Background: Nutrient intake directly affects adipose tissue function and growth.
Results: The gut peptide GLP-1 controls adipogenesis via its receptor through regulation of cell proliferation and apoptosis.
Conclusion: GLP-1 is a signaling molecule from the intestine relating nutritional status to the adipose tissue.
Significance: GLP-1 is used in treatment of type 2 diabetes, and regulation of adipose tissue mass might be one influencing factor.
Keywords: Adipocyte, Adipogenesis, Diabetes, Intestine, Obesity
Abstract
Increased nutrient intake leads to excessive adipose tissue accumulation, obesity, and the development of associated metabolic disorders. How the intestine signals to adipose tissue to adapt to increased nutrient intake, however, is still not completely understood. We show here, that the gut peptide GLP-1 or its long-lasting analog liraglutide, function as intestinally derived signals to induce adipocyte formation, both in vitro and in vivo. GLP-1 and liraglutide activate the GLP-1R, thereby promoting pre-adipocyte proliferation and inhibition of apoptosis. This is achieved at least partly through activation of ERK, PKC, and AKT signaling pathways. In contrast, loss of GLP-1R expression causes reduction in adipogenesis, through induction of apoptosis in pre-adipocytes, by inhibition of the above mentioned pathways. Because GLP-1 and liraglutide are used for the treatment of type 2 diabetes, these findings implicate GLP-1 as a regulator of adipogenesis, which could be an alternate pathway leading to improved lipid homeostasis and controlled downstream insulin signaling.
Introduction
Obesity, characterized by an excessive accumulation of adipose tissue, is a key component of the metabolic syndrome, often associated with the development of type 2 diabetes mellitus, atherosclerosis, and hyperlipidemia (1–4). The growth of adipose tissue involves cellular hypertrophy (cell size increase) and hyperplasia (cell number increase) (5). Hypertrophy is the result of excess lipid accumulation in existing adipocytes due to high energy intake (2). Obesity in adults is characterized by adipocyte hypertrophy, linked to down-regulation of adiponectin secretion, leading to the development of insulin resistance and type 2 diabetes (6). In contrast, hyperplasia results from the recruitment of new adipocytes from precursor cells in adipose tissue and involves proliferation (2) and differentiation of pre-adipocytes (7, 8). Interestingly, it was reported that hyperplasia in both visceral and subcutaneous adipose tissue might be protective against lipid, as well as glucose/insulin abnormalities in obesity (9). Adipocytes play an important role in energy homeostasis by storing energy in lipid droplets (10, 11). Furthermore, adipose tissue functions as an endocrine organ, secreting adipocytokines that regulate energy metabolism in fat and other tissues (12, 13).
Glucagon-like peptide-1 (GLP-1),2 an incretin hormone, produced by post-translational processing of proglucagon gene (14, 15) in enteroendocrine L-cells in response to food intake, is secreted as one of the gut hormones (15, 16) and collected in the intestinal lymph duct. Plasma levels of GLP-1 rise rapidly within minutes after food intake (17). The major physiological roles of this endocrine hormone include: 1) the stimulation of glucose-dependent insulin secretion from pancreatic β-cells, 2) stimulation of insulin biosynthesis and insulin sensitivity, 3) enhancement of pancreatic β-cell proliferation and protection against apoptosis, 4) inhibition of glucagon secretion and gastric emptying, and 5) inhibition of food intake (14, 18–21). As a result GLP-1 facilitates the rapid clearance and storage of blood glucose (16). For instance, subcutaneous administration of native GLP-1 to patients with type 2 diabetes mellitus lowers fasting and postprandial levels of glucose and HbA1c effectively, and reduces weight gain (22).
Given the rapid inactivation of endogenous GLP-1 (half-life of less than 2 min) by the enzyme dipeptidyl-peptidase-4 (DPP-IV), alternative therapeutic approaches have been developed using GLP-1 analogues which are resistant to DPP-IV mediated degradation (14). One of the FDA-approved GLP-1R agonists is liraglutide which is used to treat type 2 diabetes (23). Liraglutide has a 97% homology with human GLP-1 including a lysine to arginine mutation at position 34 and a palmitoyl side-chain at lysine 26 (24).
Because of the above mentioned reasons, GLP-1 and GLP-1 analogs are currently among the most promising therapeutic options for the pharmacotherapy of type 2 diabetes and obesity because these substances do not lose their eating-inhibitory and beneficial metabolic effects with chronic treatment and because they are still effective in obese patients, which show reduced GLP-1 levels. Although the cause of this association between obesity and GLP-1 is unknown (25), it might be a contributing factor toward the development of obesity as GLP-1 secretion is improved after weight loss (14, 25, 26).
The intracellular effect of GLP-1 is mediated via activation of its specific receptor (GLP-1R). GLP-1R belongs to the class B family of 7-transmembrane-spanning, heterotrimeric G-protein-coupled receptors (21). GLP-1R is expressed in many tissues, including pancreas, lung, heart, kidney, intestine, stomach, adipose tissue, muscle, as well as the central and peripheral nervous systems (17, 19). Upon its activation, GLP-1R couples with Gαs and activates adenylate cyclase (AC) to stimulate cAMP production (27), leading to the activation of second messenger pathways, such as cAMP-dependent protein kinase (PKA) and cAMP-regulated guanine nucleotide exchange factors of the Epac family (21). Here, we show that GLP-1 or its analog liraglutide stimulate pre-adipocyte differentiation via GLP-1R, both in vitro and in vivo. Suppression of Glp-1R expression reduces proliferation and differentiation while inducing apoptosis of pre-adipocytes. This mechanism is the basis for a cross talk between the intestine and adipocyte precursor cells leading to an induction of adipogenesis in response to nutrient intake.
EXPERIMENTAL PROCEDURES
Materials
Glucagon-like Peptide (7–36) GLP-1, was obtained from Bachem. Bodipy493/503, Hoechst, Syto60 were purchased from Invitrogen. Dexamethasone, IBMX, and insulin were from Sigma-Aldrich.
Differentiation of Pre-adipocytes
The 3T3-L1 fibroblasts as well as primary pre-adipocytes were grown and differentiated as described previously (7). High throughput image analysis to calculate adipogenesis was performed as described before (7, 28). In short, SVF cells and 3T3-L1 pre-adipocytes were cultured on collagen coated black 96-well plates with clear bottoms. Fluorescent images were taken with the Operetta high throughput imaging system (Perkin Elmer). Images were analyzed using either Cell Profiler or Harmony software (Perkin Elmer).
