest risk, with a nearly 4-fold mortality risk.
Conclusion. Elevated cystatin C and albuminuria are common, identify different subsets of the older population, and are independent, graded risk factors for cardiovascular disease and mortality.
Keywords: albuminuria, aging, cardiovascular diseases, kidney function, mortality
Introduction
Impaired glomerular filtration rate (eGFR < 60 ml/min/1.73 m2) or kidney damage evidenced by the presence of elevated levels of albumin in the urine affect 17% of US adults; in older adults, these findings are more common, with >40% of those over age 70 years having one or both markers of chronic kidney disease [1]. Both micro- or macroalbuminuria [2–6] and decreased GFR [7–12] have been associated with increased rates of cardiovascular disease and mortality [13]. Recent reports in select populations [6,14–17] have evaluated the relations between creatinine-based eGFR and albuminuria with outcomes. Although each study found that both impaired eGFR and albuminuria were associated with risk of cardiovascular disease or mortality, the association of eGFR with risk was limited, in the absence of microalbuminuria, to persons with stage 3 chronic kidney disease (CKD) (eGFR < 60 ml/min/1.73 m2) [18].
Several studies have now demonstrated that cystatin C is a more sensitive marker of early changes in kidney function, particularly in an older population [19,20], and has more linear associations with mortality risk [21] than Modification of Diet in Renal Disease Study (MDRD) GFR estimates [22]. No previous studies, to our knowledge, have compared associations between albuminuria, MDRD GFR estimates and cystatin C with risk of cardiovascular events and mortality in a large representative older cohort. We therefore evaluated the relative contributions of cystatin C, MDRD GFR estimates and albuminuria to cardiovascular disease and mortality risk in the Cardiovascular Health Study (CHS), a prospective cohort of older US adults.
Subjects and Methods
Study population
The CHS is a cohort of adults aged 65 years and over at enrolment, recruited from Medicare eligibility lists in four US communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh) [23]. This study, designed to examine subclinical and clinical risk factors and cardiovascular disease in older adults, was initiated in 1989 with the recruitment of 5201 individuals; during 1992–93, an additional 687 African-American participants were recruited. Albuminuria was first measured using urine samples obtained in 1996–97 (the seventh year of follow-up in the study), and serum samples for cystatin C and creatinine were available from the same visit. For the purposes of this analysis, covariates measured at the 1996–97 visit were considered the baseline for survival models. We included all 3291 individuals who participated in the 1996–97 visit and had measured urine albumin and creatinine, and serum creatinine and cystatin C. For the analysis of incident heart failure (HF, defined below), we excluded those individuals who had prevalent HF at the seventh study visit, leaving a total of 2992 individuals for analysis. For analysis of incident myocardial infarction (MI, defined below), we excluded individuals with a previously diagnosed MI (2925 individuals in the analysis). Non-fatal outcomes were analysed with censoring for death.
All participants provided written informed consent; the institutional review boards of the University of Washington and the affiliated clinical centres approved the study.
Predictor variables: cystatin C, creatinine and urinary albumin-to-creatinine ratios
Frozen sera stored at −70°C were used for measurement of cystatin C. Cystatin C was measured using a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring) with a nephelometre (BNII, Dade Behring); this assay is stable through several freeze-thaw cycles [24]. Creatinine was measured using a colorimetric method (Ektachem 700, Eastman Kodak). The mean coefficient of variation for monthly controls was 1.94 (range 1.16–3.60%). Serum creatinine was indirectly calibrated to the National Health and Nutrition Examination III study as previously described [12,25]. We then used the four-variable MDRD equation to estimate GFR [eGFR = 186.3 × (serum creatinine−1.154) × (age−0.203) × 1.212 (if black) × 0.742 (if female)] [22].
