Background: Tau phosphorylation regulates its functions and is increased in Alzheimer disease.
Results: Novel live cell assay of Tau protein-protein interaction with Pin1 showed that GABAA receptor activity regulates Tau phosphorylation.
Conclusion: GABAA receptor activity is associated with regulation of Tau phosphorylation.
Significance: Learning about Tau regulation and functions is crucial for understanding basic neurobiology, as well as mechanisms of neurodegeneration.
Keywords: Alzheimer Disease, Cytoskeleton, GABA Receptors, Neurodegeneration, Protein Phosphorylation, Protein-Protein Interactions, Tau, Pharmaceuticals, Sedative, Tauopathies
Abstract
Abnormal phosphorylation and aggregation of the microtubule-associated protein Tau are hallmarks of various neurodegenerative diseases, such as Alzheimer disease. Molecular mechanisms that regulate Tau phosphorylation are complex and currently incompletely understood. We have developed a novel live cell reporter system based on protein-fragment complementation assay to study dynamic changes in Tau phosphorylation status. In this assay, fusion proteins of Tau and Pin1 (peptidyl-prolyl cis-trans-isomerase 1) carrying complementary fragments of a luciferase protein serve as a sensor of altered protein-protein interaction between Tau and Pin1, a critical regulator of Tau dephosphorylation at several disease-associated proline-directed phosphorylation sites. Using this system, we identified several structurally distinct GABAA receptor modulators as novel regulators of Tau phosphorylation in a chemical library screen. GABAA receptor activation promoted specific phosphorylation of Tau at the AT8 epitope (Ser-199/Ser-202/Thr-205) in cultures of mature cortical neurons. Increased Tau phosphorylation by GABAA receptor activity was associated with reduced Tau binding to protein phosphatase 2A and was dependent on Cdk5 but not GSK3β kinase activity.
Introduction
Many neurodegenerative diseases are characterized by cerebral accumulation of proteinaceous aggregates. Neurodegenerative tauopathies are a group of disorders that includes Alzheimer disease (AD)2 and frontotemporal dementias (1, 2). Tauopathies share a common neuropathological hallmark, the neurofibrillary tangles that are composed of aberrantly phosphorylated forms of the microtubule (MT)-associated protein Tau. Mutations in the MAPT gene encoding the Tau protein have been associated with neurodegenerative diseases, such as familial frontotemporal dementia with parkinsonism linked to chromosome 17q21 (FTDP-17) (3). Despite their diverse disease phenotypes, degeneration of neurons and the resulting brain dysfunction in tauopathies is linked to deregulation of Tau phosphorylation and progressive intraneuronal accumulation of filamentous Tau inclusions.
In addition to its well established microtubule binding role, various cell signaling functions of Tau have been reported (reviewed in Ref. 4). Tau can modulate various neuronal functions, such as cytoskeletal reorganization, axonal transport, NGF signaling, stress response, and neurogenesis. In healthy neurons a spatial gradient of Tau, whose concentration is greater in axons than in somatodendritic compartments, is maintained. In neurodegenerative diseases, such as AD, the gradient becomes inverted, potentially disrupting normal microtubule-associated functions such as axonal transport (5, 6). Recent reports suggest that hyperphoshorylation-induced dendritic (mis)localization of Tau may directly cause synaptic abnormalities in the dendritic spines (7) and also promote synaptotoxicity of β-amyloid (8), a central pathogenic peptide accumulating in the brains of AD patients. Interestingly, changes in Tau phosphorylation status leading to Tau pathology have a temporally specific sequence (9, 10).
There are 84 potential serine and threonine phosphate acceptor residues in the longest human Tau isoform, of which ∼30 have been reported to be phosphorylated in vivo (1). The phosphorylation sites are located in regions close to the MT binding repeats, and it has been well established that increased Tau phosphorylation negatively regulates MT binding. Because of the central involvement of aberrant Tau phosphorylation in many neurodegenerative diseases, significant research efforts have focused on the protein kinases and protein phosphatases that regulate Tau phosphorylation. Currently, it is unclear whether all of them participate in Tau phosphorylation under physiological or pathological conditions in vivo (2). From the drug target perspective, particular attention has been paid to two proline-directed kinases, glycogen synthase kinase 3β (GSK3β) and cyclin-dependent kinase 5 (Cdk5), as Tau kinases. Proline-directed kinases phosphorylate Tau at 14 serine-proline (SP) or threonine-proline (TP) motifs located in the proline-rich regions flanking the microtubule binding domains of Tau (11, 12). Because phosphorylation at SP/TP sites of Tau is a striking feature in patients with AD and other tauopathies, these sites are commonly referred to as “disease-associated” sites.
