Background: High amidoxime reductase activity is found in liver and fat tissue, although its composition in cells is unknown.
Results: Amidoxime reductase is up-regulated during adipogenesis and requires MOSC2 and CYB5B.
Conclusion: MOSC2 and CYB5B are essential components of the mitochondrial amidoxime reductase, and MOSC2 is important for lipogenesis.
Significance: We suggest a role of MOSC2 in adipogenesis and fatty acid synthesis and as a potential novel drug target.
Keywords: Adipocyte, Drug Metabolism, Membrane, Enzymes, Molybdenum, Reductase, siRNA, Xenobiotics
Abstract
Reduction of hydroxylamines and amidoximes is important for drug activation and detoxification of aromatic and heterocyclic amines. Such a reductase system was previously found to be of high activity in adipose tissue and liver, and furthermore, in vitro studies using recombinant truncated and purified enzymes suggested the participation of cytochrome b5 reductase (CYB5R), cytochrome b5 (CYB5), and molybdenum cofactor sulfurase C-terminal containing 1 and 2 (MOSC1 and -2). Here, we show that purified rat liver outer mitochondrial membrane contains high amidoxime reductase activity and that MOSC2 is exclusively localized to these membranes. Moreover, using the same membrane fraction, we could show direct binding of a radiolabeled benzamidoxime substrate to MOSC2. Following differentiation of murine 3T3-L1 cells into mature adipocytes, the MOSC2 levels as well as the amidoxime reductase activity were increased, indicating that the enzyme is highly regulated under lipogenic conditions. siRNA-mediated down-regulation of MOSC2 and the mitochondrial form of cytochrome b5 type B (CYB5B) significantly inhibited the reductase activity in the differentiated adipocytes, whereas down-regulation of MOSC1, cytochrome b5 type A (CYB5A), CYB5R1, CYB5R2, or CYB5R3 had no effect. Down-regulation of MOSC2 caused impaired lipid synthesis. These results demonstrate for the first time the direct involvement of MOSC2 and CYB5B in the amidoxime reductase activity in an intact cell system. We postulate the presence of a novel reductive enzyme system of importance for lipid synthesis that is exclusively localized to the outer mitochondrial membrane and is composed of CYB5B, MOSC2, and a third unknown component (a CYB5B reductase).
Introduction
An increasing number of drugs and drug candidates contain basic functional groups such as amidines and guanidines that decrease their oral bioavailability by preventing efficient gastrointestinal adsorption as these groups are protonated under physiological pH. Instead, prodrugs have been developed by N-hydroxylation of these amidine groups, converting them into amidoximes to improve their oral bioavailability as exemplified by the direct thrombin inhibitor ximelagatran (1, 2), its follow-up compound AZD0837 (3), and a platelet inhibitor sibrafidan (4). Once these prodrugs are absorbed into the body, they are reduced back to their bioactive amidines by a reductive enzyme system believed to be composed of cytochrome b5 (CYB5),3 cytochrome b5 reductase (CYB5R), and a third unknown component and is associated with both the microsomal fraction (5) as well as the mitochondrial fraction (6, 7). The reductive metabolism of nitrogen-containing groups is not only of importance for the activation of prodrugs but also for reduction of hydroxylamines and for detoxification of carcinogenic N-hydroxylated aromatic and heterocyclic amines (8, 9). The endogenous role of this activity, however, is still unknown.
In a previous study we found high amidoxime reductase activity preferentially in outer mitochondrial membranes (OMM) associated with adipose tissue, liver, and kidney (6). Furthermore, the reductase activity was dependent on the cofactor NADH and inhibited by potassium cyanide (KCN), whereas typical CYP inhibitors, such as carbon monoxide, were ineffective (6) indicating that the reductase is unlikely to be a cytochrome P450 as was suggested previously (10). Several studies have attempted to identify the third component, and the involvement of stearoyl-CoA desaturase (11) and cytochrome P450 isoenzyme CYP2D (10) in the microsomal fraction as well as a novel molybdenum-containing enzyme in the mitochondrial fraction (12) was suggested. In subsequent studies, two highly homologous molybdenum-containing proteins, namely molybdenum cofactor sulfurase C-terminal containing 1 and 2 (MOSC1 and MOSC2, respectively, referred to as mARC1 and mARC2), were shown to display amidoxime reductase activity in vitro when incubated in a reconstituted system with CYB5 and CYB5R3 (cytochrome b5 reductase 3) (13, 14). In addition, Kurian et al. (15) showed that in a reconstituted system purified soluble CYB5 and purified soluble CYB5R were able to reduce amidoximes to amidines without the involvement of a third component. However, all studies mentioned above were performed using reconstituted systems consisting of purified and soluble truncated components, without showing the direct involvement of these components in more integrated physiological systems.
In this study, we demonstrate using a cell model commonly used to study adipogenesis and siRNA knockdown experiments that MOSC2, but not MOSC1, is critically involved in the amidoxime reductase activity in differentiated adipocytes. In addition, the mitochondrial form of cytochrome b5 type B (CYB5B) is also shown to be essential for the reductase activity in this intact cell system, which appears to be of importance for lipid formation. In contrast, the microsomal form of cytochrome b5 type A (CYB5A) and CYB5R3 as well as its homologs cytochrome b5 reductase 1 and 2 (CYB5R1 and CYB5R2) are not involved in the reductase activity in the adipocyte cell system.
EXPERIMENTAL PROCEDURES
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), sodium pyruvate, penicillin and streptomycin, insulin, and OptiMEM were obtained from Invitrogen. Bovine calf serum was from American Type Culture Collection (LGC Standards AB, Borås, Sweden). Cell culture flasks and plates were from Corning Life Science and Sarstedt (VWR International AB, Stockholm, Sweden). DharmaFECT Duo and ON-TARGETplus SMARTpool siRNA constructs (mouse MOSC1 L-044395-01, mouse MOSC2 L-044395-01, mouse CYB5R3 L-058184-01, mouse CYB5R1 L-046143-01, mouse CYB5R2 L-056941-01, mouse CYB5 L-050436-01 (CYB5A), mouse CYB5B L-059461-01, and Nontargeting Pool D-001810-10) were purchased from Thermo Scientific (VWR International AB). Dexamethasone, 3-isobutylmethylxanthine, benzamidoxime, and 6-propyl-2-thiouracil (PTU) were from Sigma. Complete protease inhibitor mixture was from Roche Applied Science. Rabbit anti-MOSC2 and anti-CYB5B antibodies were from Atlas Antibodies (Stockholm Sweden); mouse anti-mHSP70 and rabbit anti-VDAC were from Affinity Bioreagents (Thermo Scientific); and rabbit anti-CYB5 was from Santa Cruz Biotechnology (AH Diagnostics AB, Skärholmen, Sweden). Rabbit anti-CYB5R3 (16), rabbit anti-ERp27 (17), and rabbit anti-calnexin (18) were previously described. Secondary horseradish peroxidase-coupled anti-rabbit and anti-mouse antibodies were from Dako (Dako Sweden AB, Stockholm, Sweden). AR-H069637, AR-H069927, and AZ13228184-14C were from AstraZeneca R&D Mölndal, Sweden.
Purification of Outer Mitochondrial Membranes from Rat Liver
OMM were purified as described by Hovius et al. (19) and de Kroon et al. (20). Purified OMM vesicles were aliquoted, snap-frozen in liquid nitrogen, and stored at −70 °C. Also collected during fractionation were the homogenate (600 × g supernatant), P10 (10,000 × g pellet, containing mitochondria), the inner mitochondrial membrane (IMM)/mitochondrial matrix fraction, and the microsomes (100,000 × g supernatant).
Protein Concentration Determination
Protein concentrations were determined according to the method of Lowry et al. (21) using bovine serum albumin as standard.
Western Blot Analysis
Subcellular fractions isolated from rat liver and cell lysates from adipocytes and preadipocytes were subjected to Western blot analysis as described previously (22).