We defined three categories of kidney function, based on previous work [26]: (i) normal, the reference category (MDRD eGFR > 60 ml/min/1.73 m2 and cystatin C < 1.0 mg/L); (ii) preclinical kidney disease (MDRD eGFR > 60 ml/min/1.73 m2 and cystatin C > 1.0 mg/L); and (iii) CKD (MDRD eGFR < 60 ml/min/1.73 m2). Although the National Kidney Federation definition of CKD includes albuminuria as a defining factor [27]; for this analysis, CKD refers only to the filtration aspect of kidney function.
A random morning urine sample was available from each participant to assess urine albumin and creatinine. Urinary albumin was measured by rate nephelometry using the Array 360 CE Protein Analyzer (Beckman Instruments, Fullerton, CA). Urinary creatinine was measured on a Kodak Ektachem 700 Analyzer (Eastman Kodak Company, Rochester, NY). An ACR was then calculated. We first categorized ACR into sex-specific quintiles [28,29], and then dichotomized at the cutoff of 30 mg/g to define levels of albuminuria consistent with microalbuminuria (MA) [27] or greater. Those with levels >300 mg/g were included in the MA group; we performed a sensitivity analysis excluding these individuals (n = 97).
Outcome variables: cardiovascular mortality, all-cause mortality, congestive HF and MI
We analysed all events occurring from the Year 7 visit to end of available follow-up (30 June 2005). The methods used to ascertain events in CHS have been described previously [30,31]. In brief, all outcome events were adjudicated by an expert panel, according to published definitions. For the diagnosis of incident HF, a physician's diagnosis of HF was followed by review of the participant's medical records. The CHS Cardiovascular Events Committee determined the incidence of HF on the basis of diagnosis from a physician and consideration of symptoms, signs, chest radiographic findings and treatment of HF. The algorithm for classifying an incident MI included elements of chest pain, cardiac enzyme levels and changes in the electrocardiogram. Cardiovascular mortality was defined as death from coronary heart disease, HF, peripheral vascular disease or cerebrovascular disease. Vital status was determined for all participants through a combination of medical records, death certificates, obituary review, household contacts and the Centers for Medicare and Medicaid Services healthcare utilization database [30].
Covariates
We examined a number of covariates that might plausibly confound the association between albuminuria, decreased kidney function and outcomes. We included demographic variables (age, gender, race); cardiovascular risk factors including body mass index; hypertension (history and use of antihypertensive agents or an average of three seated blood pressure measurements measured with a random zero sphygmomanometre >140/90 mmHg); smoking; diabetes [use of insulin or an oral hypoglycaemic agent or a fasting blood sugar >7 mmol/L (126 mg/dL)]; and total cholesterol; subclinical markers of cardiovascular disease (C-reactive protein; electrocardiographic evidence of left ventricular hypertrophy or atrial fibrillation) and prevalent cardiovascular disease (transient ischaemic attack, stroke, coronary artery disease, MI, HF).
Analytic methods
ACR was analysed on a logarithmic scale initially, since other studies have shown linear associations of log-transformed ACR with adverse health outcomes and no clear ‘normal’ threshold for ACR [3,5], then divided into sex-specific quintiles, and finally dichotomized at the MA cutoff. For creatinine-based eGFR, we used an MDRD GFR value of 60 ml/min/1.73 m2 to define CKD [27]. We analysed cystatin C as a continuous variable per standard deviation and by quintiles. Within each of the three categories of kidney function, defined above, we dichotomized based on MA, creating six categories for analysis. The distribution of potential covariates by these six groups was compared. We examined event rates per 100 person-years across ACR quintiles by kidney disease category (normal, preclinical kidney disease and CKD). Associations of ACR and cystatin C with all-cause and cardiovascular mortality and with cardiovascular events were assessed using unadjusted and adjusted Cox proportional hazards models. The proportional hazards assumption was tested using standard residual-based techniques.
Covariates were selected as candidates for multivariate analysis based on their potential to confound the associations between albuminuria, cystatin C and mortality. We created models based on biological plausibility, including unadjusted models, followed by models adjusted for demographic factors and subclinical and clinical risk factors for cardiovascular disease. We adjusted the models of risk associated with albuminuria for cystatin C, and vice versa.