A number of phosphatases, including protein phosphatase (PP)1, PP2A, PP2B, and PP5, that mediate Tau dephosphorylation have been identified, but the exact role(s) of these phosphatases under physiological and pathological conditions remain to be addressed. Importantly, proline can adopt two completely different conformational states, providing a phosphorylation-dependent structural switch (13). Peptidyl-prolyl cis/trans-isomerase (PPIase) Pin1 (protein interacting with NIMA (never in mitosis A)-1) is a phosphorylation-specific PPIase that controls the access of phosphatases to proline-directed phosphoepitopes (14, 15). Pin1 promotes dephosphorylation of Tau at the SP/TP sites via trans-specific PP2A, and in Pin1 knock-out mice Tau is hyperphosphorylated in several SP/TP sites (16). Cis-pTau is resistant to SP/TP-dephosphorylation, and compromised Pin1 activity in neurons leads to a loss of MT binding, hyperphosphorylation of Tau, and formation of neurofibrillary tangles. Therefore, Pin1 is a critical regulator of Tau dephosphorylation and restores Tau function by catalyzing cis- to trans-isomerization (17).
Various approaches have been used to identify compounds with the potential to modulate Tau phosphorylation as a disease-modifying strategy in neurodegenerative diseases (2). Here, we have developed a dynamic assay system capable of measuring protein-protein interactions (PPIs) of Tau in live cells. A luminescence-based protein-fragment complementation assay (PCA) (or bimolecular luminescence complementation) using split humanized Gaussia princeps luciferase (hGLuc) (18) was developed for dynamic detection of Tau PPIs in cells. We used Tau and Pin1 as a reporter pair to screen a focused library of pharmaceutical compounds to test functionality of the Tau-based PCA. Several GABAA receptor modulators were found to increase Tau-Pin1 interaction. Importantly, these compounds significantly increased Tau phosphorylation at the AT8 epitope (Ser-199/Ser-202/Thr-205) in mature rat cortical neurons in a Cdk5-dependent manner. Tau phosphorylation at Ser-199/Ser-202/Thr-205 remained elevated at least for 24 h after washout of the drugs. These data suggest that hGLuc PCA is a dynamic and sensitive live cell assay to measure Tau PPIs and identify novel modulators of Tau phosphorylation. Our data suggest that GABAA activity and regulation of Tau phosphorylation are connected via a mechanism that involves both Cdk5 and PP2A.
EXPERIMENTAL PROCEDURES
Chemicals
The Pin1 inhibitor used in this study was 5-hydroxy-1,4-naphtoquinone (juglone) from Sigma. Cdk5 (roscovitine) and PP2A inhibitors (calyculin A) were from Calbiochem. GSK3β inhibitor (SB216763) and GABA receptor modulators (muscimol, picrotoxin, and bicuculline) were purchased from Tocris.
DNA Constructs
The hGLuc expression plasmids were constructed in the pcDNA3.1/zeo (Invitrogen) backbone. The original humanized G. princeps PCA plasmids (18) were donated by Prof. Stephen Michnick (Université de Montréal, Montreal, Canada). The human cDNAs for Tau (GenBankTM accession number BC114948; 0N4R), Pin1 (GenBankTM accession number NM_006221), GSK3β (GenBankTM accession number BC000251), regulatory Bα subunit of PP2A (GenBankTM accession number BC041071), and p35 (GenBankTM accession number BC020580) were purchased from Open Biosystems/Thermo Scientific. In all the hGLuc PCA constructs used in this study, the hGLuc fragment was placed in the C terminus separated by a (GGGGS)2SG linker. The fusion protein design is graphically summarized in Fig. 1A. The cDNA for human GSK3β was PCR-cloned to pcDNA3 vector (Invitrogen) using EcoRI-XhoI restriction sites. The identity of all constructs was confirmed by DNA sequencing. pEGFP-tubulin plasmid encoding human α-tubulin fused with EGFP was from Clontech.
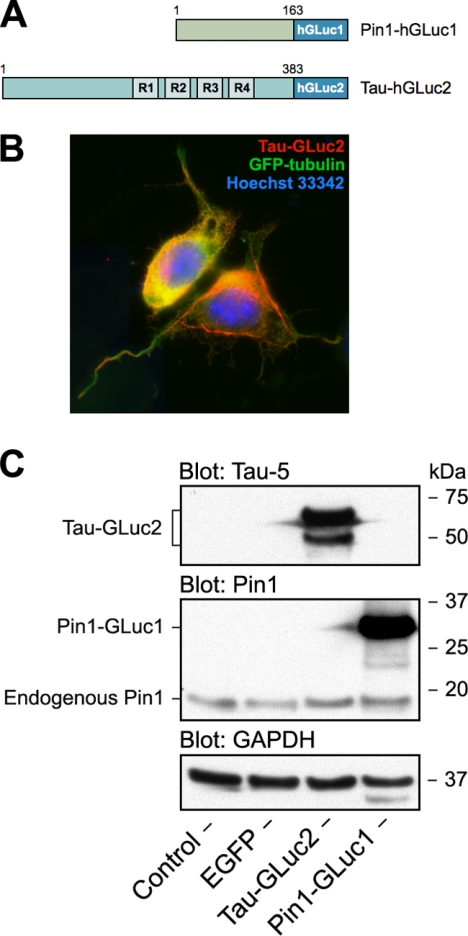
FIGURE 1.
hGLuc-based PCA strategy for studying protein-protein interactions of Tau. A, schematic presentation of hGLuc fragment-tagged reporter constructs used in this study for live cell analysis of protein-protein interactions of Tau. 0N4R isoform of human Tau was used throughout the study. B, immunofluorescence micrograph of N2a cell coexpressing Tau-GLuc2 and EGFP-tubulin. The nuclei were counter-stained with Hoechst 33342. C, Western blot analysis of expression of GLuc fragment-tagged reporter constructs in N2a cells. The blots were stained with antibodies to Tau (Tau-5), Pin1, and GAPDH as a loading control.