Lentiviral Knockdown
For propagation and downstream purification of shRNA clones, sequence-verified shRNA lentiviral plasmids (pLKO.1-puro) targeting mouse GLP-1R were obtained as frozen bacterial glycerol stocks (Sigma-Aldrich). Five different lentiviral vector plasmids constructs of mission shRNA clones were used. PLKO.1 plasmids were used to generate lentiviral particles in the packaging HEK293T cell line. 60–70% confluent pre-adipocytes were infected with GLP-1R shRNA lentivirus or a scrambled shRNA control in complete medium (10% FBS and 1% penicillin streptomycin) in the presence of polybrene (8 μg/ml). The next day, the medium was changed to complete medium, which was subsequently changed every 48 h for a total of 6 days.
RNA Isolation and Quantitative Real-time RT-PCR
Total RNA was extracted using TRIzol reagent according to the manufacturer's protocol. 1.0 μg of total RNA was converted into first-strand cDNA using the SuperScript III First-Strand kit (Invitrogen). Real-time PCR quantification was performed using platinum SYBR Green and gene-specific primer sets (sequence is available upon request).
Western Blot Analysis
For whole cell lysate preparation, differentiated cells were lysed in hypotonic buffer containing 10 mm KCL, 1.5 mm MgCl2, 0.2% Tween 20, 10 mm HEPES, pH 7.9 and 2% (v/v) protease inhibitors (Roche) for 5 min on ice. Hypertonic buffer containing 0.5% (v/v) of 150 mm NaCl, 20 mm HEPES pH 7.9, 25% glycerol, 0.2 mm EDTA, 1.5 mm MgCl2, 1.2 mm NaCl, and 2% (v/v) protease inhibitors was added, and the sample was incubated for 30 min by overhead rotation at 4 °C. Cell lysates were centrifuged at 16,000 rpm for 20 min, and protein concentrations were measured. Equal amounts of lysate proteins were resolved by SDS-PAGE (10–15%) gel and transferred onto a nitrocellulose membrane (Perkin Elmer). The following primary antibodies were used: ant-PKC (PKCβII), anti-GLP-1R, (Abcam); anti-C/EBPβ, anti-C/EBPδ, anti-PPARγ, anti-FABP4, anti-phospho-PKC (βII ser660), anti-ERK1/2, anti-phospho-ERK1/2 (Thr202/Tyr204), anti-phospho-Akt (Ser473), anti-Akt, anti-phospho-Bad (Ser136), anti-cleaved caspase-3 (Cell Signaling). Loading controls were measured with a mouse anti-γ-tubulin (Sigma-Aldrich).
Cell Proliferation and Apoptosis
Cell proliferation was determined by measuring 5-ethynyl-2′-deoxyuridine (EdU) incorporation. By using the fluorescent Alexa Fluor® 488 azide from the Click-iT® EDU Alexa Fluor® 488 Assay kit (Invitrogen). An ELISA apoptosis detection kit (Enzo) targeting denatured DNA with a monoclonal antibody to single stranded DNA (ssDNA) was used to evaluate apoptosis in adipocyte cells. The assay was carried out according to the manufacturer's instructions.
Animal Studies
All animal studies were approved by the Canton of Zurich Veterinary Office. Male 10–12-week-old C57BL/6 mice were obtained from Charles River and housed in a pathogen-free animal facility on 12 h dark/light cycle. In vivo differentiation was performed as described, previously (28). In short, male mice were sacrificed and fat tissues (subcutaneous and visceral) were minced and incubated with collagenase type II in KRB buffer for 1 h at 37 °C. SVF cells were pelleted by centrifugation and filtered with a 40 μm mesh. For in vivo adipogenesis, 106 cells were resuspended in 100 μl of Matrigel (BD) and injected into the subcutaneous subscalpular region of acceptor mice. To induce adipogenesis mice were fed a HFD for 6 weeks. To assess the influence of liraglutide (Bachem), animals were injected for the last 17 days of the study with the long lasting GLP-1 analog, liraglutide at 100 μg/kg twice daily. Mice were sacrificed and Matrigel pads were excised, adipose tissue was fixed in 5% paraformaldehyde, paraffin embedded, and cut into 10 μm sections. The cut tissues were stained with hematoxylin/eosin. The amount of adipocytes present in a tissue section was quantified using Cell Profiler (28).
Statistical Analysis
All data are expressed as mean ± S.E. The significance of differences between groups was determined using a two tailed Student's t test.
RESULTS
GLP-1 Regulates Adipogenesis
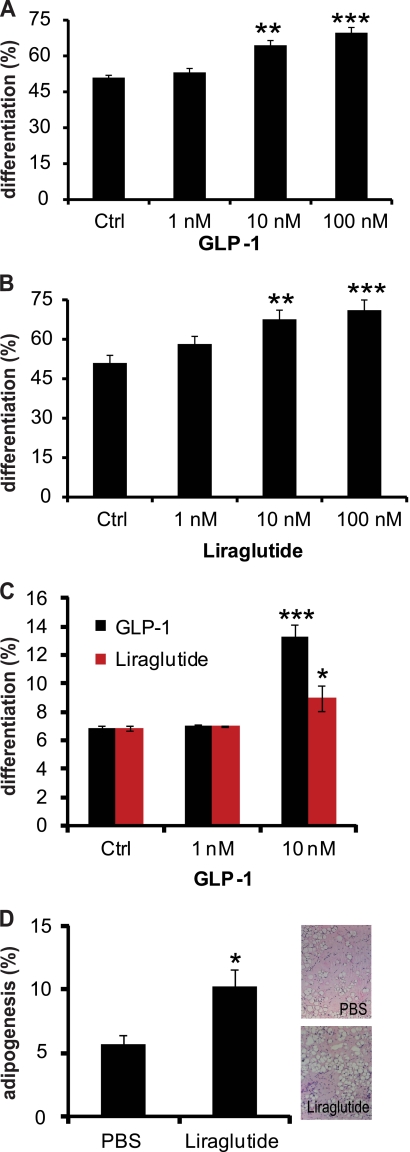
To determine the effects of GLP-1 on adipogenic differentiation, cultured 3T3-L1 cells, as well as primary pre-adipocytes were treated with or without GLP-1 or its synthetic analog liraglutide at different concentrations (Fig. 1, A–C, supplemental Fig. S1, A–D). Interestingly, cells treated with GLP-1 or liraglutide demonstrated a significantly higher degree of differentiation than control cells. Furthermore, we observed a dose-dependent increase in the number of differentiated adipocytes after GLP-1 or liraglutide treatment in 3T3-L1 cells, as well as an increase in differentiation in primary pre-adipocytes. To further examine liraglutide effects on adipogenesis in vivo, we implanted pre-adipocytes in a matrigel pad into the subscapular region of acceptor mice which were fed a HFD, in addition to treatment with liraglutide. Interestingly, 100 μg/kg twice daily IP injection of liraglutide into mice for 17 days induced pre-adipocyte differentiation significantly (Fig. 1D), even though the same mice showed a trend toward reduced weight gain and blood glucose levels (supplemental Fig. S1, E and F). Taken together these results demonstrate that nutrient intake leads to an increased circulation of GLP-1, which can regulate the process of adipogenesis.