Interactions were evaluated between cystatin C and albuminuria as well as between each of these and race. To determine whether ACR and cystatin C increased the incidence of death in an additive fashion, hazard ratios of mortality were calculated for each group, and the effect modification between ACR and cystatin C was evaluated by using the Rothman synergy index [32]. The synergy index is the ratio of the observed effect of the joint exposure divided by the sum of the effects of each factor acting separately:
where RR indicates relative risk. A value of 1 indicates no interaction and a value >1 indicates a positive interaction between the two variables.
Analyses were performed using S-Plus (release 8.0, Insightful Inc, Seattle, WA) and SPSS statistical software (release 15.0.1.1, SPSS Inc, Chicago, IL).
Results
Characteristics of study population
Of the original 5888 participants in CHS, 3291 (56%) survived to the seventh study visit and had samples available for cystatin C, creatinine and ACR. Seven percent had diabetes, and 41% had hypertension. The individuals who did not survive had more prevalent cardiovascular disease were older, and had higher cystatin C levels at baseline.
The mean (SD) age of the group included in this study was 78 (5) years at the 1996–97 CHS visit, and 16% were African-American. Those with preclinical kidney disease or kidney disease were older and more likely to have hypertension than those with normal kidney function, and within each category of kidney function, the presence of MA was associated with higher prevalence of diabetes and cardiovascular disease (Table 1). The Spearman correlation between ACR and cystatin C was 0.22 (P < 0.001). MA was present in 612 (18.6%) of the overall cohort. One thousand one hundred fifty-one individuals (35.0%) had normal kidney function, of whom 12.2% had MA; 1518 (46.1%) had preclinical kidney disease, of whom 17.9% had MA, and 622 (18.9%) had overt CKD, of whom 31.8% had MA. The group with both CKD and MA had significantly higher mean cystatin C levels than the group with CKD and no MA (1.95 mg/L vs. 1.47 mg/L).
Table 1.
Baseline (1996–97) characteristics by microalbuminuria/kidney function categories
| Normal & no MA | Normal & MA | Pre-KD & no MA | Pre-KD & MA | CKD & no MA | CKD & MA | All | |
|---|---|---|---|---|---|---|---|
| n | 1011 | 140 | 1247 | 271 | 424 | 198 | 3291 |
| Age | 76 (4) | 77 (5) | 78 (5) | 79 (5) | 79 (5) | 80 (6) | 78 (5) |
| Gender (female) | 667 (66%) | 86 (61%) | 707 (57%) | 131 (48%) | 278 (66%) | 101 (51%) | 1970 (60%) |
| Race (black) | 203 (20%) | 40 (29%) | 159 (13%) | 44 (16%) | 56 (13%) | 32 (16%) | 534 (16%) |
| BMI (kg/m2) | 26.3 (4.3) | 26.0 (4.5) | 27.3 (4.8) | 26.8 (4.7) | 27.4 (4.9) | 27.1 (4.7) | 26.9 (4.6) |
