Background: Transforming growth factor (TGF)-β signaling depends on decorin and LRP-1.
Results: Decorin internal regions 5 and 6 interact with LRP-1 to modulate TGF-β mediated signaling.
Conclusion: A specific decorin region regulates TGF-β-dependent signaling.
Significance: Identification of specific sites in decorin that are involved in TGF-β signaling might have potential therapeutic applications for treatment of fibrotic diseases.
Keywords: Cell Signaling, Lipoprotein-like Receptor (LRP), Proteoglycan, Receptor Regulation, Skeletal Muscle, Decorin, Fibrotic Diseases, Transforming Growth Factor Type β
Abstract
Decorin is a small proteoglycan, composed of 12 leucine-rich repeats (LRRs) that modulates the activity of transforming growth factor type β (TGF-β) and other growth factors, and thereby influences proliferation and differentiation in a wide array of physiological and pathological processes, such as fibrosis, in several tissues and organs. Previously we described two novel modulators of the TGF-β-dependent signaling pathway: LDL receptor-related protein (LRP-1) and decorin. Here we have determined the regions in decorin that are responsible for interaction with LRP-1 and are involved in TGF-β-dependent binding and signaling. Specifically, we used decorin deletion mutants, as well as peptides derived from internal LRR regions, to determine the LRRs responsible for these decorin functions. Our results indicate that LRR6 and LRR5 participate in the interaction with LRP-1 and TGF-β as well as in its dependent signaling. Furthermore, the internal region (LRR6i), composed of 11 amino acids, is responsible for decorin binding to LRP-1 and subsequent TGF-β-dependent signaling. Furthermore, using an in vivo approach, we also demonstrate that the LRR6 region of decorin can inhibit TGF-β mediated action in response to skeletal muscle injury.
Introduction
Transforming growth factor-β (TGF-β) is a multifunctional cytokine involved in development, cell proliferation, and extracellular matrix (ECM)2 deposition (1). This growth factor contains nine conserved cysteines and belongs to a superfamily of structurally related proteins, known as the transforming growth factor β superfamily, which includes activins, inhibins, and bone morphogenic proteins (2). TGF-β regulates cellular processes in the nucleus by binding to cell surface transmembrane receptors with serine/threonine kinase activity and cytoplasmic effectors, including Smad proteins and non-Smads proteins (3, 4).
Previously we described two novel modulators of this TGF-β-dependent signaling pathway: LDL receptor-related protein (LRP-1) (5) and decorin (6). LRP-1 is a giant receptor, belonging to the low-density lipoprotein receptor family, which bind and internalize extracellular ligands for degradation (7, 8). We showed that decorin is endocytosed and degraded by myoblasts and that both processes are blocked by siRNA suppression of LRP-1 expression (5). This observation supports the hypothesis that LRP-1 is a receptor for decorin (5, 9). These findings also point to LRP-1-mediated catabolism as a new control pathway for the biological activities of decorin, specifically for its ability to influence ECM signaling.
Following the discovery of LRP-1 as a cell surface receptor for decorin, we attempted to determine its role in decorin TGF-β-dependent signaling. Decorin-deficient myoblasts show that the decreased TGF-β response can be fully restored by decorin re-expression (6, 10). Importantly, this reactivation occurs without changes in the ability of TGF-β to bind its receptors or in intracellular signaling mediators such as Smad protein phosphorylation or Smad-4 nuclear translocation (3, 11). In myoblasts, inhibition of decorin binding to LRP-1, or depletion of LRP-1, decreased TGF-β response to levels observed in decorin-deficient myoblasts. Interestingly, re-expression of decorin in decorin-deficient myoblasts did not restore the TGF-β response when either the Smad pathway or PI3K activity was inhibited, suggesting that this LRP-1-decorin modulatory pathway requires activation of the Smad pathway by TGF-β and involves PI3K activity (6). These results reveal a new regulatory mechanism for TGF-β signaling, involving decorin and LRP-1 at the myoblast surface (5).
Decorin is one of the most well studied members of the family of small leucine-rich proteoglycans (12). Decorin is a proteoglycan formed by a core protein with 12 leucine-rich repeats (LRRs) and a chondroitin/dermatan sulfate glycosaminoglycan chain (12–15). Decorin can be localized in the ECM or at the cell plasma membrane, where it interacts with cell surface receptors. This differential localization can explain the different and sometimes contradictory functions of this proteoglycan (9). Decorin in the ECM is believed to regulate ECM structure (16, 17) and modulate the bioavailability of several growth factors, including TGF-β (18). In addition, decorin has also been reported to be associated with cell surface receptors. Furthermore, decorin has an intrinsic ability to interact with several cytokines (19). It has been shown that decorin binds to insulin growth factor-1 and the insulin growth factor-1 receptor (20), resulting in insulin growth factor-1 receptor phosphorylation and activation, followed by receptor down-regulation (21). Decorin has also been reported to cause rapid phosphorylation of the epidermal growth factor receptor (EGFR) and activation of the mitogen-activated protein kinase signaling pathway (22). In addition, decorin has been reported to modulate two pro-fibrotic molecules, TGF-β (6, 9, 23, 24) and connective tissue growth factor (CTGF) (25). Interestingly, decorin can also bind to the cMet receptor, the receptor for hepatocyte growth factor, inducing transient activation of the receptor and inhibiting cell migration and growth (26). These novel results are in agreement with our previous observations that decorin-deficient myoblasts exhibit exacerbated migration capacity after grafting in vivo (27).
Fibrotic disorders are the end point of many chronic diseases in different tissues such as kidney, skin, and skeletal muscle (28–30). Fibrosis development is caused by the action of growth factors and cytokines, which are overexpressed in fibrotic tissues. For years, TGF-β has been described as the principal inducer of fibrosis in different tissues (31). Duchenne muscular dystrophy is a disease caused by the absence of the protein dystrophin, which produces muscle weakness and leads to cycles of degeneration and regeneration of muscle fibers, resulting in a decrease in muscle mass and an increase in fibrosis, concomitant with augmented TGF-β levels in biopsies of Duchenne muscular dystrophy patients (32–34).
Elucidation of the precise binding sites of modulator molecules such as decorin is an important task with important therapeutic implications. The specific decorin-binding site on LRP-1 has not been determined. In addition, the role of this decorin-binding site on LRP-1 in TGF-β-dependent signaling remains unknown. In this study, we use decorin-deletion mutants, as well as peptides from internal LRR regions, to determine the LRRs responsible for these decorin functions. We found that LRR6 and LRR5 are responsible for LRP-1 binding and subsequent TGF-β-dependent signaling. Also we obtained evidence that the LRR6 internal region (LRR6i) participates in interaction with LRP-1- and TGF-β-dependent signaling. Furthermore, using an in vivo approach, we demonstrate that this LRR6 region can inhibit TGF-β-mediated action in response to skeletal muscle injury.
EXPERIMENTAL PROCEDURES
Animals and Experimental Muscle Injury
12-Week-old C57BL/10 ScSn male mice were used for this study. Animals were kept at room temperature with a 24-h night-day cycle and fed with pellets and water ad libitum. Injury of normal muscle tissue was performed by barium-chloride injection (35, 36) in mice under ketamine/xylazine anesthesia (80/12 mg/kg body weight, intraperitoneally). Briefly, 60 μl of an aqueous 1.2% mass/volume (m/w) BaCl2 solution was injected along the whole length of the left tibialis anterior muscle. Contralateral PBS-injected muscles were used as controls. After 3 days of BaCl2 injections, an intramuscular injection of 20 ng of TGF-β1 in the presence or absence of 10 μg of the LRR6 region was performed. 4 days post-injury, tibialis anteriors were dissected and removed under anesthesia, and animals were sacrificed. Tissues were rapidly frozen and stored at −80 °C until processing. All protocols were conducted in strict accordance and with the formal approval of the Animal Ethics Committee of the P. Universidad Católica de Chile.
Peptides Derived from LRR Regions
The following decorin LRR regions were used: LRR4, KELPEKMPKTLQELRAHENEI; LRR5, TKVRKVTFNGLNQMIVIELGTNPL; LRR6, KSSGIENGAFQGMKKLSYIRIADTNI; LRR6 external (LRR6e), KSSGIENGAFQGMKK; LRR6 internal (LRR6i), LSYIRIADTNI; LRR12, QYWEIQPSTFRCVYVRSAIQLGNYK; and Scramble peptide, SITNLDRYAII. All peptides were obtained from Peptide 2.0 Inc.
Cell Culture
The mouse skeletal muscle cell line, C2C12 (ATCC) (18), was grown and induced to differentiate as described (38). Decorin-deficient myoblasts have been previously described and characterized (6, 10).