Amidoxime Reduction Assay
Amidoxime reductase activity was determined following the benzamidoxime reduction to benzamidine as described previously (6). The amidoxime reductase activity was also monitored by the reduction of the intermediate metabolite of AZD0837, the N-hydroxylated amidine AR-H069927 to the corresponding amidine AR-H067637 (supplemental Fig. S1) (23). Incubations were composed of 0.25 μg of OMM and 20 μm AR-H069927 in 50 mm phosphate buffer, pH 6.3, in a volume of 100 μl. After a 2-min preincubation at 37 °C, the reaction was started by the addition of 250 μm NADH, and samples were incubated for 20 min at 37 °C. Reactions were terminated by the addition of 10 μl of 6 m formic acid, and precipitated proteins were settled by centrifugation 17,000 × g at 4 °C for 15 min. The supernatant was mixed with an equal volume of acetonitrile containing 1% (v/v) acetic acid. The formation of AR-H067637 was monitored by HPLC analysis using a Varian ProStar model 410 autosampler, Varian ProStar 310UV-visible detector, and Varian ProStar model 240 solvent delivery module (Agilent Technologies Sweden AB, Kista, Sweden). Samples were separated on a LiChrospher® 60 RP-select B (5 μm) column (Merck) using an isocratic mobile phase composed of 0.1% (v/v) acetic acid and 3.8 mm ammonium acetate containing 18% acetonitrile. Metabolite and parent compound were detected at 229 nm and quantified using purified standards.
Cross-linking Studies
Purified OMM (0.5 mg) was incubated with a radiolabeled (carbon-14) and cross-linkable (azide) benzamidoxime analog (AZ13228184-14C, supplemental Fig. S2) to identify putative components of the amidoxime reductase complex. OMM were diluted in phosphate-buffered saline (PBS) to a concentration of 1 mg/ml and incubated with 25 μm (1.42 μCi/ml or 52.5 kBq/ml) [14C]benzamidoxime azide in the presence and absence of 250 μm NADH for 2 min at 37 °C in the dark, and samples were cross-linked on ice by exposure to UV light for 2.5 min. The cross-linked samples were subjected to sequential detergent extraction using Triton X-100 followed by Zwittergent 3-14 (Calbiochem and VWR International AB) as described in the supplemental Experimental Procedures and supplemental Fig. S3. The detergent-extracted samples were diluted in Laemmli sample buffer, boiled. and separated by SDS-PAGE using a 10% Tris-Tricine gel. The gel was stained with Coomassie Blue R-250 and dried, and protein-[14C]benzamidoxime complexes were visualized by autoradiography (Fuji-BAS 1800, FujiFilm, Stockholm, Sweden).
Analysis of Proteins by Mass Spectrometry
Coomassie-stained protein bands with an apparent molecular mass of 30–40 kDa, which were identified by cross-linking to the radiolabeled benzamidoxime analog, were excised from the gel and processed for mass spectrometer analysis essentially as described previously (24). In brief, the gel pieces were destained and dried, and trypsin (porcine, modified, sequence grade from Promega Biotech AB, Nacka, Sweden) was allowed to soak into the swelling gel pieces on ice. After overnight incubation at 30 °C and acidification, the proteolytic peptides were subjected to mass analysis by matrix-assisted laser desorption/ionization time of flight mass spectrometry on a Bruker Ultraflex III TOF/TOF instrument from Bruker (Stockholm, Sweden), applying the manufacturer's recommendations. α-Cyano-4-hydroxycinnamic acid was used as matrix, and the spectra were externally calibrated using a 7-peptide mixture. The generated peptide mass lists were used to scan the current NCBInr sequence data base for protein identities, employing the search engine ProFound.
Immunoprecipitation Analysis
[14C]Benzamidoxime azide was incubated and cross-linked to OMM (700 μg) as described, and cross-linked samples were solubilized in 1.2% digitonin (Sigma) on ice for 30 min, conditions known to solubilize the MOSC2 protein from the OMM (data not shown). Nonsolubilized material was settled by centrifugation for 15 min at 17,000 × g, and supernatant was transferred to fresh Eppendorf tubes. The precleared supernatant was subjected to immunoprecipitation using anti-MOSC2 antibodies (0.5 μg) overnight at 4 °C. The next day protein complexes were captured by incubation with 25 μl of protein A/G gel (Thermo Scientific) for 1.5 h at 4 °C and washed two times in PBS containing 1.2% digitonin and two times in PBS. The collected protein complexes were eluted by boiling in Laemmli sample buffer, separated by SDS-PAGE, and analyzed by Western blotting for MOSC2 and subsequent autoradiography of the same nitrocellulose membrane to detect radiolabeled MOSC2.
Culture and Differentiation of 3T3-L1 Cells
Murine 3T3-L1 preadipocytes were obtained from American Type Culture Collection and cultured in DMEM with high glucose containing 10% bovine calf serum, 1% sodium pyruvate, and 100 IU/ml penicillin, and 100 μg/ml streptomycin and subcultured before they reached 80% confluency. For differentiation, preadipocytes were cultured in complete medium supplemented with 10% FCS, and fully differentiated adipocytes were obtained by treating 3-day confluent cells with a standard MDI mixture of 3-isobutylmethylxanthine (0.5 mm), dexamethasone (0.5 μm), and insulin (1.7 μm) for 2 days, followed by treatment with complete medium containing only insulin for another 5–7 days. Medium was changed every 2 days. Cells were cultured and differentiated in 6-well cluster plates or 10-cm dishes for preparation of subcellular fractions.
Subcellular Fractionation of 3T3-L1 Cells
Preadipocytes or differentiated adipocytes were washed twice with PBS, scraped into 1 ml of PBS, and centrifuged for 5 min at 750 × g and 4 °C. The cell pellet was resuspended in buffer A (10 mm Tris-HCl, pH 7.4, containing 20% (w/v) glycerol, 1 mm EDTA, and protease inhibitor mixture) and sonicated on ice with 20 times 1-s bursts. Cell lysates were centrifuged at 800 × g and 4 °C for 10 min, and supernatants were transferred and further centrifuged at 6,500 × g and 4 °C for 15 min to obtain mitochondrial pellets, which were resuspended in mitochondrial buffer (10 mm Tris acetate, pH 7.4, 0.25 m sucrose, 0.5 mm EDTA) and the 6,500 × g supernatant containing the microsomes and cytosol.
siRNA Transfection of 3T3-L1 Adipocytes
Transfection of differentiated 3T3-L1 adipocytes with siRNA was adapted from Kilroy et al. (25). Three-day confluent 3T3-L1 preadipocytes were differentiated using the standard MDI protocol for 5 days, after which they were trypsinized and resuspended in DMEM with high glucose containing 10% FCS and insulin at 6 105 cells/ml. Transfections were performed in 6-well cluster plates, and the transfection mix for one well (9.6 cm2) was composed of 200 pmol of siRNA and 14 μl of DharmaFect Duo diluted in 400 μl of OptiMEM to which 12 105 cells (2 ml) were added. Cells were analyzed for reductase activity and protein expression 5 days post-transfection (10 days post-differentiation).
Real Time PCR
The mRNA expression levels of several genes in siRNA-treated or control adipocytes was determined by ready to use TaqMan gene expression assays according to the manufacturer's instructions (Applied Biosystems, Stockholm, Sweden) using β-actin as a housekeeping gene.
Cytochrome b5 Reductase Assay
The activity of NADH CYB5R was determined by monitoring the reduction of the artificial electron acceptor 2,6-dichlorophenolindophenol at 590 nm. The incubation mixture contained 1 mm NADH, 3 mg/ml 2,6-dichlorophenolindophenol, and 15 μg of rat liver microsomes in a 100 mm phosphate buffer, pH 7.4. The CYB5R activity was determined without and in the presence of the CYB5R inhibitor PTU.
Oil Red O Staining
Intracellular lipids were measured through staining with Oil Red O (Sigma). Cells were fixed with 10% formalin for 10 min and incubated in fresh formalin for at least 1 h. After washing twice with double distilled H2O and 60% isopropyl alcohol, cells were stained for 10 min in freshly diluted Oil Red O solution (5.2 mm). Oil Red O was eluted by adding isopropyl alcohol for 10 min, and the extracted dye was measured at 510 nm on a Varian spectrophotometer (Agilent Technologies).
Fatty Acid Determination
Cell homogenates were prepared from mature adipocytes collected 5 days after transfection with control or MOSC2 siRNA. To homogenates corresponding to 0.36 mg of cell protein, nonadecanoic acid (C19:0, 20 mg) was added as an internal standard. After Folch extraction, the extracts (500 ml) were transmethylated by addition of 0.5 ml of 0.5 m sodium methoxide in methanol. Following incubation at 56 °C for 15 min, 1 ml of 1 m HCl/methanol was added to esterify free fatty acids. After incubation at 56 °C for 15 min, 2 ml of water was added, and the samples were extracted three times with 3 ml of hexane. The samples were dried under a gentle stream of argon and redissolved in 500 ml of hexane. The relative contents of fatty acids were determined on a Hewlett Packard HP 6890 gas chromatograph connected to an HP 5977 mass selective detector. The gas chromatograph was equipped with a HP-5MS column (30 m × 0.25 mm, 0.25-mm film thickness). Ratios of fatty acids (C14:0, C16:0, C16:1, C18:0, and C18:1) to the internal standard (C19:0) were calculated based on peak area. Authentic fatty acids were used to establish retention times, and mass spectra were recorded for the different fatty acids to ensure identity.