| Ever smoked | 487 (49%) | 80 (58%) | 637 (52%) | 159 (60%) | 211 (51%) | 112 (58%) | 1686 (51%) |
| Diabetes | 119 (12%) | 42 (30%) | 127 (10%) | 92 (34%) | 55 (13%) | 49 (25%) | 484 (15%) |
| Hypertension | 400 (40%) | 94 (67%) | 572 (46%) | 177 (66%) | 242 (57%) | 154 (78%) | 1639 (50%) |
| Antihypertensives | 449 (44%) | 92 (66%) | 692 (56%) | 191 (71%) | 304 (72%) | 170 (86%) | 1898 (58%) |
| Cholesterol (mmol/L) | 5.22 (0.93) | 5.17 (1.03) | 5.22 (1.03) | 5.04 (1.06) | 5.32 (1.03) | 5.32 (1.24) | 5.22 (1.02) |
| CRPa (mg/L) | 1.96 (0.93, 4.25) | 1.96 (0.84, 4.90) | 2.52 (1.16, 5.10) | 3.11 (1.53, 6.77) | 2.54 (1.25, 5.62) | 3.74 (1.82, 7.31) | 1.96 (0.93, 4.25) |
| ECG AF | 15 (2%) | 10 (7%) | 45 (4%) | 31 (11%) | 15 (4%) | 16 (8%) | 132 (4%) |
| ECG LVH | 33 (3%) | 12 (9%) | 64 (5%) | 34 (14%) | 19 (5%) | 24 (13%) | 186 (6%) |
| Prevalent disease | |||||||
| Stroke | 41 (4%) | 7 (5%) | 63 (5%) | 27 (10%) | 31 (7%) | 31 (16%) | 200 (6%) |
| MI | 63 (6%) | 11 (8%) | 126 (10%) | 54 (20%) | 64 (15%) | 48 (24%) | 366 (11%) |
| CHF | 33 (3%) | 14 (10%) | 97 (8%) | 43 (16%) | 54 (13%) | 58 (29%) | 299 (9%) |
| eGFR-MDRD | 95 (19) | 98 (20) | 80 (15) | 80 (15) | 51 (8) | 43 (14) | 79 (23) |
| Cystatin C (mg/L) | 0.88 (0.08) | 0.90 (0.08) | 1.16 (0.15) | 1.23 (0.19) | 1.47 (0.37) | 1.95 (0.82) | 1.16 (0.39) |
| ACRa (mg/g) | 7 (4, 11) | 55 (40, 103) | 7 (4, 12) | 72 (46, 154) | 8 (5,14) | 119 (62, 363) | 7 (4, 11) |
Data are presented as either mean or percentage, unless a [median (interquartile range)]; BMI = body mass index; CRP = C-reactive protein; ECG = electrocardiogram; AF = atrial fibrillation; LVH = left ventricular hypertrophy; MI = myocardial infarction; CHF = congestive heart failure; ACR = urine microalbumin/creatinine ratio.
All-cause and cardiovascular mortality
Over a median of 8.28 years of follow-up for all-cause and cardiovascular mortality, 1357/3291 (41%) of the cohort died, with 510 (15.5%) from cardiovascular causes.
Mortality rates increased both with higher cystatin C and with the presence of MA; within each cystatin C quintile (Figure 1), an ∼2-fold higher event rate was observed in those with MA as compared to those without MA. The same pattern of increasing mortality rates was observed with ACR (Figure 2); within each quintile of ACR, decreased kidney function was incrementally associated with higher risk of mortality as compared to normal kidney function.
Fig. 1.
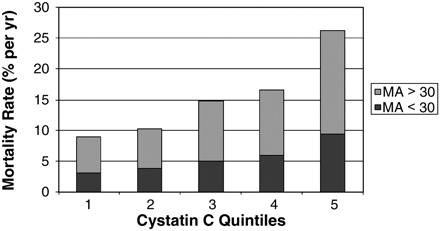
Mortality rates by quintiles of kidney function and microalbuminuria cystatin C quintiles: (1) <0.91 mg/L; (2) 0.92–1.02 mg/L; (3) 1.03–1.13 mg/L; (4) 1.14–1.32 mg/L; (5) >1.32 mg/L.
Fig. 2.
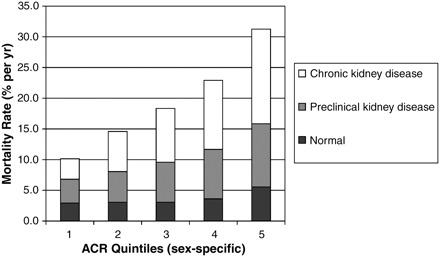
Mortality rates by ACR quintile and category of kidney disease.