FIGURE 1.
GLP-1 regulates adipocyte differentiation. A and B, 3T3-L1 pre-adipocytes were treated with indicated concentrations of GLP-1 or liraglutide during induction of adipogenesis. Differentiation was quantified using image based analysis (n = 12). C, primary pre-adipocytes were treated with indicated concentrations of GLP-1 or liraglutide during induction of adipogenesis. Differentiation was quantified as described above (n = 4). D, in vivo pre-adipocyte differentiation in mice injected with 100 μg/kg liraglutide or PBS for 17 days (n = 12). Adipogenesis was quantified using image based analysis of adipocyte formation. All values are shown as mean ± S.E. (*, <0.05, **, p < 0.01, ***, p < 0.001).
The Effects of GLP-1 on Adipogenesis Are Mediated through GLP-1R
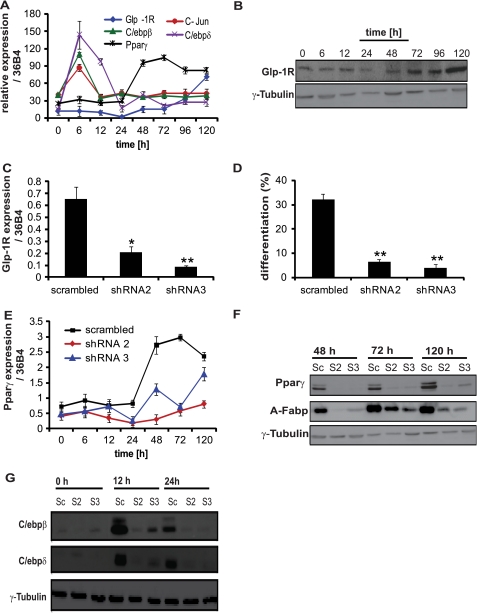
Based on our findings that GLP-1 promotes adipogenesis, we next examined the role GLP-1R during differentiation. In agreement with previous results (29), we found GLP-1R to be expressed both in undifferentiated and differentiated 3T3-L1 cells. Interestingly, the expression of Glp-1R mRNA and protein was markedly increased during late phase adipocyte differentiation (Fig. 2, A and B). This expression pattern was similar to the late phase adipogenic differentiation marker PPARγ and different from the early adipocyte differentiation markers c-Jun, C/ebpβ, and C/ebpδ. To further analyze the functionality of the GLP-1R, 3T3-L1 cells were treated with or without GLP-1 or liraglutide at different concentrations. As shown in supplemental Fig. S2A, cAMP levels increased in response to GLP-1 or liraglutide treatment and reached a maximum at 1 nm, comparable to isoproterenol, indicating the presence of a functional GLP-1R in 3T3-L1 cells.
FIGURE 2.
GLP-1 regulates adipogenesis via GLP-1R. A and B, expression of Glp-1R mRNA and protein in 3T3-L1 cells was measured at different time points during adipogenesis (n = 8). C, 3T3-L1 pre-adipocytes were infected with GLP-1R shRNA expressing lentivirus (shRNA2 and shRNA3) or scrambled shRNA control. Glp-1R expression was analyzed by qPCR (n = 4). D, quantitative analysis of adipocyte differentiation using high-throughput image analysis in cells infected with GLP-1R shRNA expressing lentivirus or scrambled shRNA control (n = 12) and treated with GLP-1. E–G, analysis of PPARγ, A-Fabp, C/ebpβ/δ mRNA and protein expression in 3T3-L1 pre-adipocytes infected with GLP-1R shRNA-expressing lentivirus (shRNA2 and shRNA3) or scrambled shRNA and treated with GLP-1 (n = 4). Cells were analyzed at indicated time points post induction of differentiation. γ-tubulin was used as a loading control. The graphs represent mean ± S.E. (**, p < 0.01, ***, p < 0.001).
To study the potential role of GLP-1R during adipocyte differentiation, 3T3-L1 cells were infected with lentivirus containing shRNA against GLP-1R or a scrambled shRNA control. GLP-1R knockdown was confirmed by RT-PCR analyses (Fig. 2C). Reduction of Glp-1R expression decreased differentiation compared with the scrambled control in the presence of GLP-1 (Fig. 2D, supplemental Fig. S2B).
To study the potential molecular mechanisms underlying GLP-l mediated activation of adipogenesis we focused on the major adipogenic differentiation markers. Knockdown of GLP-1R in the presence of GLP-1 significantly inhibited the expression of PPARγ as well as that of its target A-Fabp genes (Fig. 2, E and F, supplemental Fig. S2C). Furthermore, GLP-1R knockdown in the presence of GLP-1 led to the down-regulation of C/ebpβ and C/ebpδ expression (Fig. 2G, supplemental Fig. S2, D and E). Taken together these results indicate that GLP-1 mediates its adipogenic effect via GLP-1R through regulation of early events of differentiation, as evidenced by the down-regulation of C/ebpβ/δ, as well as the reduced expression of PPARγ and its target genes.