RESULTS
Enzyme Kinetics of the Amidoxime Reductase
The enzyme kinetics of the amidoxime reductase enzyme system were characterized using subcellular fractions isolated from rat and human liver in the absence and presence of the inhibitor potassium cyanide (KCN) (6). The reductase activity observed in the presence of KCN is considered to be the nonspecific reductase activity, especially at higher benzamidoxime concentrations. The kinetics of benzamidine formation by rat liver microsomes and mitochondria as well as human liver microsomes displayed Michaelis-Menten kinetics (supplemental Fig. S4), and the apparent Km and Vmax values are listed in Table 1. The KCN-inhibitable Km values for the rat liver microsomes and mitochondria are very similar, 24.4 and 24.6 μm, respectively, although the observed Km value in human microsomes is much higher, namely about 430 μm. The highest Vmax value was detected in rat liver mitochondria 3.2 nmol/min/mg protein, being 2–3-fold higher than those observed in rat liver microsomes and human liver microsomes.
TABLE 1.
Km and Vmax values for the amidoxime reductase activity in microsomes and mitochondria isolated from rat and human liver
Shown are the values in the absence (−KCN) and the presence (+KCN) of potassium cyanide. Values in the absence of KCN are considered nonspecific activity. The specific benzamidoxime activity is represented by the KCN inhibitable values. Further details can be found in supplemental Fig S4.
| Benzamidoxime reductase activity |
|||
|---|---|---|---|
| −KCN | +KCN | KCN inhibitable | |
| Rat liver microsomes | |||
| Vmax | 2.97 | 1.90 | 1.26 nmol/min/mg protein |
| Km | 63.1 | 155 | 24.4 μm |
| Rat liver mitochondria | |||
| Vmax | 5.65 | 2.70 | 3.19 nmol/min/mg protein |
| Km | 44.1 | 115 | 24.6 μm |
| Human liver microsomes | |||
| Vmax | 1.90 | 2.01 | 0.963 nmol/min/mg protein |
| Km | 837 | 4953 | 434 μm |
MOSC2 Is Localized in the Outer Mitochondrial Membrane
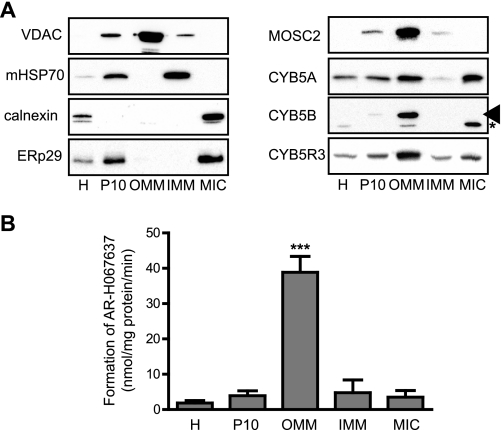
In previous studies, the highest amidoxime reductase activity was found to be associated with fractions enriched with the OMM (6, 12). We determined the amidoxime reductase activity as well as the MOSC2 protein levels in highly purified OMM isolated from female rat liver, because female rat liver displays higher activity levels than males (6). Different subcellular fractions were prepared and characterized by Western blot (Fig. 1A). A very pure OMM fraction, heavily enriched in the OMM marker protein VDAC, was isolated that was devoid of significant IMM/mitochondrial matrix and microsomal contamination (Fig. 1A, left panel). Next the amidoxime reductase activity in the different fractions was determined using the N-hydroxylamine AR-H069927 as a substrate (Fig. 1B). Over 20-fold enrichment in reductase activity was observed in the OMM fraction as compared with the homogenate and the mitochondrial fraction (P10), whereas only low activity was observed in the microsomal fraction. Similar results were obtained using benzamidoxime as a substrate (data not shown). The protein levels for MOSC2, CYB5, and CYB5R3 were determined in these fractions (Fig. 1A, right panel). MOSC2 is exclusively localized to the OMM and completely absent from the IMM and microsomal fractions, much like the OMM marker protein VDAC and correlated well with the amidoxime reductase activity. Because two forms of CYB5, the microsomal form (CYB5A) and the mitochondrial form (CYB5B) exist, the presence of both forms was determined in the different subcellular fractions. CYB5B was found to be present only in the OMM fraction (much like MOSC2), whereas CYB5A as well as CYB5R3 were found both in the microsomes and the OMM. The specificity of the two different CYB5 antibodies was confirmed using cDNA expressed CYB5A and CYB5B (supplemental Fig. S5). The distribution in the different subcellular fractions of both MOSC2 and CYB5B thus correlated well with the amidoxime reductase activity.
FIGURE 1.
Amidoxime reductase activity is enriched in the OMM isolated from female rat liver. A, characterization of the different subcellular fractions that were isolated from female rat liver as described under “Experimental Procedures.” Equal amounts of protein from the different fractions were analyzed by Western blot for the presence of specific marker proteins for the different cellular compartments (left panel). Voltage-dependent anion-selective channel protein (VDAC) was used as a marker for the OMM, mitochondrial heat shock protein 70 (mHSP70) for the mitochondrial matrix/IMM fraction, and calnexin and ERp29 for the microsomal fraction. The MOSC2, CYB5A, CYB5B, and CYB5R3 protein levels in the different subcellular fractions are shown in the right panel. Arrowhead indicates CYB5B, and asterisk indicates nonspecific band. B, amidoxime reductase activity in the different subcellular fractions isolated from female rat liver was determined by monitoring the reduction of the hydroxylamine AR-H069927. The homogenate (H), mitochondrial pellet (P10), OMM, IMM/matrix, and microsomes (MIC) were incubated with the substrate AR-H069927, and formation of the reduced metabolite AR-H067637 was determined by HPLC analysis. Results are presented as mean ± S.D. (n = 3). ***, p < 0.001 compared with the homogenate.
MOSC2 Binds a Benzamidoxime Analog
A proteomic approach using a radiolabeled (carbon-14) and cross-linkable benzamidoxime analog (supplemental Fig S2A) was set up to identify the amidoxime reductase. Incubation of the analog with OMM in the presence of NADH resulted in the appearance of a single metabolite, much like its parent compound benzamidoxime (supplemental Fig S2B) showing that it was a substrate for the enzyme, and, in addition, AR-H069927 was able to inhibit the metabolism of the radiolabeled benzamidoxime analog (data not shown).
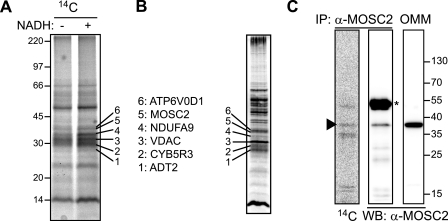
The radiolabeled benzamidoxime was incubated with the OMM in the presence of NADH to enhance binding of the substrate to the putative reductase candidate after which cross-linking was induced by exposure to UV light, as described under “Experimental Procedures.” The cross-linked protein-substrate complexes were extracted by sequential detergent extraction using Triton X-100 followed by Zwittergent 3-14, a procedure that was able to extract and partially preserve the amidoxime reductase activity from the OMM (supplemental Fig. S3). Several proteins were cross-linked in the presence of NADH to the radiolabeled benzamidoxime, in particular in the 30–40-kDa region (Fig. 2A, numbers 1–6). The protein bands corresponding to the radiolabeled bands were excised from the Coomassie-stained part of the gel containing nonlabeled OMM and were identified by mass spectrometromic analysis (Fig. 2B). Among the proteins tentatively identified were MOSC2 and CYB5R3. In addition, when MOSC2 was immunoprecipitated from OMM cross-linked with the radiolabeled benzamidoxime analog, the immunoprecipitated MOSC2 protein band was shown to contain the radiolabeled substrate (Fig. 2C). These results confirm that MOSC2 is able to bind to its substrate benzamidoxime.
FIGURE 2.