Mutant Decorin Generation
All decorins generated have an HA epitope in their C termini. Wild type decorin and decorin mutants that lack different LRR were generated by overlap PCR starting from human decorin (NM_133593) cloned in pcDNA 3.1 (kindly donated by Elke Schönherr, Institute of Physiological Chemistry and Pathobiochemistry, Münster, Germany). Generated constructs were sequenced and then stably transfected into CHO-K1 cells using medium supplemented with G418 (400 μg/ml). After 4 weeks, different clones were isolated (25). Decorin purification was conducted using conditioned medium from the CHO-K1 clones, and a DEAE-Sephacel column (Bio-Rad) as previously described (39).
Labeling of Cultures
CHO-K1 cells, stably transfected with different deletion mutant constructs of decorin, were labeled for 18 h in sulfate and serum-free DMEM/F-12 with 100 μCi/ml of H2[35S]SO4 (PerkinElmer Life Sciences, 25 mCi/ml) (39). This conditioned medium was concentrated using a DEAE-Sephacel column pre-equilibrated in 10 mm Tris-HCl, pH 7.4, 0.2 m NaCl, and 0.1% Triton X-100. The sample bound to the column was incubated with heparitinase in an appropriate buffer for 4 h at 37 °C. Samples were then eluted with 1 m NaCl and dialyzed against PBS (39).
Decorin Endocytosis Assays
Endocytosis of 35S-decorin by myoblasts in culture was determined as previously described (5).
Transient Plasmid Transfection
Cells were plated in 24-well plates until they reached 60% confluence. Cells were then incubated in Opti-MEM I containing 1 μg of TGF-β responsive plasmid p3TP-Lux (6), 0.02 μg of pRL-SV40, 2 μl of PLUS reagent, and 1 μl of Lipofectamine. After 6 h, FBS was added to the medium, and cells were cultured for a further 12 h. Medium was changed to fresh growth medium and the following reagents were added: TGF-β1 dissolved in 0.5% FBS; and different decorin mutants or decorin LRR regions in PBS. Dual luciferase activity assays (Promega) were performed after 6 h, depending on the experiment.
Short Interfering RNA (siRNA) Transfection
Annealed siRNA specific for LRP-1 and control siRNA have been previously described (5, 6). Briefly, for experiments with p3TP-Lux plasmid reporters, cells were co-transfected with siRNA. Following transfection, FBS was added to the medium, and cells were cultured for a further 24 h. Dual luciferase activity assays (Promega) were performed after 6 h depending on the experiment.
Immunoprecipitation and Immunoblot Analysis
For immunoprecipitation assays, myoblasts were lysed in 50 mm Tris-HCl, pH 7.4, 0.1 m NaCl, 0.5% Triton X-100 buffer, containing a mixture of protease inhibitors and 1 mm phenylmethylsulfonyl fluoride. Equal amounts of protein (150 μg) from pre-cleared extracts were immunoprecipitated overnight at 4 °C with 5 μg of rabbit anti-LRP-1, as previously described (5), followed by incubation for 2 h at 4 °C with 20 μl of protein A-agarose beads (Pierce). Equal volumes of immunoprecipitated protein were subjected to SDS-PAGE. For immunoblot analyses, cell extracts obtained from myoblasts were prepared in 50 mm Tris-HCl, pH 7.4, 0.1 m NaCl, 0.5% Triton X-100 with a mixture of protease inhibitors and 1 mm PMSF. Aliquots were subjected to SDS-gel electrophoresis on 5 or 10% polyacrylamide gels, electrophoretically transferred onto nitrocellulose membranes (Schleicher and Schuell), and probed with each of the following antibodies: rabbit anti-LRP-1 extracellular domain (1:1000) (Calbiochem) as previously described (5); rabbit anti-fibronectin (1:5000) and mouse anti-α-tubulin (1:5000) (Sigma); goat anti-CTGF (1:500) and rabbit anti-HA (1/2000) (Santa Cruz Biotechnology); and mouse anti-GAPDH (1:2000) (Chemicon). All immunoblots were visualized by enhanced chemiluminescence (Pierce).
RNA Isolation, Reverse Transcription and Quantitative Real Time PCR
Myoblasts were serum-starved for 18 h and then incubated for several times with the indicated decorin deletion mutants. Total RNA was isolated from cell cultures at the indicated times in the figures using TRIzol (Invitrogen) according to the manufacturer's instructions. Total RNA (1 μg) was reverse-transcribed to cDNA using random hexamers and SuperScript reverse transcriptase (Invitrogen). TaqMan quantitative real-time PCR were performed in duplicate on a Stratagene MX 3005 Termocyler (Agilent Technology), using predesigned primer sets for mouse CTGF and the housekeeping gene GAPDH (TaqMan Assays-on-Demand, Applied Biosystems). mRNA expression was quantified using the comparative ΔCt method (2−ΔΔCt), using GAPDH as the reference gene. The mRNA levels are expressed relative to the mean expression in the control group. Values correspond to the mean of the ΔCt value ± S.D. of three independent experiments.
Protein Determination
Protein levels were determined from cell extract aliquots using a bicinchoninic acid protein assay kit (Pierce) with BSA as a protein standard.
Statistics
The statistical significance of the differences between the means of the experimental groups was evaluated using one-way analysis of variance with a post hoc Bonferroni multiple comparison test (Sigma Stat 3.5 Software). A difference was considered statistically significant at a p value < 0.05.
RESULTS
The LRR4–6 Region of Decorin Is Necessary for Binding to LRP-1 and Subsequent Endocytosis
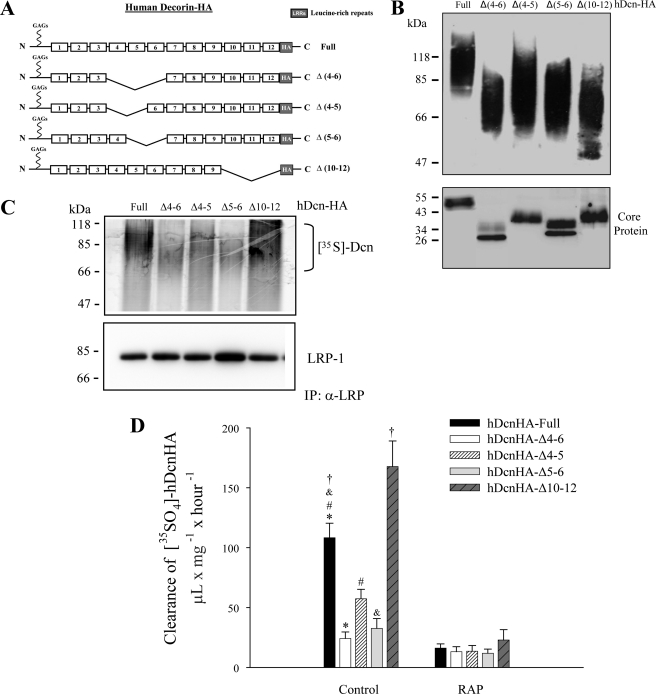
We have previously proposed a new regulatory mechanism for TGF-β signaling mediated by decorin and LRP-1 (6). One of the key features of this mechanism is decorin binding to LRP-1, followed by decorin internalization by endocytosis (5). The core protein of decorin contains 12 LRRs that interact with various growth factors, and regulate their action (9, 20, 41, 42). To analyze which decorin LRR region is important for interaction with LRP-1, we generated four decorin deletion mutants that lack different LRR regions (Fig. 1A). These mutant decorins were expressed in CHO cells and purified by DEAE. Fig. 1B shows a Western blot against the HA epitope, with or without chondroitinase ABC treatment, which degrades chondroitin/dermatan sulfate glycosaminoglycans. All of the glycanated forms, as well as their corresponding cores protein (which migrated at the expected molecular weights), are indicated. In some core protein deletion mutants, a doublet is observed that likely corresponds to the presence or absence of N-linked carbohydrates (43). To evaluate which regions of the decorin core protein are important for interaction with LRP-1, C2C12 myoblasts were co-incubated with different 35S-decorin constructs at 4 °C, to avoid endocytosis, and cells were lysed and immunoprecipitated with antibodies against the extracellular domain of LRP-1. After separation by SDS-PAGE, the immunoblot revealed that decorin co-immunoprecipitates with the LRP-1 antibody complex (Fig. 1C). In particular, the figure shows that both full-length decorin and decorin lacking LRR10–12 co-immunoprecipitates with the LRP-1 antibody complex. In contrast, less co-immunoprecipitation was observed for decorin lacking LRR4–6, LRR4–5, or LRR5–6. The characteristic decorin smear is observed after autoradiography.
FIGURE 1.