GLP-1/GLP-1R Regulate Cell Proliferation and Adipogenesis in Pre-adipocytes
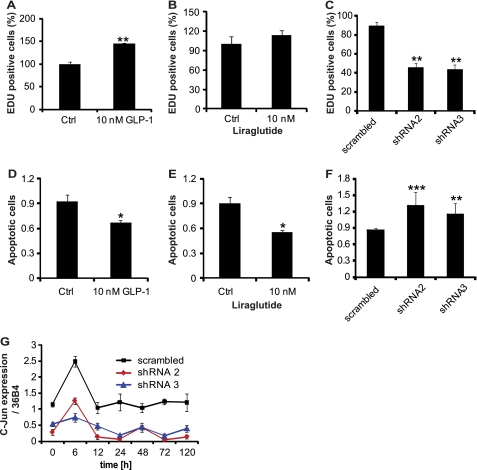
Given the fact that GLP-1 and GLP-1R regulate early adipogenic events we next examined, whether this effect is due to changes in proliferation or apoptosis of pre-adipocytes. To this end, we treated 3T3-L1 cells with GLP-1 or its synthetic analog liraglutide and analyzed the degree of proliferation during the first 24 h of adipogenesis. As seen in Fig. 3, A and B, GLP-1 and liraglutide incubation led to a significant increase in the number of proliferating cells as evidenced by EDU incorporation. In addition, we examined cell proliferation in GLP-1R knockdown cells, to evaluate whether GLP-1 acts via its receptor to regulate cell proliferation. To this end, 3T3-L1 pre-adipocytes were infected with shRNA against GLP-1R or a scrambled control. Differentiation was induced after confluence and cells were treated with 10 nm of GLP-1 (Fig. 3C). We could show that knockdown of GLP-1R led to a significant decrease of EDU-positive cells compared with the scrambled control.
FIGURE 3.
Regulation cell proliferation and apoptosis by GLP-1/GLP-1R. A and B, effect of GLP-1 and liraglutide on cell proliferation was measured by EDU incorporation into 3T3-L1 pre-adipocytes (n = 8). C, 3T3-L1 pre-adipocytes were infected with GLP-1R shRNA-expressing lentivirus (shRNA2 and shRNA3) or scrambled shRNA control and treated with 10 nm GLP-1 during induction of cell differentiation. EDU incorporation as a marker for cell proliferation was quantified 24 h postinduction (n = 8). D and E, effect of GLP-1 and liraglutide on cell apoptosis measured by TUNEL (n = 4). F, 3T3-L1 pre-adipocytes were infected with GLP-1R shRNA-expressing lentivirus (shRNA2 and shRNA3) or scrambled shRNA control and treated with 10 nm GLP-1 during induction of cell differentiation. Apopotosis was quantified by TUNEL, 24 h post induction (n = 4). G, analysis of c-Jun mRNA expression in 3T3-L1 pre-adipocytes infected with GLP-1R shRNA-expressing lentivirus (shRNA2 and shRNA3) or scrambled shRNA (n = 4) and treated with 10 nm GLP-1 during induction of cell differentiation. The graphs represent mean ± S.E. (**, p < 0.01, ***, p < 0.001).
Because adipose tissue mass is determined by the volume and number of adipocytes, factors that are dependent upon a balance between pre-adipocyte proliferation, differentiation, and cell loss by apoptosis, we quantified the effect of GLP-1 and its receptor on the regulation of apoptosis in 3T3-L1 cells. We observed a significant decrease in cell death of induced 3T3-L1 pre-adipocytes treated with GLP-1 or liraglutide (Fig. 3, D and E). In contrast, 3T3-L1 cells infected with GLP-1R shRNA and treated with GLP-1 showed significant induction of cell apoptosis compared with that of scrambled control (Fig. 3F). Thus, knockdown of GLP-1R induces apoptosis in pre-adipocytes during adipogenesis, which cannot be rescued by GLP-1.
Because c-Jun is involved in many cellular processes including cell proliferation, apoptosis, differentiation, and cell transformation, we studied the effects of GLP-1 and its receptor on the expression of c-Jun during adipogenic differentiation of 3T3-L1 cells. c-Jun was found to be expressed throughout differentiation in the scrambled control group, with a sharp increase during early differentiation (6 h) (Fig. 3G). c-Jun mRNA levels were significantly down-regulated upon GLP-1R knockdown and GLP-1 was unable to reverse down-regulation of c-Jun expression.
Taken together, our result indicated that GLP-1 regulates adipogenesis by affecting the early clonal expansion phase through regulation of c-Jun expression, as well as by shifting the balance of proliferation and apoptosis.
Effects of GLP-1/GLP-1R Are Mediated by PKC Signaling
To identify the signaling pathways mediating the effects of GLP-1 and its receptor we studied the expression of the key signaling molecules and their post-translational modifications by Western blot analysis.
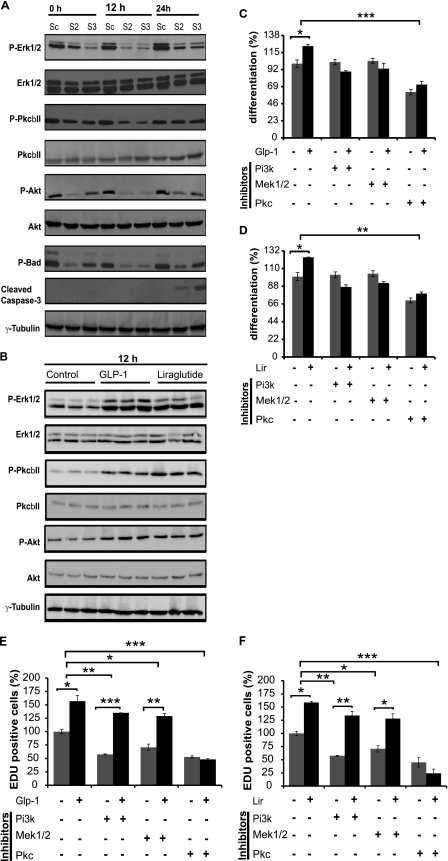
One important signaling cascade regulating proliferation and apoptosis are the extracellular signal-regulated kinases (ERK) 1 and 2 pathways. We observed GLP-1 induced Erk1/2 phosphorylation during early phases of differentiation, with maximal phosphorylation attained at 24 h post-induction (Fig. 4A). In contrast, GLP-1 induced Erk1/2 phosphorylation levels decreased in response to GLP-1R knockdown at 0, 12, and 24 h, respectively (Fig. 4A). Total Erk1/2 protein levels were not affected by either GLP-1 or GLP-1R knockdown.