MOSC2 binds to its amidoxime substrate. A, rat liver OMM was incubated with carbon-14 radiolabeled benzamidoxime azide (AZ13228184-14C) in the presence (+) or absence (−) of NADH, and the substrate was cross-linked by exposure to UV light. The cross-linked OMM proteins were extracted by sequential detergent extraction, and cross-linked complexes were separated by SDS-PAGE. The gel was dried, and 14C-labeled benzamidoxime cross-linked proteins were visualized by autoradiography. Bands that showed increased labeling in the presence of NADH are numbered 1–6. Molecular weight markers are indicated. B, Coomassie Blue stain of the detergent-extracted OMM fraction where the indicated bands that corresponded to the radiolabeled bands shown under A were excised and analyzed by mass spectrometric analysis. The proteins that were identified are shown to the right. ADT2, ADP/ATP translocase 2; CYB5R3, cytochrome b5 reductase 3; VDAC, voltage-dependent anion-selective channel protein 1; NDUFA9 protein, NADH dehydrogenase (ubiquinone) 1α subcomplex subunit 9; MOSC2, molybdenum cofactor sulfurase C-terminal containing 2; ATP6V0D1, ATPase H+, lysosomal 38 kDa, transporting V0 subunit d1. Further details for the protein identification are shown in supplemental Table S1. C, MOSC2 is cross-linked to the radiolabeled benzamidoxime analog. 14C-Labeled benzamidoxime azide was UV cross-linked to the OMM in the presence of NADH, and proteins were solubilized and immunoprecipitated with a MOSC2-specific antibody. The immunoprecipitate (IP) was analyzed by Western blot (WB) for MOSC2 (middle panel), and the same membrane was also analyzed by autoradiography (left panel). As a control for MOSC2, 5 μg of OMM was included (right panel). Arrowhead indicates MOSC2; asterisk indicates IgG.
Amidoxime Reductase Activity Is Regulated during Adipogenesis
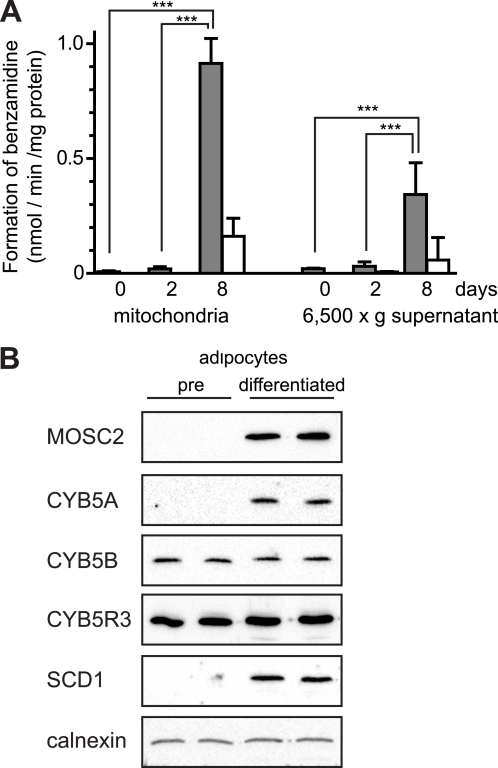
Previously, we observed high amidoxime reductase activity not only in liver and kidney isolated from both male and female rats but the highest specific activity was observed in the adipose tissue from both genders (6). We decided to use the 3T3-L1 cell line, an established cell model to study conversion of preadipocytes into mature adipocytes (26) to monitor the amidoxime reductase activity during this process. The NADH-dependent benzamidoxime reductase activity was analyzed in the mitochondrial fraction, and the 6,500 × g supernatant (containing microsomes and cytosol) was isolated from preadipocytes (day 0) and after 2 and 8 days of differentiation (Fig. 3A). Very low benzamidoxime reductase activity levels were detected in undifferentiated preadipocytes or after 2 days of differentiation. After 8 days of differentiation, however, a dramatic increase in benzamidoxime reductase activity was seen in both the mitochondrial fraction and the 6,500 × g supernatant. Moreover, a 3-fold higher amidoxime reductase activity was associated with the mitochondrial fraction as compared with the 6,500 g supernatant (Fig. 3A) and was effectively inhibited by KCN. In addition, incubations with ximelagatran, a substrate with an amidoxime moiety, in intact differentiated adipocytes revealed a 50-fold increased reduction rate as compared with undifferentiated preadipocytes (supplemental Fig. S6). Together these results suggest that the amidoxime reductase activity is present at high amounts in adipocyte mitochondria and regulated under adipogenic conditions.
FIGURE 3.
Amidoxime reductase activity is regulated during differentiation of preadipocytes into mature adipocytes. A, benzamidoxime reductase activity measured in the mitochondria and the 6,500 × g supernatant containing both the microsomes and cytosol isolated from preadipocytes (0 days) and 2- and 8-day differentiated adipocytes. Incubations were performed with 20 μm benzamidoxime in cells at 0, 2, and 8 days after differentiation start for 30, 20, and 10 min, using 30, 25, and 10 μg of mitochondria or 6,500 × g supernatant proteins, respectively. Incubations were performed in the absence (gray bars) and in the presence (white bars) of KCN. Results are presented as mean ± S.D. (n = 3). ***, p < 0.001. B, protein expression levels of MOSC2, CYB5A, CYB5B, and CYB5R3 in the cell lysates isolated from preadipocytes (pre) (0 days) and 8-day differentiated adipocytes. Also shown are the levels for SCD1 (stearoyl-CoA desaturase 1), a marker for the adipocyte differentiation and calnexin as a loading control.
Expression of MOSC2 and CYB5B in Differentiated Adipocytes
The expression of proteins suggested to be involved in the amidoxime reductase activity was also determined in the differentiated adipocyte cell model. The MOSC2 protein was undetectable in preadipocytes but highly expressed in differentiated mature adipocytes (Fig. 3B), and its presence correlated well with the amidoxime reductase activity. The MOSC1 levels were determined at the mRNA level because antibodies recognizing murine MOSC1 are unavailable. The MOSC1 mRNA level increased 170-fold in differentiated adipocytes compared with preadipocytes (data not shown), demonstrating that MOSC1 is also induced under adipogenic conditions. CYB5A was also strongly induced under adipogenic conditions, much like MOSC2 (Fig. 3B). In contrast, the mitochondrial form of CYB5B as well as CYB5R3 was expressed at similar levels in both preadipocytes and mature adipocytes and appeared not to be regulated during adipogenesis (Fig. 3B). Besides CYB5R3, two homologous cytochrome b5 reductases exist in the mammalian genome, namely CYB5R1 and CYB5R2. Because no commercial antibodies were available that recognize these proteins, their mRNA levels were determined. Both genes were induced in mature adipocytes at the mRNA level 140- and 21-fold for CYB5R1 and CYB5R2, respectively (data not shown). Stearoyl-CoA desaturase (SCD1) was included as a positive control for adipocyte differentiation and, as expected, was only expressed in mature adipocytes (Fig. 3B).
MOSC2 and CYB5B Are Both Required for Amidoxime Reductase Activity
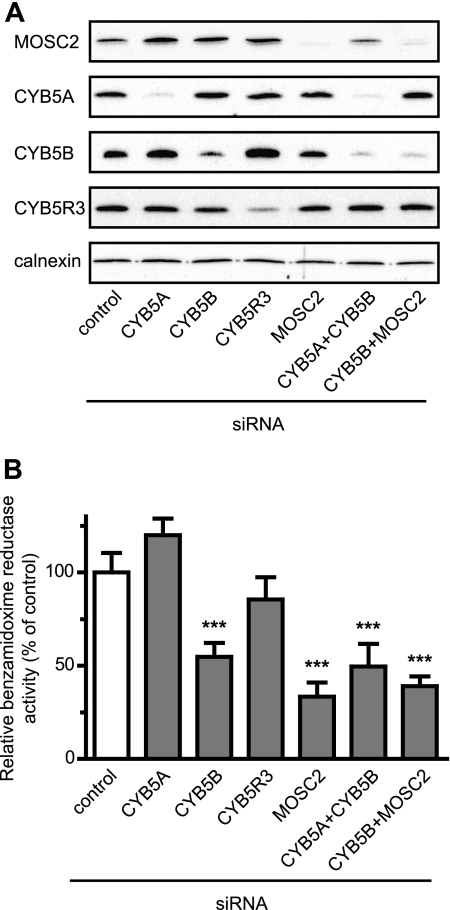
To investigate the involvement of MOSC2, CYB5A, CYB5B, and CYB5R3 in the amidoxime reductase activity further, these proteins were down-regulated by siRNA in differentiated 3T3-L1 adipocytes. In brief, preadipocytes were differentiated for 5 days, after which they were trypsinized and transfected with the different siRNA constructs specifically targeting their respective targets (25). At first, the efficiency of the siRNA-mediated down-regulation was evaluated by Western blot analysis (Fig. 4A). The efficiency of down-regulation was quantified by densitometry and is shown in supplemental Fig. S7. Compared with the cell lysates from control siRNA transfected adipocytes, MOSC2, CYB5A, and CYB5B were effectively down-regulated at the protein level by the siRNA treatment. Down-regulation of CYB5R3 also resulted in significant decrease in both CYB5R3 mRNA and protein levels with ∼21% of the protein remaining after siRNA transfection, and at the mRNA level 25% remained (Figs. 4A and 5A and supplemental Fig. S7). Simultaneous down-regulation of CYB5A together with CYB5B or MOSC2 together with CYB5B also resulted in effective down-regulation of both of the targeted proteins (Fig. 4A and supplemental Fig. S7).