Cell Culture and Transfection
Mouse Neuro-2A (N2a) neuroblastoma cells (ATCC) were grown in DMEM supplemented with 10% (v/v) FBS (Invitrogen), 1% (v/v) l-glutamine-penicillin-streptomycin solution (Lonza) at 37 °C in a water-saturated air, 5% CO2 atmosphere. N2a cells were transfected with JetPEI (Polyplus) according to the manufacturer's instructions. For the primary neuronal cultures, the cortex was dissected from E18 rat embryos, and the tissue dissociated in papain solution (50 μg/ml in 10 mg of dl-cysteine-HCl, 10 mg of BSA, 250 mg of glucose, and 50 ml of PBS for 10 min at 37 °C). Next, the cells were triturated and suspended in a medium containing 9.8 ml of Ca2+/Mg2+ free Hanks' balanced salt solution, 1 mm sodium pyruvate, 10 mm HEPES, and 10 μl of DNase I. The cells were plated onto poly-l-lysine coated 12-well culture plates at a cell density of 400 000 ml−1. The cells were maintained in neurobasal medium containing 2% B27 supplement, 1% penicillin/streptomycin, and 1% glutamine (5% CO2, +37 °C). Primary neurons at DIV 21–22 were treated with selected compounds for 6–48 h before assessment of Tau phosphorylation on Western blots.
Protein-Fragment Complementation Assay
N2a cells were plated on poly-l-lysine-coated white-wall 96-well plates (PerkinElmer Life Sciences). 100 ng of plasmid DNA was used for transient transfection per well, divided as follows: 47.5 ng of GLuc1 reporter plasmid, 47.5 ng of GLuc2 reporter plasmid, and 5 ng of pRC/CMV-β-galactosidase (βGal) as an internal vector control. Experiments were carried out 48 h post-transfection in serum-free conditions. Briefly, the cells were washed with PBS and changed to phenol red-free DMEM (Invitrogen) without serum. Test compounds were added to this medium, and the cells were incubated for 2–4 h. Me2SO was used as vehicle control. To detect the hGLuc PCA signal, the cells were injected well-by-well with 25 μl (final concentration, 20 μm) of native coelenterazine (Nanolight Technology), and emitted luminescence was detected immediately by flash luminometry using a Victor3 plate reader (PerkinElmer Life Sciences). Four replicate wells were used per experiment. Individual experiments were repeated three or four times. After measuring the PCA signal, βGal assay was performed after lysis of the cells with 20 μl of lysis buffer/well (120 mm Tris-HCl, pH 7.5, 120 mm NaCl, 6 mm MgSO4, 6% Triton X-100) and incubation for 15 min at room temperature with mild shaking. Then 45 μl of βGal substrate o-nitrophenyl-β-d-galactopyranoside (Sigma, 4 mg/ml stock in sterile water) solution was combined with 55 μl of cleavage buffer (120 mm sodium phosphate, pH 7.0, 24 mm KCl, 2.4 mm MgSO4, and 2.4 mm DTT), and 100 μl of this solution was added per well. The plates were incubated at +37 °C for a further 30 min. Finally, absorbance was read at 405 nm. PCA signals were normalized to βGal signals/well.
Screening of Pharmaceutical Compound Library
The focused chemical library used in this study contained 240 clinically approved drugs in different therapy areas, as well as some drug metabolites and other pharmaceutical reagents. These compounds were obtained mostly from commercial sources and stored as 10 mm stock solutions in Me2SO in a 96-well plate format. PCA screening was carried out with Pin1-GLuc1 and Tau-GLuc2 as reporters (50 ng + 50 ng of plasmids/well) in 96-well formats. The procedure followed the general PCA protocol described above with the following exceptions: no βGal internal vector control-based PCA signal normalization was performed, and plates were injected with coelenterazine and read with Varioskan Flash multimode plate reader (Thermo Fisher Scientific). Library compounds were diluted into assay media and added to the cells in final concentration of 50 μm by using a Biomek FX liquid handling work station (Beckman Coulter). The cells were incubated for 2 h at 37 °C before PCA analysis. Me2SO was used as vehicle control and 5 μm juglone as a negative control. Four replicate wells/compound were used. After primary screening, secondary testing and dose-response experiments (2-fold dilution series with five concentrations starting from 50 μm) were carried out with a selected set of compounds using freshly prepared stock solutions.