The LRR4–6 decorin region is necessary for LRP-1 binding and LRP-1-mediated decorin endocytosis. A, schematic showing decorin deletion mutants. B, deletion mutants generated in CHO-K1, which lack LRR4 and 5 (Δ4–5); 4, 5, and 6 (Δ4–6); 5 and 6 (Δ5–6); or 10, 11, and 12 (Δ10–12); were purified by DEAE-Sephacel and separated in a 4–12% SDS-PAGE gradient for detection of glycanated forms (upper) or protein-core after treatment with chondroitinase ABC (lower). Decorins were visualized by Western blot using anti-HA antibodies. C, C2C12 cells were incubated with 35S-labeled decorin mutants at 4 °C for 3 h. Extracts were immunoprecipitated with anti-LRP-1 antibodies and the presence of decorin deletion mutants in the immunoprecipitate (IP) was evaluated by autoradiography (18). Protein levels were detected in the immunoprecipitate with an anti-LRP-1 antibody by Western blot. D, C2C12 myoblasts were incubated with 35S-labeled decorin mutants at 37 °C for 3 h in the absence (control) or presence of 1 μm receptor-associated protein (RAP), then cells were analyzed to determine the level of endocytosis as described before (5). Values correspond to the mean ± S.D. from three independent experiments (*, #, and &, p < 0.001; †, p < 0.05).
We have shown previously that decorin is endocytosed upon binding to LRP-1 (5). Therefore, in this study, we determined the extent of endocytosis of different decorin deletion mutants. Fig. 1D shows that, among the different decorin mutants tested, decorins lacking LRR4–6, LRR4–5, and LRR5–6 displayed a diminished rate of clearance from the incubation medium compared with full-length decorin. Interestingly, in contrast, decorin lacking LRR10–12 showed an enhanced rate of endocytosis. Importantly, the clearance rate of all decorin forms was directly mediated by LRP-1, because the presence of RAP, an inhibitor of both decorin binding and endocytosis of decorin by LRP-1, completely prevented decorin clearance from the incubation medium (Fig. 1D) (6). Altogether these results suggest that the region of decorin containing LRR4–6 is important for both binding of decorin to LRP-1 and subsequent endocytosis.
The LRR4–6 Region of Decorin Is Required for Its Effect on TGF-β Signaling Mediated by LRP-1
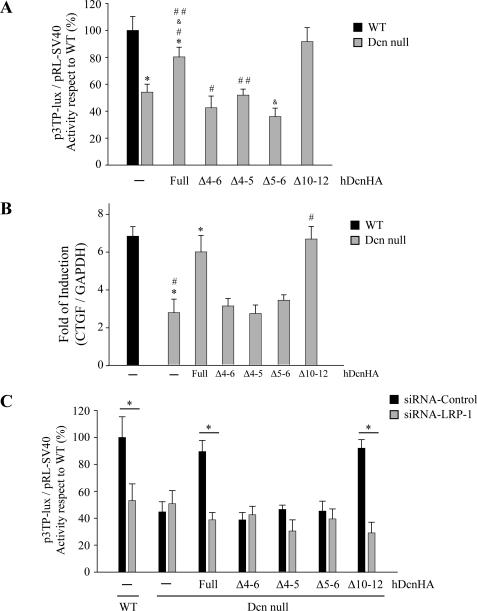
We have previously shown that decorin-deficient myoblasts display a decreased response to TGF-β1 compared with wild type myoblasts, and that this effect can be reverted upon exogenous addition of decorin core protein, a process that requires the presence of LRP-1 (6). Therefore, in this study, we evaluated which region of decorin is responsible for rescuing TGF-β-dependent activity by measuring p3TP-Lux reporter activity in response to TGF-β. Fig. 2A shows that decorin mutants lacking LRR4–6, LRR4–5, or LRR5–6 were unable to rescue p3TP-Lux reporter activity. In contrast, both full-length decorin and decorin mutant LRR10–12 were able to rescue TGF-β-dependent signaling. The inability of decorin mutants lacking LRR4–6, LRR4–5, or LRR5–6 to restore TGF-β-mediated activity was confirmed by measuring induction of CTGF mRNA in response to TGF-β. Fig. 2B shows that CTGF mRNA levels determined by quantitative RT-PCR in decorin-deficient myoblasts increase when full-length decorin, or decorin mutant lacking LRR10–12, were added to the myoblast incubation medium. In contrast, decorin mutants lacking LRR4–6, LRR4–5, or LRR5–6 did not rescue TGF-β-mediated activity as determined by CTGF expression (Fig. 2B). To confirm that this differential ability of decorin mutants to rescue TGF-β activity was dependent on LRP-1, C2C12 myoblasts were transfected with a specific siRNA for LRP-1, as we previously described (6), and TGF-β activity was determined by measuring p3TP-lux reporter activity. Under these experimental conditions, recovery of TGF-β activity was dependent on expression of LRP-1. Fig. 2D shows that the recovery of TGF-β activity mediated by full-length decorin or decorin lacking LRR10–12 is completely lost in the absence of LRP-1. Altogether, these results strongly suggest that LRR4–6, LRR4–5, and LRR5–6 are required for the decorin-mediated TGF-β response, and that this response is dependent on LRP-1.
FIGURE 2.
Decorin-mediated LRP-1-dependent TGF-β signaling requires the LRR4–6 region of decorin (Dcn). A, wild type and decorin-deficient myoblasts were transiently transfected with plasmids containing p3TP-lux and pRL-SV40 sequences and incubated with 75 nm or without complete (Full) human decorin (hDcnHA) or deletion mutants lacking LRR (Δ4–5), (Δ4–6), (Δ5–6), or (Δ10–12) as described in the legend to Fig. 1. After 6 h of TGF-β1 (1.0 ng/ml) treatment, cells were lysed and reporter activities were determined. Values correspond to the mean ± S.D. from three independent experiments (*, #, &, and ##, p < 0.05). B, decorin-deficient myoblasts were incubated with or without TGF-β1 (5.0 ng/ml) in the absence or presence of 75 nm hDcnHA or deletion mutants as described for A. Total RNA was isolated and CTGF and GAPDH expression was determined by qRT-PCR according to “Experimental Procedures.” Values correspond to the mean of dCT value ± S.D. of three independent experiments (* and #, p < 0.05). C, wild type and decorin-deficient myoblasts were transfected with control siRNA or LRP-1 siRNA, together with the TGF-β responding p3TP-lux reporter, and pRL-SV40 as a control for transfection efficiency (6). Cells were incubated with TGF-β1 in the absence or presence of 75 nm hDcnHA or deletion mutants as described in A. After 6 h, luciferase activity was determined. Values for wild type cells treated with Lipofectamine (control) and TGF-β1 (1.0 ng/ml) correspond to 100%. Values correspond to the mean ± S.D. from three independent experiments (*, p < 0.001).
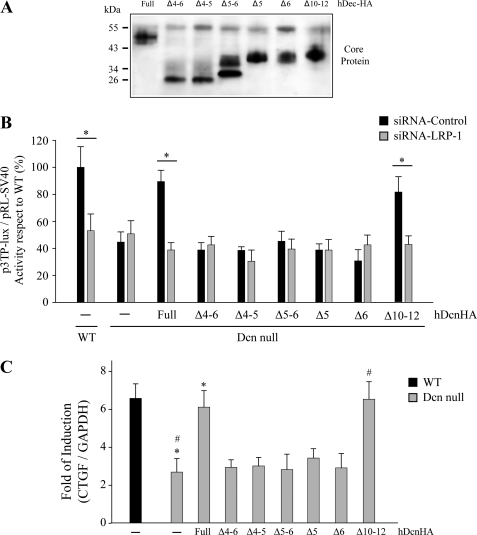
Because the results from the interaction of decorin mutants with LRP-1 as well as TGF-β activity signaling experiments suggest that LRR4–6 is required, we generated decorin deletion mutants that lack LRR5 and LRR6 regions. These mutant decorins were expressed in CHO cells and purified by DEAE as described in the legend Fig. 1A. Fig. 3A shows a Western blot against the HA epitope, with chondroitinase ABC treatment for mutants lacking LRR5 and LRR6. As shown in Fig. 3B, decorin lacking LRR5 or LRR6 was unable to recover TGF-β-dependent activity. The inability of decorin mutants lacking LRR4–6, LRR4–5, LRR5, or LRR6 to restore TGF-β-mediated activity was confirmed by measuring induction of CTGF mRNA in response to TGF-β (Fig. 3C).
FIGURE 3.
Two regions contained in decorin LRR4–6 are directly involved in decorin (Dcn) endocytosis and TGF-β-mediated signaling mediated by LRP-1. A, protein cores after treatment with chondroitinase ABC, corresponding to hDcnHA or deletion mutants generated in CHO-K1, which lack LRR4 and -5 (Δ4–5), LRR4, -5, and 6 (Δ4–6), LRR5 and -6 (Δ5–6), LRR5 (Δ5), LRR6 (Δ6) or LRR10, -11, and -12 (Δ10–12). Decorins were visualized by Western blot using anti-HA antibodies. B, wild type and decorin-deficient myoblasts were transfected with control siRNA or LRP-1 siRNA, together with the TGF-β responding p3TP-lux reporter, and pRL-SV40 as a control for transfection efficiency (6). Cells were incubated with TGF-β1 in the absence or presence of decorins or deletion mutants as described in the legend of Fig. 2A. After 6 h, luciferase activity was determined. Values for wild type cells treated with Lipofectamine (control) and TGF-β1 (1.0 ng/ml) correspond to 100%. Values correspond to the mean ± S.D. from three independent experiments (*, p < 0.001). C, decorin-deficient myoblasts were incubated with or without TGF-β1 (5.0 ng/ml) in the absence or presence of 75 nm hDcnHA or deletion mutants as described for B. CTGF and GAPDH expression was determined by qRT-PCR as described in the legend to “Experimental Procedures.” Values correspond to the mean of dCt value ± S.D. of three independent experiments. (* and #, p < 0.05).