FIGURE 4.
Signaling pathways of GLP-1/GLP-R regulating pre-adipocyte proliferation and differentiation. A, analysis of p-Erk1/2, Erk1/2, p-PKC, PKC, p-Akt, Akt, p-Bad, and cleaved caspase-3 protein expression in 3T3-L1 pre-adipocytes infected with GLP-1R shRNA-expressing lentivirus (shRNA2 and shRNA3) or scrambled shRNA (n = 4) and treated with 10 nm GLP-1 during induction of cell differentiation. B, analysis of p-Erk1/2, Erk1/2, p-PKC, PKC, p-Akt, and Akt protein expression in 3T3-L1 pre-adipocytes (n = 3) treated with or without 10 nm GLP-1or liraglutide during induction of cell differentiation. Cells were analyzed at indicated time points postinduction of differentiation. γ-Tubulin was used as a loading control. C and D, analysis of pre-adipocyte differentiation in 3T3L-1 cells induced in serum-free medium in the presence or absence of either PI3k inhibitor LY294002 (10 μm), Mek1/2 inhibitor PD-98059 (20 μm) or PKC inhibitor (1 nm). In addition cells were treated with 10 nm GLP-1 or liraglutide. Quantitative analysis of adipocyte differentiation was achieved, using high-throughput image analysis (n = 4). E and F, analysis of pre-adipocyte proliferation in 3T3L-1 cells induced in serum-free medium in the presence or absence of either PI3K inhibitor LY294002 (10 μm), MEK1/2 inhibitor PD-98059 (20 μm), or PKC inhibitor (1 nm). In addition cells were treated with10 nm GLP-1 or liraglutide. Quantitative analysis of adipocyte proliferation was measured by nuclear EDU incorporation (n = 4). The graphs represent mean ± S.E. (*, < 0.05, **, p < 0.01, ***, p < 0.001).
Another important pathway that regulates apoptosis/proliferation is the protein kinase C β pathway (PKCβ), which regulates its own expression through the (MAPK)-ERK-dependent signaling pathway. We could show increased phosphorylation of PKCβII by GLP-1 and a reduced phosphorylation by GLP-1 in the absence of GLP-1R, with no changes in total protein levels (Fig. 4A).
Lastly, the AKT signaling cascade is considered important for adipogenesis, as it appears to induce or activate PPARγ and C/ebpα expression during adipocyte differentiation. Therefore, we measured the phosphorylation states of AKT, after knockdown of GLP-1R. Consistent with Erk and PKCβII, GLP-1 treatment increased Akt phosphorylation levels in scrambled control during 3T3-L1 cell differentiation at 0, 12, and 24 h post-induction (Fig. 4A). Similarly, GLP-1R knockdown decreased GLP-1-mediated induction of p-Akt levels (Fig. 4A). Taken together these results identify three possible pathways that could explain the alterations in adipogenesis upon GLP-1R activation or knockdown.
Bcl-xL/Bcl-2-associated death promoter homolog (BAD) is pro-apoptotic member of the Bcl-2 family; its pro-apoptotic activity is regulated by phosphorylation at several sites. ERK1/2 activation mediates the Ser-112 phosphorylation of BAD, through p90RSK activation, allowing the association of BAD with the scaffold protein 14-3-3, leading to its inactivation and inhibition of β-cell apoptosis (21). Consistent with these findings we could show that the decrease in Erk1/2 and Akt phosphorylation resulted in reduced pBAD levels in response to GLP-1R knockdown compared with scrambled control cells (Fig. 4A).
Caspase-3 activation plays a pivotal role in the signal transduction pathways of cell apoptosis. Therefore, we further investigated the role of GLP-1R knockdown in caspase-3 regulation. In contrast to decreased pBAD levels during adipogenesis, cleaved caspase-3 levels were significantly increased in GLP-1R knockdown cells in the presence of GLP-1 at 24 h post- induction (Fig. 4A). The increase in cleaved caspase-3, a key executioner and marker of apoptosis, protein levels at 24 h fits with the reduction of cell proliferation and induction of apoptosis at 24 h after induction of adipogenesis. This indicates that GLP-1 and its receptor are directly involved in the regulation of adipogenesis via activation of proliferation and survival pathways.
To study whether GLP-1 directly regulates the signaling pathways downstream of GLP-1R, 3T3-L1 pre-adipocytes were treated with or without GLP-1 or liraglutide. In accordance with our previous findings, GLP-1 and liraglutide increased Erk1/2, PKCβII, and Akt phosphorylation during differentiation (Fig. 4B) while total Erk1/2, PKC, and Akt protein levels did not change (Fig. 4B). Taken together, this data indicates that GLP-1 and liraglutide activate GLP-1R and mediate cell proliferation and differentiation by regulating the ERK1/2, PKC, and AKT signaling pathways.
To determine which signaling pathways are essential for GLP-1/liraglutide-mediated adipogenic actions, we used specific pathway inhibitors. Following serum starvation, 3T3-L1 pre-adipocytes were incubated in the presence or absence of GLP-1or liraglutide and treated with different kinase inhibitors or kinases alone for 24 h in induction medium. The result showed that GLP-1/liraglutide effects were reduced in cells, where PI3K or MEK1/2 were inhibited. Inhibition of PKC led to complete ablation of GLP-1 or liraglutide-mediated induction of adipogenesis (Fig. 4, C and D). These data are supported by cell proliferation analysis, as we could show that inhibition of either PI3K or MEK1/2 significantly blunted the proliferative effect of GLP-1 or liraglutide. Similar to the differentiation results we observed a complete ablation of GLP-1 or liraglutide mediated proliferation if PKC was inhibited (Fig. 4, E and F). Taken together these data demonstrate that GLP-1, or its synthetic analog liraglutide, regulate cell proliferation through different mechanisms involving PI3K, MEK1/2, and PKC signaling to modulate adipogenesis.
DISCUSSION
It is well known that obesity and the excessive growth of adipose tissue are linked with lymphatic disease (30–32). Furthermore, abnormal subtle lymph leakage from Prox1+/− mice is known to induce adipogenesis (33). However, so far it is unclear which factors contribute to the adipogenic effects of lymph. One prominent member of the gut hormones secreted from the intestinal wall is GLP-1, which is secreted after food intake and which we show here to regulate adipogenesis via its receptor.