Western Blotting
The cells were washed twice with ice-cold PBS followed by scraping and extraction on ice for 30 min in a buffer containing 10 mm Tris-HCl, pH 6.8, 1 mm EDTA, 150 mm NaCl, 0.25% Nonidet P-40, 1% Triton X-100, 1 μm NaF, PhosStop phosphatase inhibitor, and protease inhibitor mixture tablets (both from Roche Applied Science). Cell debris was removed by a centrifugation at 16,000 × g. The protein concentrations were determined using the BCA protein assay kit (Pierce/Thermo). For Western blot analysis, equal amounts of total protein (25–40 μg) per lane was resolved in a 4–12% gradient Bis-Tris gels (Novex, Invitrogen) under reducing conditions and transferred to PVDF membranes (Amersham Biosciences/GE Healthcare). The filters were probed with the following antibodies: Tau-5 (Invitrogen), phospho-Ser9 GSK3β (Cell Signaling Technology), Tau/AT8 (Invitrogen), Tau/TG3 (Dr. Jin-Jing Pei, Karolinska Institutet, Sweden), Tau/PHF13 (Cell Signaling Technology), Pin1 (Santa Cruz Biotechnology), and GAPDH (Millipore). After incubation with horseradish-conjugated secondary antibodies, the signal was developed using ECL Western blotting detection reagent (Pierce/Thermo). Western blot images were quantitated using Quantity One software package (Bio-Rad).
Immunofluorescence Microscopy
N2a cells were plated on poly-l-lysine-coated (Sigma) glass coverslips and transiently transfected with pEGFP-tubulin and phGLuc(2)-Tau plasmids in 1:1 ratio. The cells were fixed with 3% paraformaldehyde/PBS, permeabilized with 0.1% Triton X-100/PBS for 10 min and blocked first with 10 mm NH4Cl/PBS and then with 2% BSA/PBS. Overexpressed Tau-GLuc2 was detected with Tau-5 antibody (Invitrogen) and Alexa Fluor-conjugated secondary antibody (Molecular Probes/Invitrogen). The nuclei were counterstained with Hoechst 33342 (Molecular Probes/Invitrogen). Microscope images were acquired using a Zeiss Axio Imager M1 epifluorescence microscope equipped with an AxioCam HRm CCD camera.
Protein Phosphatase 2A Assay
To evaluate protein phosphatase 2A (PP2A) enzyme activity, we performed a PP2A assay using the serine/threonine phosphatase assay system from Promega (Madison, WI) (catalogue number V2460). Rat cortical neuron cell lysates (21 DIV) were processed as suggested by the manufacturer to remove particulate matter and endogenous free phosphate. For PP2A activity, 2.0 μl of the sample solution was prepared in duplicate, and the release of phosphate from a chemically synthesized phosphopeptide was assessed over a period of 30 min in PP2A buffer (250 mm imidazole, 1 mm EGTA, 0.1% β-mercaptoethanol, 0.5 mg/ml BSA). The amount of phosphate released was measured by the absorbance of the molybdate-malachite green-phosphate complex at 620 nm.
Statistical Analyses
Statistical analyses were performed using analysis of variance (three or more groups; followed by Bonferroni's post-tests) or Student's t test (two groups) in GraphPad Prism software. Significance was placed at p < 0.05.
RESULTS
Live Cell Detection of Pin1-Tau Protein-Protein Interaction with hGLuc PCA
Because Pin1 is a critical regulator of PP2A-mediated dephosphorylation of Tau, we hypothesized that detection of Pin1-Tau interaction by a luminescence-based PCA may serve as a good starting point for developing live cell Tau PPI assays. We chose a recently developed variant of the PCA that is based on humanized form of G. princeps luciferase (hGLuc) (18) to develop a live cell assay that would be suitable for high throughput purposes. Various fusion constructs using the complementary GLuc fragments were generated (Fig. 1A) and tested in N2a mouse neuroblastoma cells. Identity and expression of the PCA constructs were verified by DNA sequencing and Western blotting (Fig. 1C). To assess the normal functionality of the Tau-GLuc2 fusion protein, we analyzed its subcellular localization by immunofluorescence microscopy. Localization to neurites and cytoskeletal structures together with GFP-tubulin suggests that the hGLuc fusion tag does not interfere with normal cellular localization and functions of Tau (Fig. 1B).
To test whether the Pin1-Tau PCA signal, indicative of protein-protein interaction between the two proteins, is responsive to Pin1 inhibition, we treated the cells with juglone for 2 h and then measured Pin1-Tau PCA signal. Juglone is a natural compound and a selective, cell-permeable, and irreversible inhibitor of parvulin-like PPIases, such as Escherichia coli parvulin and human Pin1 (IC50 of ∼1.5 μm) (19). As shown in Fig. 2A, juglone dose-dependently decreased Pin1-Tau interaction in N2a cells as measured with hGLuc PCA. Maximal inhibition (86%) was achieved at 5 μm juglone concentration.