Two Peptides Derived from Decorin LRR4–6 Are Directly Involved in Decorin Endocytosis and TGF-β Signaling Mediated by LRP-1
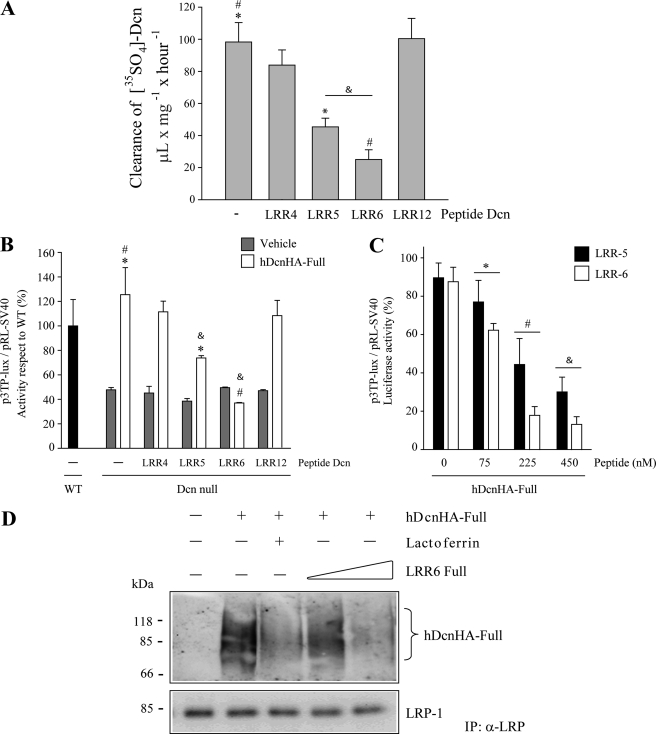
Next, we determined which LRR peptide(s) within LRR4–6 is able to compete with decorin endocytosis mediated by LRP-1. Myoblasts were incubated with 35S-decorin in the presence of LRR4, LRR5, or LRR6 peptides (or the LRR12 control peptide), and decorin clearance was determined. Fig. 4A shows that LRR6 and LRR5 peptides inhibited around 80 and 60% of decorin clearance, respectively, whereas, in contrast, LRR4 and LRR12 peptides did not display any significant effect. These results suggest that the LRR5 and LRR6 of decorin are directly involved in binding to LRP-1.
FIGURE 4.
Two synthetic peptides derived from the LRR4–6 region of decorin (Dcn) decreases LRP-1-mediated decorin endocytosis and TGF-β-mediated signaling. A, C2C12 myoblasts were incubated with 35S-decorin mutants at 37 °C for 3 h in the absence or presence of 225 nm synthetic peptides derived from the LRR4, LRR5, LRR6, or LRR12 regions of decorin. The cells were then analyzed to determine levels of decorin endocytosis as described in the legend to Fig. 1D. Values correspond to the mean ± S.D. from three independent experiments (* and &, p < 0.05; #, p < 0.001). B, wild type and decorin-deficient myoblasts were transiently transfected with plasmids containing p3TP-lux and pRL-SV40 sequences and incubated with 75 nm hDcnHA in the absence or presence of 225 nm synthetic peptides derived from LRR4, LRR5, LRR6, or LRR12 regions of decorin. After 6 h of TGF-β1 (1.0 ng/ml) treatment, cells were lysed and reporter activities were determined. Values correspond to the mean ± S.D. from three independent experiments (* and &, p < 0.05; #, p < 0.001). C, wild type and decorin-deficient myoblasts were transiently transfected with p3TP-lux and pRL-SV40, and incubated with hDcnHA in the absence or presence of the indicated amounts of synthetic LRR5 and LRR6 peptides. After 6 h of TGF-β1 (1.0 ng/ml) treatment, cells were lysed and reporter activities were determined. 100% correspond to p3TP-lux induction in WT cells. Values correspond to the mean ± S.D. from three independent experiments (*, #, and &, p < 0.05). D, C2C12 myoblasts were incubated with 75 nm hDcnHA at 4 °C for 3 h in the absence or presence of the 75 or 225 nm LRR6 peptides. Lactoferrin was used as a protein that displaces the binding of ligands to LRP-1. Cell extracts were obtained and the immunoprecipitated anti-LRP-1 antibody was evaluated by Western blot using an anti-HA and anti-LRP-1 antibodies, respectively.
Because decorin binding to LRP-1 is required for its effect on TGF-β activity, we wanted to determine which of the above tested decorin peptides affect the ability of full-length decorin to rescue TGF-β activity. Decorin-deficient myoblasts were incubated with different decorin LRR peptides, and luciferase activity, associated with a p3TP-Lux reporter, was measured. Fig. 4B shows that the LRR6 peptide, and LRR5 to a lesser extent, totally inhibits full-length decorin-mediated rescue of TGF-β activity, whereas LRR4 and LRR12 peptides did not have any effect. Fig. 4C shows a titration curve demonstrating dose-dependent displacement of the decorin-mediated rescue of TGF-β activity by LRR5 or LRR6. An IC50 of ∼90 nm for this LRR6 peptide was determined. Finally, Fig. 4D shows that increasing concentrations of LRR6 peptide competed with decorin for interaction with LRP-1, and that lactoferrin, another LRP-1 ligand, also competes with decorin binding to this endocytocic receptor. In total, these results suggest that LRR6 and LRR5 are involved in both decorin binding to LRP-1 and decorin-mediated TGF-β-dependent signaling.
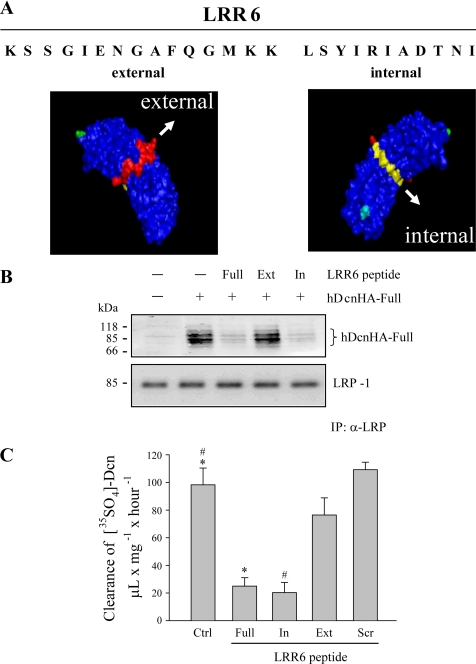
The Internal Region of LRR6 Decorin Is Required for Interaction with LRP-1 and TGF-β-dependent Signaling
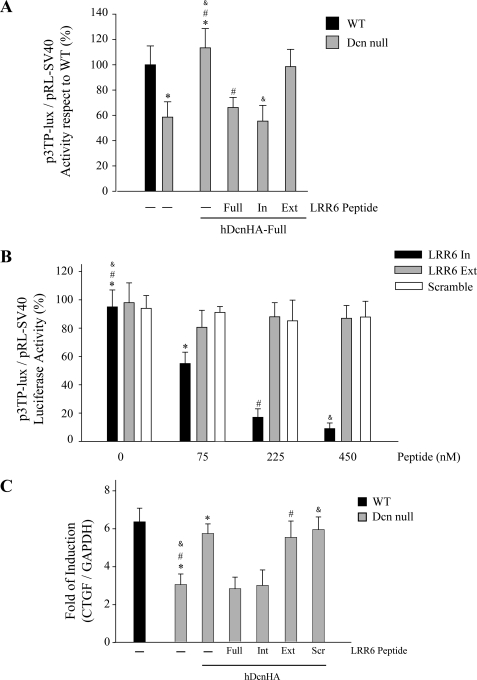
LRRs in decorin contain internal and external regions (44). The internal region is located in the concave face, and the exterior region faces the exterior of decorin. Therefore we decided to evaluate which region in LRR6 is responsible for interaction with LRP-1- and TGF-β-dependent signaling. Fig. 5A shows a sequence of peptides from the LRR6 region of decorin, LRR6 (Full), LRR6i (In), and LRR6e (Ext), and their potential locations in the decorin molecule are shown based on the structural model described by Scott et al. (44). Fig. 5B shows that a short 11-amino acid peptide from the internal region of LRR6 (LRR6i peptide) is able to compete with full-length decorin for interaction with LRP-1. The amount of LRP-1 co-immunoprecipitated is shown in the same figure. As a positive control, immunoprecipitation of decorin in the absence of any peptide is shown. Next, we evaluated if this LRR6i peptide affects clearance of 35S-decorin from myoblast incubation medium. Fig. 5C shows that the LRR6i peptide inhibits ∼80% of the clearance of decorin from the medium when compared with myoblasts incubated in the absence of or with a scramble peptide, similar to the effect observed from the whole LRR6 peptide. These results suggest that the internal region of LRR6 (LRR6i) is sufficient for binding to LRP-1 and decorin clearance. Next, we evaluated if LRR6i is able to compete with decorin for TGF-β-dependent signaling. Fig. 6A shows that the LRR6i peptide was as efficient as the full LRR6 peptide at totally inhibiting the full-length decorin-mediated rescue of TGF-β signaling. The same figure shows that a peptide corresponding to the external region of LRR6 (LRR6e) showed very little inhibitory activity. Next, we determined the IC50 for the LRR6i peptide as above. Fig. 6B shows an IC50 of around 100 nm for LRR6i, whereas the LRR6e peptide did not show any inhibitory effect nor the scramble peptide at the concentrations tested. Fig. 6C shows that the LRR6i peptide was as efficient as the full LRR6 peptide inhibiting TGF-β-mediated CTGF mRNA expression determined by quantitative RT-PCR. These results suggest that an 11-amino acid segment present in the internal region of LRR6 of decorin is involved in both decorin binding to LRP-1 and in decorin-mediated LRP-1-dependent TGF-β signaling.