In continuous analyses, both cystatin C and ACR conferred independent risks of both all-cause and cardiovascular mortality; with simultaneous adjustment, each log increase in albuminuria or standard deviation increase in cystatin C was associated with ∼20% increase in risk {for log increase in albuminuria, hazard ratio (HR) 1.20 [95% confidence interval (CI) 1.15, 1.25]; for standard deviation increase in cystatin C, HR 1.18 (95% CI 1.14, 1.24)}. There was an additive interaction between ACR and cystatin C [Rothman synergy index 1.91 (1.30–2.51)]. After adjustment for demographic factors, cardiovascular risk factors and cystatin C level, increasing quintiles of albuminuria conferred steadily increasing risk of all-cause and cardiovascular mortality, with the uppermost quintile having a doubled risk of all-cause mortality and cardiovascular mortality (Table 2). After similar adjustment for ACR and other factors, increasing quintiles of cystatin C also conferred increasing risk of all-cause and cardiovascular mortality; again, the uppermost quintile was associated with doubling of risk for all-cause and cardiovascular mortality as compared to the first quintile (Table 2).
Table 2.
Association of categories of ACR and cystatin C with adjusted hazard ratios for mortality, MI or CHF
| All-cause mortalitya | Cardiovascular mortalitya | MIa | CHFa | |
|---|---|---|---|---|
| Quintiles of ACR (mg albumin/g creatinine) | ||||
| <4.35 (w); < 4.02 (m) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 4.36-6.78 (w); 4.03-6.86 (m) | 1.17 (0.95, 1.43) | 1.10 (0.75, 1.6) | 0.97 (0.68, 1.39) | 1.49 (1.09, 2.05) |
| 6.79-11.24 (w); 6.87-12.77 (m) | 1.36 (1.11, 1.66) | 1.56 (1.10, 2.23) | 0.83 (0.57, 1.20) | 1.59 (1.16, 2.18) |
| 11.25–23.19 (w); 12.78–35.71 (m) | 1.56 (1.28, 1.90) | 1.80 (1.25, 2.54) | 1.18 (0.83, 1.68) | 2.02 (1.49, 2.74) |
| >23.19 (w); >35.71 (m) | 2.05 (1.67, 2.50) | 2.45 (1.74, 3.46) | 1.15 (0.79, 1.69) | 2.57 (1.89, 3.51) |
| Quintiles of cystatin C (mg/L) | ||||
| <0.92 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 0.92–1.02 | 1.10 (0.89, 1.36) | 1.17 (0.80, 1.71) | 1.19 (0.82, 1.75) | 1.05 (0.78, 1.41) |
| 1.03–1.13 | 1.35 (1.10, 1.65) | 1.65 (1.16, 2.36) | 1.26 (0.86, 1.85) | 1.26 (0.94, 1.67) |
| 1.14–1.32 | 1.58 (1.29, 1.93) | 1.69 (1.18, 2.42) | 1.45 (0.99, 2.11) | 1.22 (0.91, 1.63) |
| >1.32 | 2.01 (1.64, 2.46) | 2.24 (1.57, 3.19) | 1.92 (1.30, 2.83) | 1.62 (1.21, 2.17) |
W = women; m = men; ACR = albumin/creatinine ratio; MA = albumin/creatinine ratio >30 mg/g
Adjusted for the other marker of kidney function (for ACR models, adjustment is for cystatin C; for cystatin C models, adjustment is for ACR); age, gender, race, diabetes, systolic and diastolic blood pressure, hypertension medications, body mass index, smoking, C-reactive protein, prevalent MI (except for analysis of MI), CHF (except for analysis of CHF) and stroke.
Each group, as defined by the presence of MA or an abnormal category of kidney function, had increased risk of cardiovascular and all-cause mortality compared to the group with normal kidney function and no MA. Preclinical kidney disease with MA and MA with normal kidney function were each associated with an >50% increase in all-cause and cardiovascular mortality, while the presence of both was associated with a 2.4-fold mortality risk. Those with CKD and MA were at the highest risk, with a nearly 4-fold increase in all-cause and cardiovascular mortality (Figure 3).