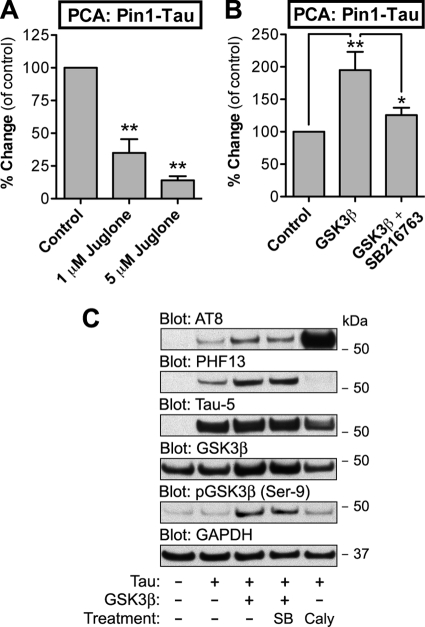
FIGURE 2.
Validation of GLuc-based PCA for detection of Tau-Pin1 interaction. A, N2a cells were transiently transfected with Pin1-GLuc1 and Tau-GLuc2 constructs. 48 h post-transfection, the cells were treated for 2 h with 1 or 5 μm juglone. Luminescence signal was measured by flash luminometry in live cells. The luminescence values were normalized by corresponding data from β-galactosidase assay (internal vector control, per well). B, N2a cells were transfected as in A with either empty mock plasmid or pcDNA3-GSK3β. Some of the cells expressing GSK3β were treated with GSK3β inhibitor SB216763 for 2 h, whereas other cells were treated with vehicle. PCA signal was measured as in A. The average values are displayed as percentages of change as compared with vehicle-treated control cells (means ± S.E.; four replicate wells/experiment, four independent experiments). C, N2a cells were transiently transfected with mock plasmid or plasmids encoding Tau-GLuc2 or GSK3β as indicated. The cells were treated with vehicle, 100 nm SB216763 (SB) or 10 nm calyculin A for 2 h before harvest. Cell extracts were analyzed on Western blots with AT8 and PHF13 phospho-specific Tau antibodies and Tau-5 for total Tau levels. GSK3β and phospho-GSK3β (Ser9) antibodies were used to verify GSK3β overexpression. GAPDH antibody was used as a loading control. * and ** indicate significant differences with p < 0.05 and p < 0.01, respectively.
Glycogen synthase kinase-3β (GSK3β) is one of the central Tau kinases (2, 20), and its overexpression in mammalian cells results in increased Tau phosphorylation in several sites including the AT8 epitope (21). We measured Pin1-Tau interaction by PCA in cells cotransfected with empty mock plasmid or plasmid encoding GSK3β. As shown in Fig. 2B, overexpression of GSK3β nearly doubled Pin1-Tau interaction as measured by the PCA. This effect was abolished if the GSK3β-expressing cells were treated with a specific GSK3β inhibitor SB216763. Western blots of N2a cells transfected with Tau-GLuc2 alone or together with GSK3β showed that GSK3β overexpression promotes phosphorylation of the Tau-GLuc2 fusion protein in at least AT8 and PHF13 epitopes (Fig. 2C). These functional validation data suggest that the Pin1-Tau PCA reporter system responds bidirectionally to stimuli that alter either Pin1 or Tau status in the cells.
Screening of Small Molecule Modulators of Tau-Pin1 Interaction with hGLuc PCA
Next, we screened a focused library of 240 pharmaceutical compounds to identify modulators of Tau-Pin1 interaction. Before the screen, the Tau-Pin1 PCA assay was optimized for parameters, such as vehicle (Me2SO) tolerance and intra- and interplate variance of signal (data not shown). Four replicate wells were used. The primary screen yielded 25 initial hits of which 21 increased (PCA signal more than 200% of control) and four decreased (PCA signal less than 75% of control) the Pin1-Tau interaction compared with vehicle-treated control (Table 1 and supplemental Table S1). The relatively high hit rate (10.4%) may be partially explained by the focused nature of the library. Also, cell-based screens tend to have higher hit rates than biochemical screens. Two compounds were determined to be incompatible with flash luminometry-based live cell PCA because they produced almost a complete loss of signal in the Tau-based screen, as well as in a screening assay on metabolism of β-amyloid precursor protein, which we performed simultaneously.3 A set of 14 compounds was selected for secondary screening and dose-response experiments based on primary screening data and compound characteristics (e.g. known therapeutic function; supplemental Table S2). In the secondary screening, the responses of selected compounds were consistently somewhat lower than in the primary screen. This may be explained by small differences in the assay setup between the primary and secondary screens, such as the use of β-galactosidase normalization and the lower sensitivity of plate reader used.
TABLE 1.