FIGURE 5.
A peptide containing the internal region of LRR6 decorin competes with full-length decorin for binding to LRP-1 and reduces LRP-1-mediated decorin endocytosis. A, sequence of peptides from the LRR6 region of decorin, LRR6 (Full), LRR6i (In), and LRR6e (Ext), are depicted and their potential locations in the three-dimensional decorin molecule are shown based on the structural model described by Scott et al. (44). B, C2C12 myoblasts were incubated with 75 nm hDcnHA at 4 °C for 3 h in the absence or presence of 225 nm of the synthetic peptides derived from LRR6 as described in A, cell extracts were immunoprecipitated with anti-LRP-1 antibody and the presence of hDcnHA and LRP-1 in the immunoprecipitate were evaluated by Western blot using anti-HA and anti-LRP-1 antibodies, respectively. C, C2C12 myoblasts were incubated with 35S-decorin mutants at 37 °C for 3 h in the absence or presence of synthetic peptides from the LRR6 region of decorin as described in A and B, or scramble peptide. The cells were then analyzed to determinate decorin endocytosis levels as described in the legend to Fig. 1C. Values correspond to the mean ± S.D. from three independent experiments (* and #, p < 0.001).
FIGURE 6.
TGF-β activity is decreased by a synthetic peptide derived from the LRR6 internal region of decorin (Dcn). A, wild type and decorin-deficient myoblasts were transiently transfected with plasmids containing p3TP-lux and pRL-SV40 sequences and incubated with 75 nm hDcnHA in the absence or presence of 225 nm synthetic peptides from the LRR6 region of decorin, LRR6 (Full), LRR6i (In), or LRR6e (Ext). After 6 h of TGF-β1 (1.0 ng/ml) treatment, cells were lysed and reporter activities were determined. Values correspond to the mean ± S.D. from three independent experiments (*, #, and &, p < 0.05). B, wild type and decorin-deficient myoblasts were transiently transfected with p3TP-lux and pRL-SV40 plasmids and incubated with 75 nm hDcnHA-Full in the absence or presence of different amounts of LRR6 (Full, In, or Ext) or scramble peptide (Scramble). Treatment with TGF-β1 (1.0 ng/ml) and reporter activities were determined as in A. Values correspond to the mean ± S.D. from three independent experiments (*, p < 0.05; # and &, p < 0.001). C, decorin-deficient myoblasts were incubated with TGF-β1 (5.0 ng/ml) plus 75 nm hDcnHA in the absence or presence of 225 nm synthetic peptides LRR6: Full, In, or Ext, or scramble (Scr) peptide. Total RNA was isolated and CTGF and GAPDH expression was determined by qRT-PCR according to “Experimental Procedures.” Values correspond to the mean of dCT value ± S.D. of three independent experiments (* and #, p < 0.05).
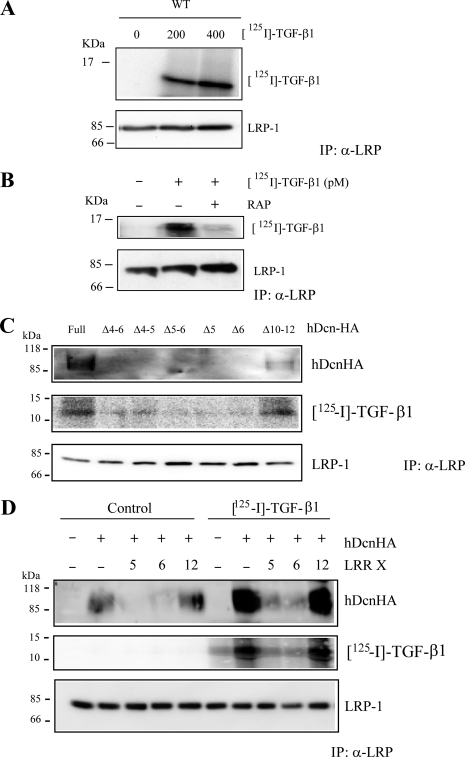
The LRR4–6 Region of Decorin Participates in Interaction of Decorin with TGF-β
It is well known that decorin interacts with TGF-β (45). We evaluated if the LRR4–6 region of decorin is required for the binding of TGF-β to decorin. To this, C2C12 myoblasts were co-incubated with two 125I-TGF-β concentrations at 4 °C, to avoid endocytosis, and cells were lysed and immunoprecipitated with antibodies against the extracellular domain of LRP-1. Fig. 7A shows that after separation by SDS-PAGE, the immunoblot revealed that the 125I-TGF-β1 co-immunoprecipitates with the LRP-1 antibody complex. The total LRP-1 immunoprecipitated is shown. Fig. 7B shows that RAP strongly inhibited the co-immunoprecipitation of 125I-TGF-β1 with LRP-1. Next, we evaluated if full decorin or decorin lacking LRR4–6, LRR4–5, LRR5–6, LRR5, LRR6, and LRR10–12 have an effect on the immunoprecipitation of 125I-TGF-β1 mediated by LRP-1. Fig. 7C shows that only the full decorin or decorin lacking LRR10–12 were able to co-immunoprecipitate 125I-TGF-β1. The immunoprecipitation of LRP-1 is shown in the same figure. Finally, we evaluated which LRR peptide(s) within LRR4–6 is able to compete for the binding of TGF-β to decorin mediated by LRP-1. Fig. 7D shows that LRR5 and LRR6 strongly inhibited decorin and 125I-TGF-β1 co-immunoprecipitation with the LRP-1 antibody complex. The total amount of LRP-1 immunoprecipitated is shown. Interestingly, the presence of TGF-β1 increased the amount of decorin immunoprecipitated by LRP-1. These results strongly suggest that the LRR5–6 decorin domain is required for interaction between TGF-β and decorin as well as decorin and LRP-1.
FIGURE 7.
The region contained in LRR4–6 of decorin (Dcn) contains the binding site for TGF-β1. A, C2C12 myoblasts were incubated with the indicated amount of 125I-TGF-β1 at 4 °C for 3 h. Extracts were immunoprecipitated with anti-LRP-1 antibodies and the presence of 125I-TGF-β1 in the immunoprecipitate was evaluated by autoradiography. Levels of LRP-1 were detected by Western blot assay. B, C2C12 myoblasts were incubated with 125I-TGF-β1 at 4 °C for 3 h in the absence or presence of RAP (1 μm). Extracts were immunoprecipitated with anti-LRP-1 antibodies. The presence of 125I-TGF-β1 in the immunoprecipitate was evaluated by autoradiography and the protein levels of LRP-1 by Western blot. C, C2C12 myoblasts were incubated with 125I-TGF-β1 as in A in the absence or presence of 75 nm hDcnHA or deletion mutants lacking LRR (Δ4–6), (Δ4–5), (Δ5–6), (Δ5), (Δ6), or (Δ10–12). Extracts were immunoprecipitated with anti-LRP-1 antibodies as in A. The presence of hDcnHA or the corresponding deletion mutants in the immunoprecipitate was evaluated by Western blot using an anti-HA antibodies. 125I-TGF-β1 in the immunoprecipitate (IP) was evaluated by autoradiography. LRP-1 levels were detected by Western blot analysis. D, C2C12 myoblasts were incubated with 75 nm hDcnHA in the absence or presence of 125I-TGF-β1 as in A. In some cases different LRR-derived peptides (LRR5, LRR6, LRR12) were added to the cells. Extracts were immunoprecipitated with anti-LRP-1 antibodies as in A. The presence of hDcnHA and LRP-1 in the immunoprecipitate was evaluated by Western blot using anti-HA and LRP-1 antibodies, respectively. 125I-TGF-β1 in the immunoprecipitate was evaluated by autoradiography.