Fig. 3.
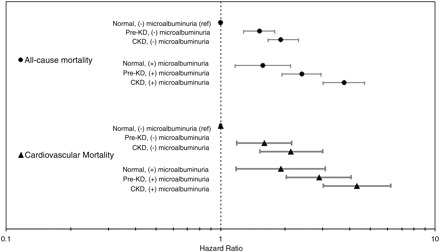
Adjusted hazards for all-cause and cardiovascular mortality based on kidney function and microalbuminuria adjusted for age, gender, race, diabetes, systolic and diastolic blood pressure, hypertension medications, body-mass index, smoking, CRP, prevalent myocardial infarction, congestive heart failure and stroke.
There was no interaction of race with either MA or cystatin C (P = 0.913 and 0.233, respectively). A sensitivity analysis excluding the subgroup with ACR >300 mg/g (97 individuals) did not alter the results.
MI and HF
Two thousand nine hundred twenty-five individuals were at risk for incident MI over a median of 8.24 years of follow-up; there was a weak association between albuminuria and MI, with each log increase in ACR associated with an 8% higher MI risk. Only the highest quintile of cystatin C was significantly associated with incident MI in adjusted analysis. Among the six subgroups, those with CKD were at significantly increased risk of incident MI; 1.5-fold risk for those with CKD but no MA, and a 2.5-fold risk for those with both CKD and MA (Figure 4).
Fig. 4.
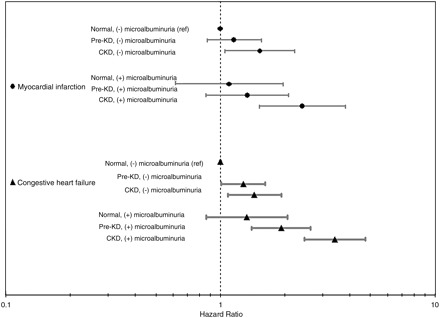
Adjusted hazards for incident myocardial infarction and congestive heart failure based on kidney function and albuminuria adjusted for age, gender, race, diabetes, systolic and diastolic blood pressure, hypertension medications, body-mass index, smoking, CRP, prevalent myocardial infarction (for CHF only), congestive heart failure (for MI only) and stroke.
Two thousand nine hundred ninety-two individuals were at risk for incident HF over a median of 8.22 years of follow-up. The risk pattern for HF was similar to that seen with all-cause or cardiovascular mortality. Graded increases in risk were seen with increasing quintiles of ACR or cystatin C, with significantly increased risk by the second quintile of ACR (Table 2). MA conferred an increased risk of congestive HF (CHF) in those with preclinical kidney disease or CKD (Figure 4).
Discussion
In this cohort of older adults, both ACR and impaired GFR, as measured by elevated serum cystatin C, were independently associated with increased risk of all-cause and cardiovascular mortality in older adults. Among persons without CKD (eGFR > 60 ml/min/1.73 m2), MA and pre-CKD identified different segments of the population and were associated with increased mortality risk; together, they were associated with more than 2-fold risk of death. In addition, we observed a linear gradient in mortality risk associated with albuminuria levels below the threshold considered for MA. A weaker association was observed between ACR and MI or CHF, particularly in those with normal kidney function. The risks associated with impaired GFR and ACR remained significant after adjustment for each other and numerous potential confounders including diabetes, hypertension and prevalent cardiovascular disease, and were not affected by race.
The reasons for the additive risks associated with the presence of albuminuria or impaired GFR are not entirely clear. The two measures are weakly correlated, and a significant proportion of those with impaired GFR does not have MA, and vice versa, as noted above [13]. The pattern of independent mortality risk that we observed suggests that these two clinical markers may operate through different pathophysiologic mechanisms.