FIGURE 4.
MOSC2 and CYB5B are essential for the amidoxime reductase activity. A, efficiency of siRNA-mediated down-regulation of MOSC2, CYB5A, CYB5B, CYB5R3, CYB5A + CYB5B, and CYB5B + MOSC2 in differentiated 3T3-L1 adipocytes as determined by Western blot analysis. Cell lysates prepared from siRNA-transfected adipocytes were separated by SDS-PAGE and analyzed by Western blot using specific antibodies as shown. Calnexin is included as a loading control. Quantification of the down-regulated proteins is shown in supplemental Fig. S7. B, effect of siRNA-mediated down-regulation of MOSC2, CYB5A, CYB5B, CYB5R3, CYB5A + CYB5B, and CYB5B + MOSC2 in differentiated 3T3-L1 adipocytes on the benzamidoxime reductase activity. The same cell lysates analyzed by Western blot under A were used. Activity data are shown relative to the benzamidoxime reductase activity in control siRNA transfected adipocytes (0.56 nmol of benzamidine formed per min/mg protein). Results are presented as mean ± S.D. (n = 3). ***, p < 0.001.
FIGURE 5.
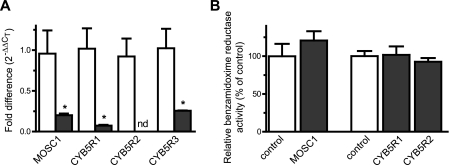
MOSC1 is not involved in the amidoxime reductase activity. A, efficiency of the siRNA-mediated down-regulation of MOSC1, CYB5R1, CYB5R2, and CYB5R3 in differentiated adipocytes as determined by RT-PCR. nd means not detected. B, effect of the siRNA-mediated down-regulation of MOSC1, CYB5R1, and CYB5R2 on the benzamidoxime reductase activity in differentiated adipocytes. Activity data are shown relative to the benzamidoxime reductase activity in control siRNA-transfected adipocytes. Results are presented as mean ± S.D. (n = 3). *, p < 0.05.
Down-regulation of MOSC2 alone resulted in a 65% decrease in the amidoxime reductase activity as compared with control transfected cells (Fig. 4B), although the protein levels were down-regulated to 6% of those present in control transfected cells (Fig. 4A and supplemental Fig. S7). Down-regulation of CYB5A had a slightly simulating effect on the reductase activity, and although reproducible this was not statistically significant (Fig. 4B), although protein levels were down-regulated to 6% of the levels present in control cells (Fig. 4A and supplemental Fig. S7). In contrast, down-regulation CYB5B resulted in a significant decrease in activity of about 50% (Fig. 4B), with 31% of the protein remaining (Fig. 4A and supplemental Fig. S7). Also, when both forms of CYB5 (CYB5A + CYB5B) were simultaneously knocked down, activity was decreased by about 50% (Fig. 4B) with 10% or less of both proteins remaining (Fig. 4A and supplemental Fig. S7). These results clearly indicated that MOSC2 and the mitochondrial form of cytochrome b5 (CYB5B), but not the microsomal CYB5A, are involved in the amidoxime reductase reaction.
Simultaneous down-regulation of MOSC2 and CYB5B did not further decrease the reductase activity as compared with down-regulation of the individual proteins (Fig. 4B). Down-regulation of CYB5R3 did not significantly alter the reductase activity, even though the observed activity was slightly lower than control (Fig. 4B). All activities were efficiently inhibited by KCN indicating that the reductase activity was indeed specific (data not shown). Together, these data clearly demonstrate that MOSC2 as well as CYB5B, but not CYB5A and CYB5R3, are important components of the amidoxime reductase enzyme complex in differentiated adipocytes.
MOSC1, CYB5R1, and CYB5R2 Are Not Involved in the Amidoxime Reductase Activity
MOSC1 has been shown to be able to catalyze the reduction of amidoximes in reconstituted systems containing soluble and truncated forms of MOSC1, CYB5, and CYB5R3 (13, 14). The involvement of MOSC1 in the amidoxime reductase activity in mature adipocytes was studied also by down-regulation of MOSC1 in these cells. As shown in Fig. 5A, MOSC1 siRNA down-regulated MOSC1 mRNA levels to about 20% of the control levels. The amidoxime reductase activity in MOSC1 down-regulated adipocytes was not significantly affected (Fig. 5B).
The involvement of CYB5R1 and CYB3R2 was also examined by siRNA-mediated down-regulation in the mature adipocyte cell model. Both CYB5R1 and CYB5R2 are efficiently down-regulated at the mRNA level with 20% or less of the levels observed in control transfected cells (Fig. 5A). The amidoxime reductase activity in the CYB5R1 and CYB5R2 down-regulated adipocytes was unaffected as compared with control cells (Fig. 5B). In addition, simultaneous down-regulation of CYB5R1, CYB5R2, and CYB5R3 did not inhibit the amidoxime reductase activity either (data not shown).
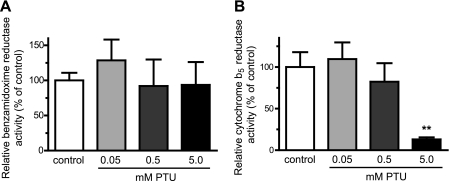
Finally, the involvement of CYB5R in the amidoxime reductase activity was studied using the specific CYB5R inhibitor PTU (27). Although PTU was able to efficiently inhibit the CYB5R activity with almost 90% efficiency (Fig. 6B), it was not able to significantly inhibit the benzamidoxime reductase activity in the OMM fraction (Fig. 6A), again confirming that CYB5R is not an essential component of the amidoxime reductase enzyme system in these cells.
FIGURE 6.
Inhibition of the CYB5R activity by PTU does not affect the amidoxime reductase activity. A, increasing concentrations of PTU do not inhibit the benzamidoxime reductase activity. OMM was incubated with benzamidoxime in the absence (control) and presence of 0.05, 0.5, and 5.0 mm PTU, and the benzamidoxime reductase activity was determined. Activity data are shown relative to the benzamidoxime activity in control OMM. B, efficiency of the CYB5R inhibitor PTU was evaluated in rat liver microsomes by monitoring the reduction of 2,6-dichlorophenolindophenol in the absence (control) and the presence of 0.05, 0.5, and 5.0 mm PTU. Activity data are shown relative to control microsomes. Results are presented as mean ± S.D. (n = 3). **, p < 0.005.
Down-regulation of MOSC2 Affects Intracellular Lipid Levels
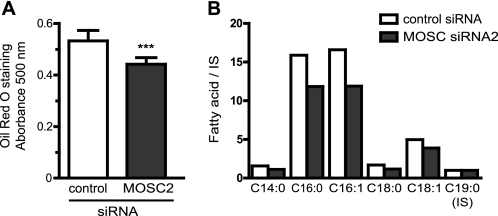
Because MOSC2 is up-regulated during adipogenesis, we investigated the effect of down-regulation of MOSC2 on the intracellular lipid content in mature adipocytes. Adipocytes were transfected with MOSC2 siRNA, and 5 days after transfection cells were stained for intracellular lipids using Oil Red O (Fig. 7A). Down-regulation of MOSC2 resulted in a significant decrease (17%, n = 6; p < 0.001) of the intracellular lipid levels as compared with control transfected cells. The intracellular fatty acids were also analyzed by GC/MS, and the amounts for C14:0, C16:0, C16:1, C18:0, and C18:1 were determined relative to the internal standard (C19:0) (Fig. 7B). Down-regulation of MOSC2 resulted in decreased levels of these fatty acids, the reduction being most pronounced for C16:0 and C16:1 with about a 28% decrease.
FIGURE 7.
MOSC2 levels affect the intracellular lipid content. A, mature adipocytes were transfected with control or MOSC2 siRNA and 5 days post-transfection were stained with Oil Red O. The dye was eluted in isopropyl alcohol and quantified spectrophotometrically at 510 nm. Results are presented as mean ± S.D. (n = 6). ***, p < 0.001. B, relative amounts of fatty acids in control and MOSC2 siRNA-transfected adipocytes. Transfected adipocytes were harvested 5 days post-transfection, and cellular fatty acids were extracted, converted into methyl esters, and analyzed by GC-MS. An internal standard (IS), nonadecanoic acid (C19:0), was added to the cells before extraction as described under “Experimental Procedures.”