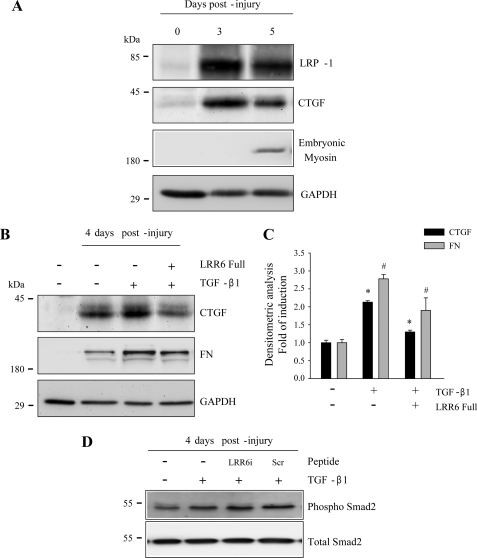
Inhibitory Effects of LRR6 on TGF-β-mediated Target Molecules in a Mouse Model of Skeletal Muscle Damage
Finally, we evaluated if LRR6 is able to inhibit TGF-β-mediated expression of CTGF (38) and fibronectin (46) in a mouse model of induced damage after addition of TGF-β. Tibialis anterior skeletal muscle degeneration was induced by BaCl2 injection as previously described (36). Because decorin-mediated TGF-β signaling requires LRP-1, first we determined the amount of LRP-1 present in the damaged muscle. Fig. 8A shows a significant increase in LRP-1 after 3 and 5 days of induced muscle damage. The figure also shows that CTGF is expressed as a consequence of the damage, because very little expression was observed in undamaged contralateral muscle. Skeletal muscle regeneration occurred as a result of the damage and was confirmed by the expression of embryonic myosin after 5 days of induced damage (36). Fig. 8B shows a similar experiment measured on post-injury day 4, where TGF-β1 was injected in the regenerating muscle on day 3 after damage induction. The figure shows that CTGF expression increases in response to TGF-β. However, when LRR6 peptide was co-injected with TGF-β1 on day 3 after damage induction, inhibition of CTGF induction was observed. In addition, LRR6 peptide co-injection resulted in a decrease in TGF-β-dependent induction of fibronectin. Fig. 8C shows a quantification of this experiment. Finally, we found that the level of phospho-Smad2 in response to TGF-β1 was not affected by LRR6i. As control a null effect of a scramble peptide on phospho-Smad2 is shown. This result is in concordance with our previous observation that suggests that levels of phospho-Smad2 are not affected when the LRP-1-decorin-dependent TGF-β-mediated signaling pathway is activated (6). This in vivo experiment strongly suggests that LRR6 inhibits the induction of fibronectin and CTGF, mediated by TGF-β1 signaling, in damaged muscle.
FIGURE 8.
A synthetic peptide from the LRR6 region of decorin decreases TGF-β activity in an injured skeletal muscle mouse model. A, tibialis anterior muscles of C57BL10 mice were injured by BaCl2 injection (36). Tibialis anterior muscles were removed at different times post-injury and protein extracts were separated by SDS-PAGE. Levels of LRP-1, CTGF, and embryonic myosin were determined by Western blot analysis. GAPDH levels are shown as a loading control. B, tibialis anterior muscles of C57BL10 mice were injured as in A. At day 3 post-injury, tibialis anterior muscles were injected with TGF-β1 (20 ng) in the absence or presence of complete synthetic LRR6 peptide (10 μg). Tibialis anterior muscles were removed on day 4 post-injury and protein extracts were separated by SDS-PAGE. Levels of CTGF and fibronectin (FN) were determined by Western blot analysis. GAPDH levels are shown as a loading control. C, quantification of three independent experiments as shown in B. Values correspond to the mean ± S.D. for fold-induction relative to control cells without TGF-β1 and LRR6 (* and #, p < 0.05). D, tibialis anterior muscles of C57BL10 mice were injured as in B. TGF-β1 (20 ng) was injected in the absence or presence of complete synthetic LRR6i peptide (10 μg) or scramble peptide (Scr, 10 μg). Tibialis anterior muscles were removed on day 4 post-injury and protein extracts were separated by SDS-PAGE. Levels of phospho-Smad-2 and total Smad-2 were determined by Western blot analysis.
DISCUSSION
In this article, we show that the LRR6 and LRR5 regions of decorin are vital for binding to LRP-1 and TGF-β-mediated signaling. Furthermore, we determined that the internal region of LRR6, an 11-amino acid peptide on the concave face of decorin, is critical for the two functions described above. Using different deletions mutants we found that LRR4–6 is essential for interaction with LRP-1, the endocytic receptor for decorin (5), interacting with TGF-β, and induction of decorin-mediated LRP-1-dependent TGF-β signaling (6). As expected, decorin mutants lacking LRR4–6, LRR5–6, LRR5, or LRR6 had diminished LRP-1-mediated endocytosis rates. Interestingly, the same LRR4–6 region is required for decorin-mediated LRP-1-dependent TGF-β signaling. We dissected the LRR4–6 region, using individual peptides from the LRR4–6 region, and found that the LRR6 peptide was able to inhibit nearly 80% of LRP-1-mediated decorin endocytosis. LRR6 and LRR5 peptides were able to inhibit decorin-mediated rescue of TGF-β signaling. Thus the interaction of decorin with LRP-1 and decorin-mediated LRP-1-dependent TGF-β signaling requires the LRR6 region of decorin. Also we found that decorin mutants lacking LRR4–6, LRR4–5, LRR5–6, LRR5, or LRR6 had diminished 125I-TGF-β1 and decorin co-immunoprecipitation with the LRP-1 antibody.
Furthermore, we found that an 11-amino acid peptide, corresponding to the internal region of LRR6 in decorin, is required for the interaction of decorin with LRP-1 and subsequent TGF-β-mediated signaling. Previously we have shown that a peptide derived from decorin, Leu155–Val260 (105 amino acids), forms a complex with TGF-β, and when immobilized on a column can bind TGF-β1 and -β2 with nanomolar dissociation constants, indicative of high-affinity binding (45). Interestingly, we show that a region contained in LRR4–6 is required for the interaction between TGF-β and decorin. Furthermore, the LRR6 region presented an inhibitory effect on TGF-β-dependent signaling in the nanomolar range. However, whether the same decorin sequence is responsible for TGF-β binding, interaction with LRP-1, and TGF-β-mediated signaling (47) requires further investigation. We have previously demonstrated that decorin, among other proteoglycans, is able to bind and sequester TGF-β from its transducing receptors, thus modulating the bioavailability of this growth factor (18). It would be interesting to evaluate if the LRR6 region can compete with decorin for TGF-β binding.
A sequence similar to LRR6i has been reported to bind collagen type I (48). Furthermore, as indicated in the Introduction, decorin interacts with EGFR (14, 50). Santra et al. (51) investigated the structural requirements of the decorin/EGFR interaction, and their results suggest that the LRR6 region is also required for proper interaction of decorin and EGFR. It is striking that several of the different biological functions attributed to decorin, such as receptor or ligand binding, reside in the same structural region. Some small LRR proteoglycans, such as decorin, have been shown to dimerize with high affinity (13, 44, 52). Interestingly, the crystal structure of decorin indicates that decorin dimerizes through the concave surfaces of its LRR domains, some of which correspond to the sequence described to be responsible for its protein-ligand interactions (44). It has been argued that the sequence of decorin that binds to collagen I (which is similar to the sequence described in this paper for LRP-1 binding and TGF-β signaling) is located on the concave face of LRR6, a position that would be less accessible for triple helical collagen (14). It has also been argued that, given the overall dimensions of the decorin core protein, a dimeric decorin would not fit in the EGFR receptor groove where EGF binds (14). In contrast, Scott et al. (44) argue, based on their crystal structure studies, that decorin has a more open structure that seems incompatible with a tight interaction with a single collagen triple helix. Furthermore, the concave surface of decorin has been postulated to be involved in a high-affinity dimer interaction, and is thus unlikely to be available for ligand binding (44). However, we have experimental evidence from chemical cross-linking assays that the LRR4, LRR5, and LRR6 regions of decorin do not affect decorin dimer formation (25). Clearly the role of the dimer-monomer transition and its function in the binding of decorin to specific ligands and receptors requires further investigation. Interestingly, we have recently demonstrated that decorin interacts with CTGF, this interaction is mediated by LRR12 (25). The fact that decorin is able to interact with two different pro-fibrotic molecules, TGF-β and CTGF, and these molecules also interact with LRP-1 (25, 53), open interesting possibilities of fine tune regulation mediated by decorin and these growth factors. Furthermore, an increase in TGF-β activity mediated by CTGF has been described (54). If decorin modulates then this augmented activity requires further investigation.