We have demonstrated that injection of liraglutide into mice fed a HFD induces in vivo adipocyte differentiation. Liraglutide has been reported to enhance glucose-dependent insulin secretion and inhibit postprandial glucagon secretion (34). Clinical studies have found liraglutide to enhance β-cell function and prevent insulin resistance (35). Other groups have demonstrated the beneficial effects of liraglutide, independent of weight loss, since liraglutide at low concentrations improved cardiac function and increased the survival of the mice without reducing their weight, (36). Interestingly, we observed a stimulation of in vivo adipogenesis, albeit reduced glucose level and reduced weight gain as reported, previously (36). This suggests that even though these mice lose their adipogenic insulin stimulus, liraglutide itself is still able to activate adipogenesis. Moreover, it has been shown that GLP-1 secretion is reduced in obesity (14) and that GLP-1 secretion improves after weight loss (26). Based on our findings it can be speculated that GLP-1 receptor activation acts locally at the tissue level to maintain adipogenesis and mass at an equilibrium set point.
Suppressed adipogenesis, accompanied by an increase in adipocytes size, is linked to increased insulin resistance (7). In contrast, up-regulation of adipocyte differentiation results in increased glucose disposal (37) and high adiponectin secretion (38), both of which are known to enhance insulin sensitivity and prevent excess lipid storage in the liver, heart, or muscle (39). Based on our studies we suggest that in response to food intake, GLP-1 increases the differentiation of adipocyte precursors into mature adipocytes, which may further contribute to enhanced whole body insulin sensitivity (3) by decelerating ectopic lipid accumulation and weight gain.
We show, that GLP-1 and liraglutide mediate their effect on adipogenesis by regulating cell proliferation and apoptosis of pre-adipocytes. Consistent with these results, others have also reported that GLP-1 and liraglutide regulate islet growth and act as differentiation factors of the endocrine pancreas (40). Our data, however, are inconsistent with previous findings showing that GLP-1 prevents human bone marrow-derived mesenchymal stem cell (hMSCs) differentiation into adipocytes (41). Given the fact that hMSCs are not committed adipocyte precursors, this discrepancy might be due to cell line differences.
In agreement with previous data (29), we found that 3T3-L1 cells express GLP-1R. The expression of Glp-1R correlated with PPARγ, a late phase marker of adipocyte differentiation, suggesting that GLP-1R might be a direct target gene of PPARγ and also play a role in mature adipocyte function. Furthermore, we confirmed the function of GLP-1R as a GPCR by measuring an elevation in cAMP levels after GLP-1 or liraglutide treatment. We also show that knockdown of GLP-1R markedly decreases 3T3-L1 pre-adipocyte differentiation. Consistent with this finding, a previous study indicated that male Glp-1r−/− mice are protected from HF diet-induced weight gain (42). Others have reported that Glp-1r−/− mice exhibit glucose intolerance, due to diminished insulin levels, albeit having normal body weight and feeding behavior (43). This could be due to impaired adipogenesis in Glp-1r−/− mice, leading to a decreased glucose uptake and reduced insulin sensitivity. To directly analyze the effect in vivo it would be necessary to knock out GLP-1R in pre-adipocytes, however, unfortunately so far no markers exists that would allow such an experiment.
In line with the suppressed Pparγ expression, we observe here a reduced expression of C/ebpβ and C/ebpδ upon GLP-1R knockdown. These actions of GLP-1 and GLP-1R on early adipogenic regulation events seem to be mediated through cellular proliferation. This is consistent with previous results demonstrating that GLP-1 promotes cellular proliferation of hMSC cells and significantly reduces cell apoptosis (41). These findings, combined with our results, strengthen the notion that GLP-1 or liraglutide regulate adipogenesis via suppression of apoptosis and stimulation of proliferation.
GLP-1, via GLP-1R, activates ERK1/2 in β-cells by Gαs/cAMP/PKA and β-arrestin dependent pathways controlling cell proliferation and apoptosis (21). We observe here, that GLP-1R silencing markedly reduced Erk1/2 phosphorylation, compared with control cells. These findings confirmed the observed decrease in cell proliferation and increase in apoptosis upon GLP-1R knockdown, suggesting that activation of ERK1/2 might be required for the proliferative effect of GLP-1 via its receptor (44).
In addition to ERK signaling, the PKC family plays important roles in various biological functions such as proliferation, differentiation, cell migration and apoptosis (45). Studies have suggested that the PKC pathway is involved in the regulation of adipogenesis (46). In agreement with our findings, increased PKCβII expression has been associated with increased cell proliferation and suppression of apoptosis, which was reported in distal colon crypt cells (47).
Previous studies reported that interference with the IRS-1 and AKT associated signaling pathways represses adipogenesis (48). Activation of these pathways induces Pparγ and C/ebpα expression during 3T3-L1 adipocyte differentiation and inactivation of the AKT signal can induce apoptosis (49, 50). In line with these findings we observed decreased pAkt levels upon GLP-1R knockdown. Activation of the AKT pathway is also known to regulate BAD activity as a survival response (51). Here we show that GLP-1R silencing suppresses BAD phosphorylation, while increasing cleaved-caspase-3 and caspase-3 activity, which could explain the induction of apoptosis. Thus, our data suggest that the reduced adipogenic differentiation of 3T3-L1 cells with a GLP-1R knockdown is partly due to a decrease Erk1/2, PKCβII, and Akt phosphorylation.
To further confirm the mechanism behind the regulation of adipogenesis by GLP-1R, we investigated the effects of different kinase inhibitors. Consistent with our data, it has been shown that inhibiting the PKC pathway with pharmacological inhibitors, such as Ro318220 and Go6976, inhibits adipocyte differentiation (52). At low doses inhibition of PKC signaling significantly decreased both GLP-1 and liraglutide mediated induction of both cell proliferation and differentiation. These results further confirm that the PKC pathway is involved in mediating liraglutide and GLP-1 signals that regulate pre-adipocyte proliferation, apoptosis, and differentiation.