DISCUSSION
In this study, we demonstrate for the first time in an intact cell system (mature adipocytes) that the mitochondrial amidoxime activity is dependent on the expression of MOSC2 and CYB5B. CYB5A, the microsomal form of cytochrome b5, although present in the OMM, is not critically involved in the mitochondrial amidoxime reductase activity. Furthermore, MOSC1 and CYB5R1, CYB5R2, or CYB5R3 were found not to be crucial components of the mitochondrial amidoxime reductase enzyme system in mature adipocytes. In addition, our data show that MOSC2 affects the fatty acid levels in adipocytes suggesting a role of MOSC2 in lipogenesis, a finding consistent with its lipogenic regulation in the adipocytes.
Further characterization established that MOSC2 is exclusively localized to the OMM isolated from rat liver, and its expression correlated well with the amidoxime reductase activity. Using a cross-linkable benzamidoxime analog and immunoprecipitation, we could show direct binding of a benzamidoxime substrate to MOSC2. Differentiation of preadipocytes into mature adipocytes resulted in an over 100-fold increase in mitochondrial associated amidoxime reductase activity and was paralleled by an increase in MOSC2 expression. Moreover siRNA-mediated down-regulation of MOSC2 attenuated the amidoxime reductase activity. An interesting observation was that although CYB5A was regulated under adipogenic conditions and CYB5B was not, down-regulation of CYB5B and not CYB5A inhibited the reductase activity, showing the involvement of the mitochondrial form but not the microsomal form of cytochrome b5 in the amidoxime reductase activity. In our adipocyte cell model, we were unable to demonstrate the involvement of MOSC1 in the reductase activity. MOSC1 was like its homolog MOSC2 up-regulated in differentiated adipocytes, but down-regulation of MOSC1 in these cells did not affect the amidoxime reductase activity. MOSC1 has previously been shown to be able to reduce amidoximes to amidines in vitro (13, 14). The reason for this discrepancy is unclear, but in the in vitro system the amidoxime reductase activity was reconstituted with recombinantly expressed truncated and soluble components CYB5B and CYB5R3 and MOSC1 or MOSC2.
Although soluble CYB5R3 can support the amidoxime reductase activity in vitro together with truncated soluble MOSC2 and CYB5B (13, 14), we were unable to confirm its involvement in the adipocyte cell system. This is despite the fact that we identified CYB5R3 as one of the proteins that was cross-linked by the radiolabeled substrate in the OMM and might possibly reflect not direct binding of the substrate to CYB5R3 but background caused by nonspecific cross-linking background. The closely related genes CYB5R1 and CYB5R2 were also found not to be crucially involved in the reductase activity, although they were up-regulated in mature adipocytes. Moreover, the selective CYB5R inhibitor PTU (27) was unable to inhibit the amidoxime reductase activity in the OMM. Together, these data suggest that another presently unknown reductase is involved in the NADH-supported mitochondrial amidoxime reductase activity. Because the other two components MOSC2 and CYB5B are shown to be exclusively localized to the mitochondrial outer membrane, it is suggested that the unknown reductase is also present in this compartment.
CYB5B and CYB5A share considerable homology with each other (28), and although much is known about the function of CYB5A not much is known about the function of CYB5B. CYB5B has been suggested to be involved in the semi-dehydroascorbic acid reductase activity (29) and is thought to function as an activator of androgen synthesis by CYP17A1 (30). More recently, increased CYB5B levels were observed in Hodgkin and aggressive non-Hodgkin lymphomas (31) and luteinizing hormone stimulated prostate cancer cell lines (32), although the exact role of elevated CYB5B levels in these cancers remains unclear. Here, we show that CYB5B is exclusively associated with the OMM, although it has been suggested that CYB5B was present in both mitochondria and microsomes (30). In contrast, CYB5A and CYB5R3 display a less defined localization and are found to be associated with both the microsomal membrane and the OMM, confirming previous observations (16, 33). Based on the findings presented here, we propose a novel function for CYB5B, namely in the reduction of N-hydroxylated amines together with MOSC2.
The amidoxime reductase enzyme kinetic parameters differed considerably between human liver microsomes and rat liver microsomes and mitochondria, with rat liver mitochondria being the most efficient. The amidoxime reductase activity in isolated rat liver OMM (data not shown) and in mitochondria isolated from differentiated adipocytes was effectively inhibited by KCN. Cyanide has been shown to be an inhibitor of the reduced form of molybdenum-containing proteins such as xanthine oxidase, aldehyde oxidase, and sulfite oxidase (34). The inhibitory effect of KCN on the amidoxime reductase activity in our system is in good agreement with these observations. It was recently reported that an in vitro recombinant system using truncated MOSC1 and MOSC2 that cyanide was not able to inhibit the reductase activity, and it was subsequently concluded that MOSC1 and MOSC2 do not contain a terminal sulfide ligand on the molybdenum as is the case in xanthine oxidase, for example (14). One explanation for this inconsistency might be that we included the cyanide directly in the incubation mixture containing OMM or the mitochondrial fraction from adipocytes together with cofactor and substrate under aerobic conditions, although Wahl et al. (14) incubated the recombinant proteins with cyanide under anaerobic conditions prior to the incubation with cofactor and substrate.
Molybdenum is an essential trace element for nearly all organisms, and molybdenum-dependent enzymes, such as sulfite oxidase and xanthine oxidase, are conserved in all three domains of life and usually catalyze redox reactions on carbon, nitrogen, or sulfur centers (35, 36). The MOSC (molybdenum cofactor sulfurase C-terminal) family of proteins is conserved in most eukaryotes and is responsible for the sulfuration of the molybdenum cofactor present in xanthine dehydrogenase and aldehyde oxidase, a modification essential for their catalytic activity (37). In eukaryotes, this modification is catalyzed by molybdenum cofactor sulfurase, a protein consisting of the following two domains: an N-terminal NifS domain, which possesses cysteine desulfurase activity, and a C-terminal MOSC domain responsible for the transfer of the sulfur to the molybdenum cofactor (35, 37). Two other conserved members of the MOSC family only possess the MOSC domain and are called MOSC1 and MOSC2. The physiological role of these two proteins is at present not known, but MOSC2 or CDK7 (candidate diabetes-associated kidney gene 7) was shown to be up-regulated in the kidney in a type II diabetes rat model and by glucose in human kidney cells (38). In Escherichia coli, hypersensitivity toward N-hydroxylated derivatives of purines and pyrimidines was shown to be caused by inactivating mutations of two members of the MOSC superfamily, namely YcbX and YiiM (39). Moreover, it was suggested that both proteins were involved in the detoxification of these N-hydroxylated base analogs by reducing them to their corresponding amines. This finding together with ours presented here suggests that the MOSC domain-containing proteins are capable of catalyzing reduction of N-hydroxylamines and that this activity could be one of the physiological functions of these proteins. In a recent integrated proteomic and transcriptomic high throughput study, MOSC2 mRNA and protein levels were found to be down-regulated in colon cancer, and it was suggested that MOSC2 could be used as a colon tumor biomarker (40).
Down-regulation of MOSC2 in mature adipocytes not only affected the amidoxime activity but also decreased the intracellular lipid levels as shown by Oil Red O staining, and also the fatty acid levels were decreased in these MOSC2 down-regulated adipocytes. Together with the fact that high amidoxime reductase activity was found in the adipose tissue in rats (6), these results imply that this activity is the result of a lipogenic enzyme. In contrast, the amidoxime reductase activity in liver fractions from starvation-treated rats was decreased by 75% as compared with liver fractions from rats that received food ad libitum suggesting that during lipolytic conditions the activity is down-regulated (data not shown). Recently, a genome-wide association study identified a single nucleotide polymorphism linked to the MOSC1 locus that was associated with altered plasma concentrations of total cholesterol and low density lipoprotein cholesterol, two important risk factors for coronary artery disease, suggesting a possible role of MOSC in lipoprotein metabolism (41). It must be noted that in the 3T3-L1 adipocyte cell model, the up-regulation of the reductase activity also coincided with that of the adipocyte differentiation marker SCD1 suggesting its regulation during adipogenesis. The exact role of the outer mitochondrial electron transport chain and MOSC2 in lipid synthesis requires further research.