Summary of results from Pin1-Tau PCA screen
| Compounds screened | 240 |
| Total number of hits | 27 |
| Total number of excluded hits based on assay incompatibility | 2 |
| Hit rate (%) | 10.4 |
| Total number of compounds increasing the interaction | 21 |
| Total number of compounds decreasing the interaction | 4 |
| Most commonly repeated drug classes | |
| Sedatives (barbiturates or benzodiazepines) | 5 |
| Sulfonamide antibacterials | 4 |
Two compound classes were strongly presented among the hits. Sedative-hypnotics and sulfonamide antibacterials presented 43% of all hit compounds increasing the Pin1-Tau signal. The activity of benzodiazepines and barbiturates was confirmed in secondary testing and dose-response experiments, whereas a consistent effect of sulfonamides could not be verified in the follow-up studies (supplemental Table S2). Depending on the sedative, the average increase in Pin1-Tau interaction varied between 1.4- and 2.0-fold as compared with vehicle-treated cells. Desalkylflurazepam, an active metabolite of the benzodiazepine flurazepam, and butethal, a barbiturate, were chosen as representative sedative hit compounds for further studies. As shown in Fig. 3A, both compounds elicited a dose-dependent increase in Pin1-Tau interaction.
FIGURE 3.
Validation of hit compounds with GLuc-based PCA for detection of Tau-Pin1 interaction. N2a cells were transiently transfected with Pin1-GLuc1 and Tau-GLuc2 constructs. 48 h post-transfection, the cells were treated for 2 h with increasing concentrations of desalkylflurazepam or butethal (A) or folic acid (C). In B, cells were treated for 2 h with either 100 nm muscimol or with 100 nm muscimol together with 1 μm picrotoxin. Luminescence signal was measured by flash luminometry in live cells. The luminescence values were normalized by corresponding data from β-galactosidase assay (internal vector control, per well). The average values are displayed as percentages of change as compared with vehicle-treated control cells (means ± S.E.; n = 3). *, **, and *** indicate significant differences with p < 0.05, p < 0.01, and p < 0.001, respectively.
Benzodiazepines (BZ) and barbiturates are widely used sedatives and hypnotics that promote the binding of the major inhibitory neurotransmitter GABA to the GABAA receptors enhancing the GABA-induced ionic currents through these ligand-gated chloride channels (22, 23). Because N2a cells are known to express functional GABAA receptors (24) and both barbiturates and BZ were found among the hit compounds, it is likely that the increased Pin1-Tau interaction results from increased GABA signaling. To confirm this, we used muscimol, a psychoactive alkaloid and a selective agonist of the GABAA receptor, in Pin1-Tau PCA in N2a cells. We also tested whether picrotoxin, a noncompetitive antagonist and channel blocker of GABAA receptors, could block the effect of muscimol. After a 2-h treatment, 100 nm muscimol increased Pin1-Tau PCA signal by nearly 2-fold (Fig. 3B), confirming that increased GABAA receptor activity enhances Pin1-Tau interaction in N2a cells. Moreover, this effect of muscimol was completely blocked by 1 μm picrotoxin, suggesting that the effect of muscimol was specific and dependent on GABAAR activity.
Only four of the 25 hit compounds decreased Pin1-Tau interaction (supplemental Tables S1 and S2). We tested one of these compounds, folic acid, in secondary assays. As shown in Fig. 3C, folic acid caused a dose-dependent decrease in Pin1-Tau interaction, with a maximal −40% effect, as measured by the Pin1-Tau PCA. Interestingly, folate deficiency has been shown to promote Tau phosphorylation in both neuroblastoma cells and in mouse brain (25).
Pharmacological Modulation of GABAA Receptor Promotes Tau Phosphorylation at Ser-199/Ser-202/Thr-205 in Neurons
Next, we tested whether the GABAA modulators identified in the screen affect Tau phosphorylation in mature cortical neurons. Phosphorylation of Tau in Pin1−/− mouse brain is significantly increased in several proline-directed sites relevant to AD pathology, including the AT8, AT180, TG3, MC1, and Alz50 epitopes (16). Because the hit compounds identified in the Pin1-Tau PCA screen may be specific for certain phosphoepitopes recognized by Pin1, we used antibodies AT8 and TG3 that detect Tau phosphorylated at Ser-199/Ser-202/Thr-205 and Thr-231/Ser-235, respectively. As a third antibody we used PHF13 (Ser 396). AT8 and TG3 epitopes but not the PHF13 were reported to be hyperphosphorylated in the Pin1−/− mouse brain (16). The effects of desalkylflurazepam and butethal were tested in cultures of mature (DIV 21) rat cortical neurons that are known to display synaptic activity and GABAergic responses (26, 27). Neurons were treated with increasing concentrations of desalkylflurazepam or butethal for 6 h in normal culture medium and then analyzed for Tau phosphorylation on Western blots. As shown in Fig. 4 (A and B), both compounds increased Tau phosphorylation specifically at the AT8 epitope with no effect on the TG3 and PHF13 epitopes. Desalkylflurazepam appeared to be more potent in these experimental conditions as compared with butethal with a maximum increase of 72% at 1 μm.
FIGURE 4.
GABAA receptor activation promotes Tau phosphorylation at the AT8 epitope in mature cortical neurons. A, primary rat cortical neurons (21 DIV) were treated with indicated concentrations of butethal and desalkylflurazepam for 6 h. As a control, the neurons were treated with vehicle only. Cell extracts were analyzed on Western blots with AT8, TG3, and PHF13 phospho-specific Tau antibodies. GAPDH antibody was used as a loading control. B, optical density quantification of Tau phosphorylation at the AT8 epitope from the Western blot (Fig. 4A). C, primary rat cortical neurons (21 DIV) were treated with 50 μm butethal (BUT) and desalkylflurazepam (DAF) for 48 h or treated for 24 followed by a 24-h washout period. As a control, the neurons were treated with vehicle only. The samples were analyzed as in Fig. 4A. Veh, vehicle.