In conclusion, our data show cross-talk between the intestinal wall and adipose tissue through GLP-1. GLP-1 directly activates adipogenesis through GLP-1R. The underlying signaling pathway involves activation of PKC, ERK, and AKT, which leads to altered proliferation, apoptosis and differentiation. Our findings suggest that GLP-1 and its receptor might contribute to nutrient-induced adipocyte development and thereby might influence whole body energy metabolism.
Supplementary Material
This work was supported by the ERC (AdipoDif) and by the SNF.

This article contains supplemental Figs. S1 and S2.
- GLP
- glucagon-like peptide
- DPP
- dipeptidyl-peptidase
- BAD
- Bcl-xL/Bcl-2-associated death
- PPAR
- peroxisome proliferator-activated receptor.
REFERENCES
- 1. Bays H. E., González-Campoy J. M., Bray G. A., Kitabchi A. E., Bergman D. A., Schorr A. B., Rodbard H. W., Henry R. R. (2008) Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev. Cardiovasc. Ther. 6, 343–368 [DOI] [PubMed] [Google Scholar]
- 2. Hausman D. B., DiGirolamo M., Bartness T. J., Hausman G. J., Martin R. J. (2001) The biology of white adipocyte proliferation. Obes. Rev. 2, 239–254 [DOI] [PubMed] [Google Scholar]
- 3. Kahn B. B., Flier J. S. (2000) Obesity and insulin resistance. J. Clin. Invest. 106, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaplan N. M. (1998) Obesity in hypertension effects on prognosis and treatment. J. Hypertens. Suppl. 16, S35–S37 [PubMed] [Google Scholar]
- 5. Jo J., Gavrilova O., Pack S., Jou W., Mullen S., Sumner A. E., Cushman S. W., Periwal V. (2009) Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput. Biol. 5, e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kadowaki T., Yamauchi T. (2005) Adiponectin and adiponectin receptors. Endocr. Rev. 26, 439–451 [DOI] [PubMed] [Google Scholar]
- 7. Meissburger B., Stachorski L., Röder E., Rudofsky G., Wolfrum C. (2011) Tissue inhibitor of matrix metalloproteinase 1 (TIMP1) controls adipogenesis in obesity in mice and in humans. Diabetologia 54, 1468–1479 [DOI] [PubMed] [Google Scholar]
- 8. Wolfrum C., Shih D. Q., Kuwajima S., Norris A. W., Kahn C. R., Stoffel M. (2003) Role of Foxa-2 in adipocyte metabolism and differentiation. J. Clin. Invest. 112, 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffstedt J., Arner E., Wahrenberg H., Andersson D. P., Qvisth V., Löfgren P., Rydén M., Thörne A., Wirén M., Palmér M., Thorell A., Toft E., Arner P. (2010) Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 53, 2496–2503 [DOI] [PubMed] [Google Scholar]
- 10. Cornelius P., MacDougald O. A., Lane M. D. (1994) Regulation of adipocyte development. Annu. Rev. Nutr. 14, 99–129 [DOI] [PubMed] [Google Scholar]
- 11. Hwang C. S., Loftus T. M., Mandrup S., Lane M. D. (1997) Adipocyte differentiation and leptin expression. Annu. Rev. Cell Dev. Biol. 13, 231–259 [DOI] [PubMed] [Google Scholar]
- 12. Körner A., Blüher S., Kapellen T., Garten A., Klammt J., Kratzsch J., Kiess W. (2005) Obesity in childhood and adolescence: a review in the interface between adipocyte physiology and clinical challenges. Hormones 4, 189–199 [DOI] [PubMed] [Google Scholar]
- 13. Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K. L., Armstrong D., Ducy P., Karsenty G. (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 [DOI] [PubMed] [Google Scholar]
- 14. Holst J. J. (2007) The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 [DOI] [PubMed] [Google Scholar]
- 15. Lovshin J. A., Drucker D. J. (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 5, 262–269 [DOI] [PubMed] [Google Scholar]
- 16. Hayes M. R., Kanoski S. E., Alhadeff A. L., Grill H. J. (2011) Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity 19, 1342–1349 [DOI] [PubMed] [Google Scholar]
- 17. Drucker D. J. (2006) The biology of incretin hormones. Cell Metab. 3, 153–165 [DOI] [PubMed] [Google Scholar]
- 18. Huang Y., Wilkinson G. F., Willars G. B. (2010) Role of the signal peptide in the synthesis and processing of the glucagon-like peptide-1 receptor. Br. J. Pharmacol. 159, 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y., Tweedie D., Mattson M. P., Holloway H. W., Greig N. H. (2010) Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem. 113, 1621–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montrose-Rafizadeh C., Yang H., Wang Y., Roth J., Montrose M. H., Adams L. G. (1997) Novel signal transduction and peptide specificity of glucagon-like peptide receptor in 3T3-L1 adipocytes. 37 J. Cell. Physiol. 172, 275–283 [DOI] [PubMed] [Google Scholar]
- 21. Quoyer J., Longuet C., Broca C., Linck N., Costes S., Varin E., Bockaert J., Bertrand G., Dalle S. (2010) GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β-arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J. Biol. Chem. 285, 1989–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zander M., Madsbad S., Madsen J. L., Holst J. J. (2002) Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359, 824–830 [DOI] [PubMed] [Google Scholar]
- 23. Drucker D. J., Dritselis A., Kirkpatrick P. (2010) Liraglutide. Nat. Rev. Drug Discov. 9, 267–268 [DOI] [PubMed] [Google Scholar]
- 24. Knudsen L. B., Nielsen P. F., Huusfeldt P. O., Johansen N. L., Madsen K., Pedersen F. Z., Thøgersen H., Wilken M., Agersø H. (2000) Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J. Med. Chem. 43, 1664–1669 [DOI] [PubMed] [Google Scholar]
- 25. Rask E., Olsson T., Söderberg S., Johnson O., Seckl J., Holst J. J., Ahrén B. (2001) Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 24, 1640–1645 [DOI] [PubMed] [Google Scholar]
- 26. Verdich C., Toubro S., Buemann B., Lysgård Madsen J., Juul Holst J., Astrup A. (2001) The role of postprandial releases of insulin and incretin hormones in meal-induced satiety–effect of obesity and weight reduction. Int. J. Obes. Relat. Metab. Disord. 25, 1206–1214 [DOI] [PubMed] [Google Scholar]