Albuminuria is indicative of damage to glomerular basement membranes and may be reflective of vascular and endothelial damage [33]; it has been linked with inflammation and dysfunction of the coagulation system, possibly explaining its association with higher rates of cardiovascular events and cardiovascular mortality [34,35]. Impaired glomerular filtration rate leads to decreased clearance of numerous potentially toxic endogenous and exogenous by-products, and is also associated with higher levels of systemic inflammation [25,36]. Inflammation is noted with small changes in GFR, but other effects of impaired GFR have been noted primarily at more advanced degrees of kidney dysfunction. Our results persisted despite adjustment for measured comorbidities, but it is possible that preclinical kidney disease is also a marker for more severe or long-standing diseases such as hypertension, diabetes and dyslipidaemia.
Our study provides more detailed information about early kidney dysfunction in older adults, more complete assessment of outcomes and a more diverse cohort of older adults than those seen in previous studies of albuminuria and impaired eGFR. The recent studies using ACR and creatinine-based eGFR noted additive relationships between decreased eGFR and albuminuria, but did not use cystatin C, and either did not examine [14] or found equivocal or minimally increased risk [6,15] of mortality above an estimated eGFR of 60 or 75 ml/min/1.73 m2. Although the populations under study had slightly different demographic characteristics, both include a substantial proportion of older adults and are, we believe, comparable. Our study, by contrast, showed increased risk of mortality in the subgroup with preclinical kidney disease independent of albuminuria.
Our results were similar for CHF, with graded increases in risk based on increasing levels of ACR and decreasing kidney function. This pattern was attenuated for MI, and MA and preclinical kidney disease had only a modest relationship with this outcome. This is likely due to different underlying mechanisms, but may also partially reflect a lack of statistical power due to smaller numbers of events.
We noted that those with both MA and CKD were at markedly increased risk of deleterious outcomes in our study, with a nearly 4-fold risk of all-cause mortality even after adjustment for other comorbidities. This group also had the highest rates of incident CHF and MI. This emphasizes the importance of assessing MA in older adults with CKD, and vice-versa, to improve risk estimation and to target interventions to this extremely high-risk group.
These findings support the concept that kidney disease should be defined using albuminuria and impaired GFR as separate markers, since they contribute separately to cardiovascular risk. Since these markers are elevated in different segments of the population, screening strategies should be tailored based on age and likelihood of having these abnormalities [13].
Our results support the use of cystatin C as an independent risk marker in older adults. The subgroup in our study with preclinical kidney disease and no MA had a 50% increased risk of all-cause mortality in follow-up. This group had an average MDRD eGFR of 80 ml/min/1.73 m2, a range which has not previously been associated with increased risk in the absence of proteinuria.
As noted, incorporation of cystatin C in addition to microalbuminuria allows the detection of a substantial number of older adults at increased risk of cardiovascular events (69% of individuals had either a cystatin C >1 mg/L, MA or both). We acknowledge that it remains to be determined whether these individuals have kidney disease per se. Ultimately, rather than labelling 69% of older adults with a disease, we should focus our efforts on understanding why the remaining 31% are at comparatively low risk.
The limitations of our study include the presence of only one sample of urine for ACR estimation; this level is known to vary considerably on a day-to-day basis. However, random fluctuations in ACR would have tended to bias our results to the null. Additionally, our estimates of kidney function are limited in that we do not have actual GFR measurements and so cannot comment on discrepancies between cystatin C or creatinine measurements and true GFR. Because urine samples were only collected at the seventh study visit, our study is subject to some degree of survivor bias; however, since the individuals who did not survive to the seventh study visit were sicker and had lower eGFR at baseline, we suspect that survivor bias would attenuate our findings.
In conclusion, we found independent risks of all-cause and cardiovascular mortality as well as incident CHF associated with both albuminuria and impaired kidney function as measured by cystatin C. Added risk was seen in those with abnormal cystatin C levels even in those without albuminuria. Abnormal cystatin C and albuminuria identify different segments of the population, and have useful, independent roles in providing prognostic information about future cardiovascular disease and mortality risk in older adults.