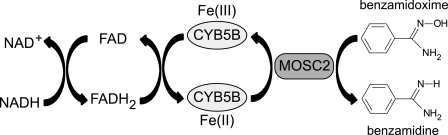
In conclusion, we demonstrate using an intact cell system that MOSC2 and CYB5B are essential components of the mitochondrial amidoxime reductase enzyme system. The identification of MOSC2 in the amidoxime reductase system is in accordance with results obtained with soluble protein components in a reconstituted system (13, 14). Our findings are schematically summarized in Fig. 8. Electrons from NADH are transferred via an as yet unidentified reductase (FAD) to CYB5B, which in its turn will reduce the MOSC2-benzamidoxime complex and produce the reduced product benzamidine. As both MOSC2 and CYB5B are exclusively localized in the OMM, it is hypothesized that all components of the amidoxime reductase enzyme system are embedded in the outer mitochondrial membrane. Moreover, the reductase activity is regulated under adipogenic conditions, and down-regulation of the terminal component MOSC2 resulted in decreased lipid synthesis, suggesting a possible physiological role of this enzyme system and its component MOSC2 in lipogenesis.
FIGURE 8.
Proposed reaction scheme for the mitochondrial amidoxime reductase enzyme system. For more details see under “Discussion.” FAD, flavin adenine dinucleotide (oxidized); FADH2, flavin adenine dinucleotide (reduced).
Supplementary Material
Acknowledgments
We thank Prof. N. Borgese (Milan, Italy) and Dr. S. Mkrtchian (Stockholm, Sweden) for the gift of antibodies. We also thank Malin Lemurell (Lead Generation, AstraZeneca R&D) for the idea to synthesize the radiolabeled azide compound; the isotope group at AstraZeneca R&D, Mölndal, Sweden, for synthesizing the labeled azide used in the study, and Stefan C. Carlsson, AstraZeneca, for initial support.
This work was supported by grants from AstraZeneca and from the Swedish Research Council.

This article contains supplemental “Experimental Procedures,” Figs. S1–S7, and Table S1.
- CYB5
- cytochrome b5
- CYB5A
- cytochrome b5 type A
- CYB5B
- cytochrome b5 type B
- MOSC1
- molybdenum cofactor sulfurase C terminus containing 1
- MOSC2
- molybdenum cofactor sulfurase C terminus containing 2
- CYB5R
- cytochrome b5 reductase
- OMM
- outer mitochondrial membrane
- PTU
- 6-propyl-2-thiouracil
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- IMM
- inner mitochondrial membrane.
REFERENCES
- 1. Eriksson U. G., Bredberg U., Hoffmann K. J., Thuresson A., Gabrielsson M., Ericsson H., Ahnoff M., Gislén K., Fager G., Gustafsson D. (2003) Absorption, distribution, metabolism, and excretion of ximelagatran, an oral direct thrombin inhibitor, in rats, dogs, and humans. Drug Metab. Dispos. 31, 294–305 [DOI] [PubMed] [Google Scholar]
- 2. Gustafsson D., Nyström J., Carlsson S., Bredberg U., Eriksson U., Gyzander E., Elg M., Antonsson T., Hoffmann K., Ungell A., Sörensen H., Någård S., Abrahamsson A., Bylund R. (2001) The direct thrombin inhibitor melagatran and its oral prodrug H 376/95. Intestinal absorption properties, biochemical and pharmacodynamic effects. Thromb. Res. 101, 171–181 [DOI] [PubMed] [Google Scholar]
- 3. Deinum J., Mattsson C., Inghardt T., Elg M. (2009) Biochemical and pharmacological effects of the direct thrombin inhibitor AR-H067637. Thromb. Haemost. 101, 1051–1059 [PubMed] [Google Scholar]
- 4. Timm U., Zumbrunnen R., Erdin R., Singer M., Steiner B. (1997) Oral platelet aggregation inhibitor Ro 48-3657. Determination of the active metabolite and its prodrug in plasma and urine by high performance liquid chromatography using automated column switching. J. Chromatogr. B Biomed. Sci. Appl. 691, 397–407 [DOI] [PubMed] [Google Scholar]
- 5. Kadlubar F. F., Ziegler D. M. (1974) Properties of an NADH-dependent N-hydroxyamine reductase isolated from pig liver microsomes. Arch. Biochem. Biophys. 162, 83–92 [DOI] [PubMed] [Google Scholar]
- 6. Andersson S., Hofmann Y., Nordling A., Li X. Q., Nivelius S., Andersson T. B., Ingelman-Sundberg M., Johansson I. (2005) Characterization and partial purification of the rat and human enzyme systems active in the reduction of N-hydroxymelagatran and benzamidoxime. Drug Metab. Dispos. 33, 570–578 [DOI] [PubMed] [Google Scholar]
- 7. Bernheim M. L., Hochstein P. (1968) Reduction of hydroxylamine by rat liver mitochondria. Arch. Biochem. Biophys. 124, 436–442 [DOI] [PubMed] [Google Scholar]
- 8. King R. S., Teitel C. H., Shaddock J. G., Casciano D. A., Kadlubar F. F. (1999) Detoxification of carcinogenic aromatic and heterocyclic amines by enzymatic reduction of the N-hydroxy derivative. Cancer Lett. 143, 167–171 [DOI] [PubMed] [Google Scholar]
- 9. Kurian J. R., Chin N. A., Longlais B. J., Hayes K. L., Trepanier L. A. (2006) Reductive detoxification of arylhydroxylamine carcinogens by human NADH cytochrome b5 reductase and cytochrome b5. Chem. Res. Toxicol. 19, 1366–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clement B., Lomb R., Möller W. (1997) Isolation and characterization of the protein components of the liver microsomal O2-insensitive NADH-benzamidoxime reductase. J. Biol. Chem. 272, 19615–19620 [DOI] [PubMed] [Google Scholar]
- 11. Reh R., Ozols J., Clement B. (2008) Involvement of stearoyl-CoA desaturase in the reduction of amidoxime prodrugs. Xenobiotica 38, 1177–1190 [DOI] [PubMed] [Google Scholar]
- 12. Havemeyer A., Bittner F., Wollers S., Mendel R., Kunze T., Clement B. (2006) Identification of the missing component in the mitochondrial benzamidoxime prodrug-converting system as a novel molybdenum enzyme. J. Biol. Chem. 281, 34796–34802 [DOI] [PubMed] [Google Scholar]
- 13. Gruenewald S., Wahl B., Bittner F., Hungeling H., Kanzow S., Kotthaus J., Schwering U., Mendel R. R., Clement B. (2008) The fourth molybdenum containing enzyme mARC. Cloning and involvement in the activation of N-hydroxylated prodrugs. J. Med. Chem. 51, 8173–8177 [DOI] [PubMed] [Google Scholar]
- 14. Wahl B., Reichmann D., Niks D., Krompholz N., Havemeyer A., Clement B., Messerschmidt T., Rothkegel M., Biester H., Hille R., Mendel R. R., Bittner F. (2010) Biochemical and spectroscopic characterization of the human mitochondrial amidoxime reducing components hmARC-1 and hmARC-2 suggests the existence of a new molybdenum enzyme family in eukaryotes. J. Biol. Chem. 285, 37847–37859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurian J. R., Bajad S. U., Miller J. L., Chin N. A., Trepanier L. A. (2004) NADH cytochrome b5 reductase and cytochrome b5 catalyze the microsomal reduction of xenobiotic hydroxylamines and amidoximes in humans. J. Pharmacol. Exp. Ther. 311, 1171–1178 [DOI] [PubMed] [Google Scholar]
- 16. Borgese N., Aggujaro D., Carrera P., Pietrini G., Bassetti M. (1996) A role for N-myristoylation in protein targeting. NADH-cytochrome b5 reductase requires myristic acid for association with outer mitochondrial but not ER membranes. J. Cell Biol. 135, 1501–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mkrtchian S., Fang C., Hellman U., Ingelman-Sundberg M. (1998) A stress-inducible rat liver endoplasmic reticulum protein, ERp29. Eur. J. Biochem. 251, 304–313 [DOI] [PubMed] [Google Scholar]
- 18. Andersson A. M., Pettersson R. F. (1998) Targeting of a short peptide derived from the cytoplasmic tail of the G1 membrane glycoprotein of Uukuniemi virus (Bunyaviridae) to the Golgi complex. J. Virol. 72, 9585–9596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hovius R., Lambrechts H., Nicolay K., de Kruijff B. (1990) Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim. Biophys. Acta 1021, 217–226 [DOI] [PubMed] [Google Scholar]