- 27. Buteau J. (2008) GLP-1 receptor signaling: effects on pancreatic beta-cell proliferation and survival. Diabetes Metab. 34, Suppl. 2, S73–S77 [DOI] [PubMed] [Google Scholar]
- 28. Meissburger B., Ukropec J., Roeder E., Beaton N., Geiger M., Teupser D., Civan B., Langhans W., Nawroth P. P., Gasperikova D., Rudofsky G., Wolfrum C. (2011) EMBO Mol. Med. 3, 637–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egan J. M., Montrose-Rafizadeh C., Wang Y., Bernier M., Roth J. (1994) Glucagon-like peptide-1(7–36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology 135, 2070–2075 [DOI] [PubMed] [Google Scholar]
- 30. Lo Y. F., Cheung Y. C., Hsueh S., Ho K. C. (2009) Feasibility of sentinel lymph node biopsy in multifocal/multicentric breast cancer. Chang Gung Med. J. 32, 51–58 [PubMed] [Google Scholar]
- 31. Tong J., Tschöp M. H., Aulinger B. A., Davis H. W., Yang Q., Liu J., Gaylinn B. D., Thorner M. O., D'Alessio D., Tso P. (2010) The intestinal lymph fistula model–a novel approach to study ghrelin secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G474–G480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von der Weid P. Y., Rainey K. J. (2010) Review article: lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Aliment Pharmacol. Ther. 32, 697–711 [DOI] [PubMed] [Google Scholar]
- 33. Harvey N. L., Srinivasan R. S., Dillard M. E., Johnson N. C., Witte M. H., Boyd K., Sleeman M. W., Oliver G. (2005) Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 37, 1072–1081 [DOI] [PubMed] [Google Scholar]
- 34. Bregenholt S., Møldrup A., Blume N., Karlsen A. E., Nissen Friedrichsen B., Tornhave D., Knudsen L. B., Petersen J. S. (2005) The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits β-cell apoptosis in vitro. Biochem. Biophys. Res. Commun. 330, 577–584 [DOI] [PubMed] [Google Scholar]
- 35. Mari A., Degn K., Brock B., Rungby J., Ferrannini E., Schmitz O. (2007) Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care 30, 2032–2033 [DOI] [PubMed] [Google Scholar]
- 36. Noyan-Ashraf M. H., Momen M. A., Ban K., Sadi A. M., Zhou Y. Q., Riazi A. M., Baggio L. L., Henkelman R. M., Husain M., Drucker D. J. (2009) GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58, 975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dubuisson O., Dhurandhar E. J., Krishnapuram R., Kirk-Ballard H., Gupta A. K., Hegde V., Floyd E., Gimble J. M., Dhurandhar N. V. (2011) PPARgamma-independent increase in glucose uptake and adiponectin abundance in fat cells. Endocrinology, 152, 3649–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee N. K., Karsenty G. (2008) Reciprocal regulation of bone and energy metabolism. Trends Endocrinol. Metab. 19, 161–166 [DOI] [PubMed] [Google Scholar]
- 39. Berg A. H., Scherer P. E. (2005) Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 96, 939–949 [DOI] [PubMed] [Google Scholar]
- 40. Egan J. M., Bulotta A., Hui H., Perfetti R. (2003) GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab. Res. Rev. 19, 115–123 [DOI] [PubMed] [Google Scholar]
- 41. Sanz C., Vázquez P., Blázquez C., Barrio P. A., Alvarez Mdel M., Blázquez E. (2010) Signaling and biological effects of glucagon-like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am. J. Physiol. Endocrinol. Metab. 298, E634–643 [DOI] [PubMed] [Google Scholar]
- 42. Hansotia T., Maida A., Flock G., Yamada Y., Tsukiyama K., Seino Y., Drucker D. J. (2007) Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J. Clin. Invest. 117, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thorens B. (1992) Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci. U.S.A. 89, 8641–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bost F., Aouadi M., Caron L., Binétruy B. (2005) The role of MAPKs in adipocyte differentiation and obesity. Biochimie 87, 51–56 [DOI] [PubMed] [Google Scholar]
- 45. Clemens M. J., Trayner I., Menaya J. (1992) The role of protein kinase C isoenzymes in the regulation of cell proliferation and differentiation. J. Cell Sci. 103, 881–887 [DOI] [PubMed] [Google Scholar]
- 46. Lacasa D., Agli B., Giudicelli Y. (1995) Zeta PKC in rat preadipocytes: modulation by insulin and serum mitogenic factors and possible role in adipogenesis. Biochem. Biophys. Res. Commun. 217, 123–130 [DOI] [PubMed] [Google Scholar]
- 47. Davidson L. A., Brown R. E., Chang W. C., Morris J. S., Wang N., Carroll R. J., Turner N. D., Lupton J. R., Chapkin R. S. (2000) Morphodensitometric analysis of protein kinase C β(II) expression in rat colon: modulation by diet and relation to in situ cell proliferation and apoptosis. Carcinogenesis 21, 1513–1519 [PubMed] [Google Scholar]
- 48. Baudry A., Yang Z. Z., Hemmings B. A. (2006) PKBα is required for adipose differentiation of mouse embryonic fibroblasts. J. Cell Sci. 119, 889–897 [DOI] [PubMed] [Google Scholar]
- 49. Kim J. E., Chen J. (2004) Regulation of peroxisome proliferator-activated receptor-γ activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 53, 2748–2756 [DOI] [PubMed] [Google Scholar]
- 50. Kortum R. L., Costanzo D. L., Haferbier J., Schreiner S. J., Razidlo G. L., Wu M. H., Volle D. J., Mori T., Sakaue H., Chaika N. V., Chaika O. V., Lewis R. E. (2005) The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol. Cell. Biol. 25, 7592–7604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Friedrichsen B. N., Neubauer N., Lee Y. C., Gram V. K., Blume N., Petersen J. S., Nielsen J. H., Møldrup A. (2006) Stimulation of pancreatic β-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signaling pathways. J. Endocrinol. 188, 481–492 [DOI] [PubMed] [Google Scholar]
- 52. Zhou Y., Wang D., Li F., Shi J., Song J. (2006) Different roles of protein kinase C-βI and -δ in the regulation of adipocyte differentiation. Int. J. Biochem. Cell Biol. 38, 2151–2163 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.