Fibrotic disorders are characterized by excessive connective tissue and ECM deposition that preclude the normal healing of different tissues (55). Marked overexpression of TGF-β and CTGF, both profibrotic cytokines, is strongly linked to the pathogenesis of these diseases. CTGF is selectively induced by TGF-β1 (38, 40, 56), and TGF-β and CTGF are coordinately expressed at sites of tissue repair and fibrosis (57, 58). In addition, production of TGF-β is altered in many pathologic conditions. For example, TGF-β is overproduced as in pulmonary fibrosis, cirrhosis, glomerulosclerosis, cardiomyopathy, Crohn disease, scleroderma and chronic graft versus host disease (55, 59). Several studies have indicated that TGF-β is a potent inducer of the myofibroblast phenotype (60, 61), which correlates with induction of elevated collagen synthesis but is prevented by induction of cell proliferation. Thus, although it is apparent that targeting TGF-β for therapy is of major clinical interest, to date no therapeutic treatment for fibrosis exist (30).
We present experimental evidence that indicates that LRR6 inhibits (at least in part) expression of TGF-β target proteins in vivo in response to skeletal muscle damage. We evaluated a mouse model where the expression of TGF-β is elevated (62) concomitant with the expression of CTGF and fibronectin: an induced skeletal muscle damage model (36). Thus, induced skeletal muscle damage (63) was associated with reduced expression of CTGF and fibronectin if the LRR6 region was co-injected with TGF-β. Because the effect of LRR6 on TGF-β-mediated activity requires the presence of LRP-1, we determined levels of this endocytic receptor protein. Muscle damage strongly induced LRP-1 expression in skeletal muscles. In this experimental model it has been shown that decorin is also present (63). Furthermore, there is some evidence in the literature that LRP-1 is required for fibrotic responses mediated by TGF-β1 (64). It has also been demonstrated that TGF-β receptor-V is identical to thr LRP-1/α2-macrogloblin receptor (53). Thus, the CTGF response would be mediated by LRP-1 expression (49). Results3 from our laboratory show elevated levels of LRP-1 in dystrophic skeletal muscles compared with wild type mice. Therefore the use of LRR6 peptides, or a modified LRR6 molecule with higher biological activity, to modulate decorin-mediated LRP-1-dependent TGF-β signaling, opens the possibility of designing pharmaceutical strategies for use in the fight against fibrosis, a severe consequence of many chronic diseases.
Excess connective tissue in dystrophic skeletal muscles, as well as in other skeletal muscular dystrophies, could be a barrier for successful cell therapy approaches, as exemplified by the use of tendon fibroblasts expressing a metalloproteinase (matrix metalloproteinase-9), which restored the vascular network and significantly reduced collagen deposition, allowing for efficient cell therapy in aged dystrophic mice (37). Thus, the fact that this short LRR6 peptide derived from decorin is directly involved in TGF-β signaling is an important finding with potential therapeutic implications.
This work was supported by FONDAP-Biomedicine Grants 13980001, CARE PFB12/2007, FONDECYT 11080212, FONDECYT 1110400, ICM P09-016-F, and MDA 89419 and Fundación Chilena para Biología Celular Proyecto MF-100.
C. Cabello-Verrugio, C. Santander, C. Cofré, M. J. Acuña, F. Melo, and E. Brandan, unpublished results.
- ECM
- extracellular matrix
- CTGF
- connective tissue growth factor
- EGFR
- epidermal growth factor receptor
- LRR6i
- LRR6 internal region
- LRR6e
- LRR6 external region
- LRRs
- leucine-rich repeats
- LRP-1
- low density lipoprotein receptor-related protein
- TGF-β
- transforming growth factor β.
REFERENCES
- 1. Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem. 67, 753–791 [DOI] [PubMed] [Google Scholar]
- 2. Gray P. C., Bilezikjian L. M., Vale W. (2002) Antagonism of activin by inhibin and inhibin receptors. A functional role for betaglycan. Mol. Cell. Endocrinol. 188, 254–260 [DOI] [PubMed] [Google Scholar]
- 3. Massagué J., Gomis R. R. (2006) The logic of TGF-βa signaling. FEBS Lett. 580, 2811–2820 [DOI] [PubMed] [Google Scholar]
- 4. Kang J. S., Liu C., Derynck R. (2009) New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 19, 385–394 [DOI] [PubMed] [Google Scholar]
- 5. Brandan E., Retamal C., Cabello-Verrugio C., Marzolo M. P. (2006) The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J. Biol. Chem. 281, 31562–31571 [DOI] [PubMed] [Google Scholar]
- 6. Cabello-Verrugio C., Brandan E. (2007) A novel modulatory mechanism of transforming growth factor-β signaling through decorin and LRP-1. J. Biol. Chem. 282, 18842–18850 [DOI] [PubMed] [Google Scholar]
- 7. Herz J., Strickland D. K. (2001) LRP, a multifunctional scavenger and signaling receptor. J. Clin. Invest. 108, 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonias S. L., Wu L., Salicioni A. M. (2004) Low density lipoprotein receptor-related protein. Regulation of the plasma membrane proteome. Thromb. Haemost. 91, 1056–1064 [DOI] [PubMed] [Google Scholar]
- 9. Brandan E., Cabello-Verrugio C., Vial C. (2008) Novel regulatory mechanisms for the proteoglycans decorin and biglycan during muscle formation and muscular dystrophy. Matrix Biol. 27, 700–708 [DOI] [PubMed] [Google Scholar]
- 10. Riquelme C., Larrain J., Schonherr E., Henriquez J. P., Kresse H., Brandan E. (2001) Antisense inhibition of decorin expression in myoblasts decreases cell responsiveness to transforming growth factor beta and accelerates skeletal muscle differentiation. J. Biol. Chem. 276, 3589–3596 [DOI] [PubMed] [Google Scholar]
- 11. Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signaling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 12. Iozzo R. V. (1999) The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J. Biol. Chem. 274, 18843–18846 [DOI] [PubMed] [Google Scholar]
- 13. McEwan P. A., Scott P. G., Bishop P. N., Bella J. (2006) Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J. Struct. Biol. 155, 294–305 [DOI] [PubMed] [Google Scholar]
- 14. Schaefer L., Iozzo R. V. (2008) Biological functions of the small leucine-rich proteoglycans. From genetics to signal transduction. J. Biol. Chem. 283, 21305–21309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobe B., Kajava A. V. (2001) The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11, 725–732 [DOI] [PubMed] [Google Scholar]
- 16. Cáceres S., Cuellar C., Casar J. C., Garrido J., Schaefer L., Kresse H., Brandan E. (2000) Synthesis of proteoglycans is augmented in dystrophic mdx mouse skeletal muscle. Eur. J. Cell Biol. 79, 173–181 [DOI] [PubMed] [Google Scholar]
- 17. Fadic R., Mezzano V., Alvarez K., Cabrera D., Holmgren J., Brandan E. (2006) Increase in decorin and biglycan in Duchenne muscular dystrophy. Role of fibroblasts as cell source of these proteoglycans in the disease. J. Cell. Mol. Med. 10, 758–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Droguett R., Cabello-Verrugio C., Riquelme C., Brandan E. (2006) Extracellular proteoglycans modify TGF-β bioavailability attenuating its signaling during skeletal muscle differentiation. Matrix Biol. 25, 332–341 [DOI] [PubMed] [Google Scholar]
- 19. Iozzo R. V., Schaefer L. (2010) Proteoglycans in health and disease. Novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 277, 3864–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schönherr E., Sunderkötter C., Iozzo R. V., Schaefer L. (2005) Decorin, a novel player in the insulin-like growth factor system. J. Biol. Chem. 280, 15767–15772 [DOI] [PubMed] [Google Scholar]
- 21. Schaefer L., Tsalastra W., Babelova A., Baliova M., Minnerup J., Sorokin L., Gröne H. J., Reinhardt D. P., Pfeilschifter J., Iozzo R. V., Schaefer R. M. (2007) Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and mammalian target of rapamycin. Am. J. Pathol. 170, 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moscatello D. K., Santra M., Mann D. M., McQuillan D. J., Wong A. J., Iozzo R. V. (1998) Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J. Clin. Invest. 101, 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takeuchi Y., Kodama Y., Matsumoto T. (1994) Bone matrix decorin binds transforming growth factor-β and enhances its bioactivity. J. Biol. Chem. 269, 32634–32638 [PubMed] [Google Scholar]
- 24. Yamaguchi Y., Mann D. M., Ruoslahti E. (1990) Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 346, 281–284 [DOI] [PubMed] [Google Scholar]