To understand the stability of the effect of GABAA modulators on Tau phosphorylation, we next performed a washout experiment. 21 DIV cortical neurons were treated with 50 μm desalkylflurazepam or butethal for 48 h or treated for 24 h followed by a 24-h washout period before the analysis of Tau phosphorylation. The phosphorylation at the AT8 epitope remained at an elevated level even after the 24-h washout (Fig. 4C). These results suggest that GABAA activity can cause a specific increase of Tau phosphorylation at the AT8 epitope, and this effect remains even after a 24-h washout period.
GABAA Receptor Modulators Do Not Affect PP2A Activity in Cell-free System but Reduce PP2A-Tau Interaction in Live N2a Cells
A recent study found that anesthesia promotes hyperphosphorylation of Tau in several epitopes, including AT8 (28). The authors suggested that the anesthesia effect on Tau phosphorylation was due to PP2A inhibition, likely because of anesthesia-induced hypothermia. On the other hand, barbiturates have been shown to directly inhibit phosphatases, such as calcineurin/PP2B, another phosphatase associated with Tau dephosphorylation (29). We used an in vitro PP2A assay to test direct effects of desalkylflurazepam and butethal on PP2A activity. As shown in Fig. 5A, no effects on PP2A activity were detected using desalkylflurazepam or butethal, whereas calyculin A (PP2A inhibitor) significantly reduced PP2A activity in this cell-free enzyme activity assay.
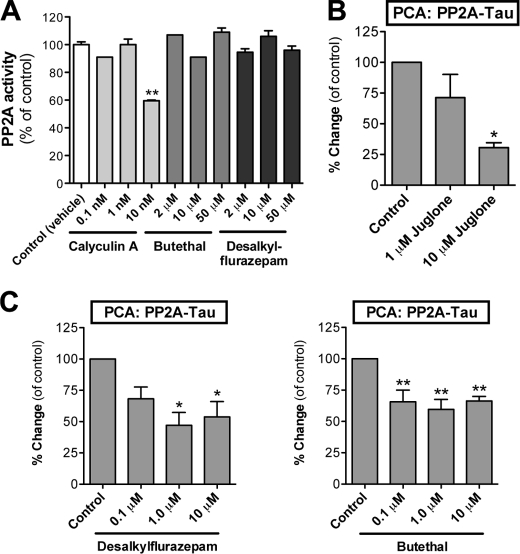
FIGURE 5.
GABAA modulators do not directly affect PP2A activity in vitro but reduce interaction of PP2A with Tau in N2a cells. A, the direct effect of a benzodiazepine and a barbiturate on PP2A enzyme activity was evaluated in a cell-free system using a PP2A-specific phosphopeptide substrate. The samples were preincubated with the indicated concentrations of butethal, desalkylflurazepam, or calyculin A as a control. Average values are displayed as percentages of change as compared with vehicle-treated control cells (means ± S.E.; n = 3). B, N2a cells were transiently transfected with PP2A-GLuc1 and Tau-GLuc2 plasmids. 48 h post-transfection, the cells were treated with Pin1 inhibitor juglone. Luminescence signal was measured by flash luminometry in live cells. The luminescence values were normalized by corresponding data from β-galactosidase assay (internal vector control, per well). C, N2a cells were transfected and analyzed as in Fig. 5B. The cells were treated with the indicated concentrations of desalkylflurazepam (left panel) or buthethal (right panel) for 2 h. The average values are displayed as percentages of change as compared with vehicle-treated control cells (means ± S.E.; n = 3). * and ** indicate significant differences with p < 0.05 and p < 0.01, respectively.
Next, we generated a PP2A PCA reporter with GLuc1 fusion attached to the regulatory Bα subunit of PP2A. The PP2A-Tau PCA assay was first pharmacologically validated with juglone. As expected based on previous reports (30–32), inhibition of Pin1 activity in cells by juglone reduced PP2A interaction with Tau (Fig. 5B). When N2a cells expressing PP2A-Tau PCA reporters were treated with desalkylflurazepam and butethal, nearly ∼50% reduction in PP2A-Tau interaction was found (Fig. 5C). These data suggest that although GABAAR modulators do not directly affect PP2A enzyme activity, the interaction of PP2A with Tau in intact cells is reduced when GABAAR is activated.