Acknowledgments
The research reported in this article was supported by contract numbers R01-AG-027002, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Conflict of interest statement. None declared.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Sukhija R, Aronow WS, Kakar P, et al. Relation of microalbuminuria and coronary artery disease in patients with and without diabetes mellitus. Am J Cardiol. 2006;98:279–281. doi: 10.1016/j.amjcard.2006.01.098. [DOI] [PubMed] [Google Scholar]
- 3.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 4.Cao JJ, Barzilay JI, Peterson D, et al. The association of microalbuminuria with clinical cardiovascular disease and subclinical atherosclerosis in the elderly: the Cardiovascular Health Study. Atherosclerosis. 2006;187:372–377. doi: 10.1016/j.atherosclerosis.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 6.Astor BC, Hallan SI, Miller ER, 3rd, et al. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- 8.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 9.Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 12.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 13.Garg AX, Kiberd BA, Clark WF, et al. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. 2002;61:2165–2175. doi: 10.1046/j.1523-1755.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 14.Foster MC, Hwang SJ, Larson MG, et al. Cross-classification of microalbuminuria and reduced glomerular filtration rate: associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med. 2007;167:1386–1392. doi: 10.1001/archinte.167.13.1386. [DOI] [PubMed] [Google Scholar]
- 15.Hallan S, Astor B, Romundstad S, et al. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med. 2007;167:2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 16.Cirillo M, Lanti MP, Menotti A, et al. Definition of kidney dysfunction as a cardiovascular risk factor: use of urinary albumin excretion and estimated glomerular filtration rate. Arch Intern Med. 2008;168:617–624. doi: 10.1001/archinte.168.6.617. [DOI] [PubMed] [Google Scholar]
- 17.Tonelli M, Jose P, Curhan G, et al. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. Bmj. 2006;332:1426. doi: 10.1136/bmj.38814.566019.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 19.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 20.Rule AD, Bergstralh EJ, Slezak JM, et al. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69:399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Fried LBN, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 24.Finney H, Newman DJ, Gruber W, et al. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II) Clin Chem. 1997;43:1016–1022. [PubMed] [Google Scholar]
- 25.Shlipak MG, Katz R, Cushman M, et al. Cystatin-C and inflammatory markers in the ambulatory elderly. 2005;1416 doi: 10.1016/j.amjmed.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 26.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 27.K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 28.Dyer AR, Greenland P, Elliott P, et al. Evaluation of measures of urinary albumin excretion in epidemiologic studies. Am J Epidemiol. 2004;160:1122–1131. doi: 10.1093/aje/kwh326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattix HJ, Hsu CY, Shaykevich S, et al. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13:1034–1039. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 30.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 31.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 32.Hallqvist J, Ahlbom A, Diderichsen F, et al. How to evaluate interaction between causes: a review of practices in cardiovascular epidemiology. J Intern Med. 1996;239:377–382. doi: 10.1046/j.1365-2796.1996.431782000.x. [DOI] [PubMed] [Google Scholar]
- 33.Hermans MM, Henry RM, Dekker JM, et al. Albuminuria, but not estimated glomerular filtration rate, is associated with maladaptive arterial remodeling: the Hoorn Study. J Hypertens. 2008;26:791–797. doi: 10.1097/HJH.0b013e3282f50066. [DOI] [PubMed] [Google Scholar]
- 34.Kario K, Matsuo T, Kobayashi H, et al. Activation of tissue factor-induced coagulation and endothelial cell dysfunction in non-insulin-dependent diabetic patients with microalbuminuria. Arterioscler Thromb Vasc Biol. 1995;15:1114–1120. doi: 10.1161/01.atv.15.8.1114. [DOI] [PubMed] [Google Scholar]
- 35.Stehouwer CD, Nauta JJ, Zeldenrust GC, et al. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340:319–323. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- 36.Stuveling EM, Hillege HL, Bakker SJ, et al. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63:654–661. doi: 10.1046/j.1523-1755.2003.00762.x. [DOI] [PubMed] [Google Scholar]