- 25. Vial C., Gutiérrez J., Santander C., Cabrera D., Brandan E. (2011) Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J. Biol. Chem. 286, 24242–24252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldoni S., Humphries A., Nyström A., Sattar S., Owens R. T., McQuillan D. J., Ireton K., Iozzo R. V. (2009) Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 185, 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olguin H. C., Santander C., Brandan E. (2003) Inhibition of myoblast migration via decorin expression is critical for normal skeletal muscle differentiation. Dev. Biol. 259, 209–224 [DOI] [PubMed] [Google Scholar]
- 28. Bolster M. B., Silver R. M. (1993) Lung disease in systemic sclerosis (scleroderma). Baillieres Clin. Rheumatol. 7, 79–97 [DOI] [PubMed] [Google Scholar]
- 29. Ziyadeh F. N. (1993) The extracellular matrix in diabetic nephropathy. Am. J. Kidney. Dis. 22, 736–744 [DOI] [PubMed] [Google Scholar]
- 30. Wynn T. A. (2008) Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Denton C. P., Abraham D. J. (2001) Transforming growth factor-β and connective tissue growth factor. Key cytokines in scleroderma pathogenesis. Curr. Opin. Rheumatol. 13, 505–511 [DOI] [PubMed] [Google Scholar]
- 32. Bernasconi P., Di Blasi C., Mora M., Morandi L., Galbiati S., Confalonieri P., Cornelio F., Mantegazza R. (1999) Transforming growth factor-β1 and fibrosis in congenital muscular dystrophies. Neuromusc. Disord. 9, 28–33 [DOI] [PubMed] [Google Scholar]
- 33. Alvarez K., Fadic R., Brandan E. (2002) Augmented synthesis and differential localization of heparan sulfate proteoglycans in Duchenne muscular dystrophy. J. Cell. Biochem. 85, 703–713 [DOI] [PubMed] [Google Scholar]
- 34. Schiaffino S., Partridge T. (2008) Advances in Muscle Research, Vol. 3, pp. XIV-380, Springer, The Netherlands [Google Scholar]
- 35. Caldwell C. J., Mattey D. L., Weller R. O. (1990) Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol. Appl. Neurobiol. 16, 225–238 [DOI] [PubMed] [Google Scholar]
- 36. Casar J. C., Cabello-Verrugio C., Olguin H., Aldunate R., Inestrosa N. C., Brandan E. (2004) Heparan sulfate proteoglycans are increased during skeletal muscle regeneration. Requirement of syndecan-3 for successful fiber formation. J. Cell Sci. 117, 73–84 [DOI] [PubMed] [Google Scholar]
- 37. Gargioli C., Coletta M., De Grandis F., Cannata S. M., Cossu G. (2008) PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat. Med. 14, 973–978 [DOI] [PubMed] [Google Scholar]
- 38. Vial C., Zúñiga L. M., Cabello-Verrugio C., Cañón P., Fadic R., Brandan E. (2008) Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J. Cell. Physiol. 215, 410–421 [DOI] [PubMed] [Google Scholar]
- 39. Brandan E., Carey D. J., Larraín J., Melo F., Campos A. (1996) Synthesis and processing of glypican during differentiation of skeletal muscle cells. Eur. J. Cell Biol. 71, 170–176 [PubMed] [Google Scholar]
- 40. Cabello-Verrugio C., Córdova G., Vial C., Zúñiga L. M., Brandan E. (2011) Connective tissue growth factor induction by lysophosphatidic acid requires transactivation of transforming growth factor type β receptors and the JNK pathway. Cell. Signal. 23, 449–457 [DOI] [PubMed] [Google Scholar]
- 41. Kresse H., Schönherr E. (2001) Proteoglycans of the extracellular matrix and growth control. J. Cell. Physiol. 189, 266–274 [DOI] [PubMed] [Google Scholar]
- 42. Giri S. N. (2003) Novel pharmacological approaches to manage interstitial lung fibrosis in the twenty-first century. Annu. Rev. Pharmacol. Toxicol. 43, 73–95 [DOI] [PubMed] [Google Scholar]
- 43. Krusius T., Ruoslahti E. (1986) Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc. Natl. Acad. Sci. U.S.A. 83, 7683–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scott P. G., McEwan P. A., Dodd C. M., Bergmann E. M., Bishop P. N., Bella J. (2004) Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan. Proc. Natl. Acad. Sci. U.S.A. 101, 15633–15638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schönherr E., Broszat M., Brandan E., Bruckner P., Kresse H. (1998) Decorin core protein fragment Leu155–Val260 interacts with TGF-β but does not compete for decorin binding to type I collagen. Arch. Biochem. Biophys. 355, 241–248 [DOI] [PubMed] [Google Scholar]
- 46. Varga J., Rosenbloom J., Jimenez S. A. (1987) Transforming growth factor β (TGF-β) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem. J. 247, 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hildebrand A., Romarís M., Rasmussen L. M., Heinegård D., Twardzik D. R., Border W. A., Ruoslahti E. (1994) Interaction of the small interstitial proteoglycans biglycan, decorin, and fibromodulin with transforming growth factor β. Biochem. J. 302, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalamajski S., Aspberg A., Oldberg A. (2007) The decorin sequence SYIRIADTNIT binds collagen type I. J. Biol. Chem. 282, 16062–16067 [DOI] [PubMed] [Google Scholar]
- 49. Segarini P. R., Nesbitt J. E., Li D., Hays L. G., Yates J. R., 3rd, Carmichael D. F. (2001) The low density lipoprotein receptor-related protein/α2-macroglobulin receptor is a receptor for connective tissue growth factor. J. Biol. Chem. 276, 40659–40667 [DOI] [PubMed] [Google Scholar]
- 50. Iozzo R. V., Karamanos N. (2010) Proteoglycans in health and disease. Emerging concepts and future directions. FEBS J. 277, 3863. [DOI] [PubMed] [Google Scholar]
- 51. Santra M., Reed C. C., Iozzo R. V. (2002) Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J. Biol. Chem. 277, 35671–35681 [DOI] [PubMed] [Google Scholar]
- 52. Orgel J. P., Eid A., Antipova O., Bella J., Scott J. E. (2009) Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PloS One 4, e7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang S. S., Ling T. Y., Tseng W. F., Huang Y. H., Tang F. M., Leal S. M., Huang J. S. (2003) Cellular growth inhibition by IGFBP-3 and TGF-β1 requires LRP-1. FASEB J. 17, 2068–2081 [DOI] [PubMed] [Google Scholar]
- 54. Abreu J. G., Ketpura N. I., Reversade B., De Robertis E. M. (2002) Connective tissue growth factor (CTGF) modulates cell signaling by BMP and TGF-β. Nat. Cell Biol. 4, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prud'homme G. J. (2007) Pathobiology of transforming growth factor β in cancer, fibrosis, and immunologic disease, and therapeutic considerations. Lab. Invest. 87, 1077–1091 [DOI] [PubMed] [Google Scholar]
- 56. Duncan M. R., Frazier K. S., Abramson S., Williams S., Klapper H., Huang X., Grotendorst G. R. (1999) Connective tissue growth factor mediates transforming growth factor β-induced collagen synthesis. Down-regulation by cAMP. FASEB J. 13, 1774–1786 [PubMed] [Google Scholar]
- 57. Frazier K., Williams S., Kothapalli D., Klapper H., Grotendorst G. R. (1996) Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J. Invest. Dermatol. 107, 404–411 [DOI] [PubMed] [Google Scholar]
- 58. Shinozaki M., Kawara S., Hayashi N., Kakinuma T., Igarashi A., Takehara K. (1997) Induction of subcutaneous tissue fibrosis in newborn mice by transforming growth factor β. Simultaneous application with basic fibroblast growth factor causes persistent fibrosis. Biochem. Biophys. Res. Commun. 240, 292–297 [PubMed] [Google Scholar]
- 59. Pohlers D., Brenmoehl J., Löffler I., Müller C. K., Leipner C., Schultze-Mosgau S., Stallmach A., Kinne R. W., Wolf G. (2009) TGF-β and fibrosis in different organs. Molecular pathway imprints. Biochim. Biophys. Acta 1792, 746–756 [DOI] [PubMed] [Google Scholar]
- 60. Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. (1993) Transforming growth factor-β 1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 122, 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Folger P. A., Zekaria D., Grotendorst G., Masur S. K. (2001) Transforming growth factor-β-stimulated connective tissue growth factor expression during corneal myofibroblast differentiation. Invest. Ophthalmol. Vis. Sci. 42, 2534–2541 [PubMed] [Google Scholar]
- 62. McLennan I. S., Koishi K. (1997) Cellular localization of transforming growth factor-β2 and -β3 (TGF-β2, TGF-β3) in damaged and regenerating skeletal muscles. Dev. Dyn. 208, 278–289 [DOI] [PubMed] [Google Scholar]
- 63. Casar J. C., McKechnie B. A., Fallon J. R., Young M. F., Brandan E. (2004) Transient up-regulation of biglycan during skeletal muscle regeneration. Delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev. Biol. 268, 358–371 [DOI] [PubMed] [Google Scholar]
- 64. Hu K., Wu C., Mars W. M., Liu Y. (2007) Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J. Clin. Invest. 117, 3821–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]