- 20. de Kroon A. I., Dolis D., Mayer A., Lill R., de Kruijff B. (1997) Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim. Biophys. Acta 1325, 108–116 [DOI] [PubMed] [Google Scholar]
- 21. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 22. Sivertsson L., Ek M., Darnell M., Edebert I., Ingelman-Sundberg M., Neve E. P. (2010) CYP3A4 catalytic activity is induced in confluent Huh7 hepatoma cells. Drug Metab. Dispos. 38, 995–1002 [DOI] [PubMed] [Google Scholar]
- 23. Johansson S., Cullberg M., Eriksson U. G., Elg M., Dunér K., Jensen E., Wollbratt M., Wåhlander K. (2011) Single-dose pharmacokinetics, pharmacodynamics, and safety of AZD0837, a novel oral direct thrombin inhibitor, in young healthy male subjects. Int. J. Clin. Pharmacol. Ther. 49, 258–267 [DOI] [PubMed] [Google Scholar]
- 24. Hellman U. (2000) in Proteomics in Functional Genomics. Protein Structure Analysis (Jollès P., Jörnvall H., eds) pp. 43–54, Birkhauser Verlag AG, Basel: [PubMed] [Google Scholar]
- 25. Kilroy G., Burk D. H., Floyd Z. E. (2009) High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS One 4, e6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ntambi J. M., Young-Cheul K. (2000) Adipocyte differentiation and gene expression. J. Nutr. 130, 3122S-3126S [DOI] [PubMed] [Google Scholar]
- 27. Kariya K., Lee E., Yamaoka M., Ishikawa H. (1984) Selective induction of cytochrome b5 and NADH cytochrome b5 reductase by propylthiouracil. Life Sci. 35, 2327–2334 [DOI] [PubMed] [Google Scholar]
- 28. Altuve A., Wang L., Benson D. R., Rivera M. (2004) Mammalian mitochondrial and microsomal cytochromes b5 exhibit divergent structural and biophysical characteristics. Biochem. Biophys. Res. Commun. 314, 602–609 [DOI] [PubMed] [Google Scholar]
- 29. Ito A., Hayashi S., Yoshida T. (1981) Participation of a cytochrome b5-like hemoprotein of outer mitochondrial membrane (OM cytochrome b) in NADH-semidehydroascorbic acid reductase activity of rat liver. Biochem. Biophys. Res. Commun. 101, 591–598 [DOI] [PubMed] [Google Scholar]
- 30. Ogishima T., Kinoshita J. Y., Mitani F., Suematsu M., Ito A. (2003) Identification of outer mitochondrial membrane cytochrome b5 as a modulator for androgen synthesis in Leydig cells. J. Biol. Chem. 278, 21204–21211 [DOI] [PubMed] [Google Scholar]
- 31. Murphy D., Parker J., Zhou M., Fadlelmola F. M., Steidl C., Karsan A., Gascoyne R. D., Chen H., Banerjee D. (2010) Constitutively overexpressed 21-kDa protein in Hodgkin lymphoma and aggressive non-Hodgkin lymphomas identified as cytochrome Bb5 (CYB5B). Mol. Cancer 9, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinski J., Xiong S., Wang Q., Stanczyk F., Hawes D., Liu S. V. (2011) Effect of luteinizing hormone on the steroidogenic pathway in prostate cancer. Prostate 71, 892–898 [DOI] [PubMed] [Google Scholar]
- 33. Carlsen J., Christiansen K. (1995) The subcellular localization of newly synthesized cytochrome b5. Cell Biol. Int. 19, 759–767 [DOI] [PubMed] [Google Scholar]
- 34. Coughlan M. P., Johnson J. L., Rajagopalan K. V. (1980) Mechanisms of inactivation of molybdoenzymes by cyanide. J. Biol. Chem. 255, 2694–2699 [PubMed] [Google Scholar]
- 35. Schwarz G., Mendel R. R., Ribbe M. W. (2009) Molybdenum cofactors, enzymes, and pathways. Nature 460, 839–847 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y., Gladyshev V. N. (2008) Molybdoproteomes and evolution of molybdenum utilization. J. Mol. Biol. 379, 881–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anantharaman V., Aravind L. (2002) MOSC domains. Ancient, predicted sulfur-carrier domains, present in diverse metal-sulfur cluster biosynthesis proteins, including molybdenum cofactor sulfurases. FEMS Microbiol. Lett. 207, 55–61 [DOI] [PubMed] [Google Scholar]
- 38. Malik A. N., Rossios C., Al-Kafaji G., Shah A., Page R. A. (2007) Glucose regulation of CDK7, a putative thiol related gene, in experimental diabetic nephropathy. Biochem. Biophys. Res. Commun. 357, 237–244 [DOI] [PubMed] [Google Scholar]
- 39. Kozmin S. G., Leroy P., Pavlov Y. I., Schaaper R. M. (2008) YcbX and yiiM, two novel determinants for resistance of Escherichia coli to N-hydroxylated base analogs. Mol. Microbiol. 68, 51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mikula M., Rubel T., Karczmarski J., Goryca K., Dadlez M., Ostrowski J. (2010) Integrating proteomic and transcriptomic high throughput surveys for search of new biomarkers of colon tumors. Funct. Integr. Genomics 11, 215–224 [DOI] [PubMed] [Google Scholar]
- 41. Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., Johansen C. T., Fouchier S. W., Isaacs A., Peloso G. M., Barbalic M., Ricketts S. L., Bis J. C., Aulchenko Y. S., Thorleifsson G., Feitosa M. F., Chambers J., Orho-Melander M., Melander O., Johnson T., Li X., Guo X., Li M., Shin Cho Y., Jin Go M., Jin Kim Y., Lee J. Y., Park T., Kim K., Sim X., Twee-Hee Ong R., Croteau-Chonka D. C., Lange L. A., Smith J. D., Song K., Hua Zhao J., Yuan X., Luan J., Lamina C., Ziegler A., Zhang W., Zee R. Y., Wright A. F., Witteman J. C., Wilson J. F., Willemsen G., Wichmann H. E., Whitfield J. B., Waterworth D. M., Wareham N. J., Waeber G., Vollenweider P., Voight B. F., Vitart V., Uitterlinden A. G., Uda M., Tuomilehto J., Thompson J. R., Tanaka T., Surakka I., Stringham H. M., Spector T. D., Soranzo N., Smit J. H., Sinisalo J., Silander K., Sijbrands E. J., Scuteri A., Scott J., Schlessinger D., Sanna S., Salomaa V., Saharinen J., Sabatti C., Ruokonen A., Rudan I., Rose L. M., Roberts R., Rieder M., Psaty B. M., Pramstaller P. P., Pichler I., Perola M., Penninx B. W., Pedersen N. L., Pattaro C., Parker A. N., Pare G., Oostra B. A., O'Donnell C. J., Nieminen M. S., Nickerson D. A., Montgomery G. W., Meitinger T., McPherson R., McCarthy M. I., McArdle W., Masson D., Martin N. G., Marroni F., Mangino M., Magnusson P. K., Lucas G., Luben R., Loos R. J., Lokki M. L., Lettre G., Langenberg C., Launer L. J., Lakatta E. G., Laaksonen R., Kyvik K. O., Kronenberg F., König I. R., Khaw K. T., Kaprio J., Kaplan L. M., Johansson A., Jarvelin M. R., Janssens A. C., Ingelsson E., Igl W., Kees Hovingh G., Hottenga J. J., Hofman A., Hicks A. A., Hengstenberg C., Heid I. M., Hayward C., Havulinna A. S., Hastie N. D., Harris T. B., Haritunians T., Hall A. S., Gyllensten U., Guiducci C., Groop L. C., Gonzalez E., Gieger C., Freimer N. B., Ferrucci L., Erdmann J., Elliott P., Ejebe K. G., Döring A., Dominiczak A. F., Demissie S., Deloukas P., de Geus E. J., de Faire U., Crawford G., Collins F. S., Chen Y. D., Caulfield M. J., Campbell H., Burtt N. P., Bonnycastle L. L., Boomsma D. I., Boekholdt S. M., Bergman R. N., Barroso I., Bandinelli S., Ballantyne C. M., Assimes T. L., Quertermous T., Altshuler D., Seielstad M., Wong T. Y., Tai E. S., Feranil A. B., Kuzawa C. W., Adair L. S., Taylor H. A., Jr., Borecki I. B., Gabriel S. B., Wilson J. G., Holm H., Thorsteinsdottir U., Gudnason V., Krauss R. M., Mohlke K. L., Ordovas J. M., Munroe P. B., Kooner J. S., Tall A. R., Hegele R. A., Kastelein J. J., Schadt E. E., Rotter J. I., Boerwinkle E., Strachan D. P., Mooser V., Stefansson K., Reilly M. P., Samani N. J., Schunkert H., Cupples L. A., Sandhu M. S., Ridker P. M., Rader D. J., van Duijn C. M., Peltonen L., Abecasis G. R., Boehnke M., Kathiresan S. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.