Cdk5 but Not GSK3β Mediates GABAA Receptor Activity-induced Phosphorylation of Tau
Phosphorylation of Tau is catalyzed by a large number of kinases (2). The AT8 epitope (Ser-199/Ser-202/Thr-205) can be phosphorylated by at least GSK3β, Cdk5, PKA, and ERK1/2 (4). GSK3β and Cdk5 are often considered as prime candidates mediating aberrant Tau phosphorylation at disease-associated sites. Moreover, GSK3β, Cdk5, and PP2A have been reported to associate in a functional complex (33) and cross-regulate each other's activities (34). We tested whether GSK3β or Cdk5 inhibition could affect the ability of GABAAR activation to enhance Tau phosphorylation. SB216763, a GSK3β inhibitor, and roscovitine, a Cdk5 inhibitor, were used together with desalkylflurazepam in 21 DIV cortical neuron cultures. Muscimol and bicuculline, a competitive GABAAR antagonist, were used as positive and negative controls. Although SB216763 had no effect, roscovitine effectively inhibited desalkylflurazepam-induced Tau phosphorylation at the AT8 epitope (Fig. 6A). This indicates that Cdk5 but not GSK3β activity mediates the effects of GABAAR activity on Tau. In accordance with these data, no change in GSK3β serine 9 phosphorylation status was found in desalkylflurazepam-treated neurons (Fig. 6A).
FIGURE 6.
GABAA receptor-induced Tau phosphorylation requires Cdk5 but not GSK3β activity. A, primary rat cortical neurons (21 DIV) were treated with desalkylflurazepam for 6 h together with GSK3β inhibitor SB216763 (100 nm) or Cdk5 inhibitor roscovitine (Rosco, 10 μm). The cells were pretreated with the inhibitors for 30 min before the addition of desalkylflurazepam. As controls, neurons were treated with vehicle only or with desalkylflurazepam and 5 μm bicuculline (Bicu) or 2 μm muscimol (Musci). The cell extracts were analyzed on Western blots with AT8 antibody as in Fig. 4. B, Pin1-Tau PCA was performed otherwise as in Fig. 2 with the exception that pCMV/SPORT6-p35 or an empty mock plasmid was included in the transfection. The cells were treated vehicle or with increasing concentrations of roscovitine for 4 h before PCA analysis. C, Pin1-Tau PCA was performed as in B. N2a cells were transfected with equal amounts of Pin1-Tau PCA reporter constructs in combination with either mock or pCMV/SPORT6-p35 plasmids. The cells were treated with vehicle or 10 μm desalkylflurazepam for 2 h before PCA analysis. The average values are displayed as percentages of change as compared with vehicle-treated control cells (means ± S.E.; n = 3). *, **, and *** indicate significant differences with p < 0.05, p < 0.01, and p < 0.001, respectively.
Finally, we modulated Cdk5 activity in N2a cells to measure its effects on Pin1-Tau interaction. For this purpose, we overexpressed p35, the activating regulatory subunit of Cdk5 (35, 36), in parallel Pin1-Tau PCA reporter constructs. In agreement with previously published data, overexpression of p35 in N2a cells enhanced Pin1-Tau interaction indicative of increased Tau phosphorylation (Fig. 6B). The effect of p35 on Pin1-Tau interaction was inhibited by the addition of roscovitine to the culture media. Finally, the effect of desalkylflurazepam on Pin1-Tau interaction was enhanced by more than 3-fold in cells overexpressing p35 as compared with mock-transfected PCA reporter cells (Fig. 6C). Taken together, these results suggest that GABAAR activity modulates Tau phosphorylation at the AT8 epitope in a Cdk5-dependent manner.
DISCUSSION
Regulation of Tau phosphorylation is a dynamic and tightly controlled event in neurons. Deregulation of this system may result in development of tauopathies, such as Alzheimer disease or frontotemporal dementia. Interestingly, recent studies have identified novel functions for Tau beyond the conventional regulation of microtubules (reviewed in Ref. 4). Detailed understanding of the complex protein-protein interactions of Tau is becoming increasingly important for development of Tau-targeting therapies for many aging-related CNS disorders. We have developed a novel protein fragment complementation-based assay system for studying Tau PPIs in live cells. Because Pin1 is a critical regulator of Tau dephosphorylation in neurons (37), we first focused on Pin1-Tau interaction as a dynamic live cell PPI reporter. Based on the data reported here, we conclude that hGLuc-based PCA suits well for studying PPIs of Tau in a live cell assay. Functional validation data showed that Pin1-Tau PCA is responsive to physiological stimulation (increased GSK3β level/activity) and inhibition of Pin1 (juglone). Moreover, the proof-of-concept screen of the focused pharmaceutical library showed that the PCA method is capable of identifying compounds increasing and decreasing the Pin1-Tau interaction. Folic acid, one of the few compounds that decreased the Pin1-Tau interaction, has previously been shown to affect Tau phosphorylation by promoting methylation and maturation of the PP2A complex (25, 38). This is consistent with our data showing that the Pin1-Tau PCA signal levels correlates with Tau phosphorylation and that folic acid reduced Pin1-Tau signal in cells. Interestingly, higher folate intake in the elderly (>65 years) has been associated with lower incidence of AD (39).
Unexpectedly, the proof-of-concept screen identified several GABAA receptor modulators belonging to the barbiturate and benzodiazepine classes as hit compounds increasing Pin1-Tau interaction. Previously, Tau has been functionally linked to NMDA receptor trafficking (reviewed in Refs. 4 and 40). Other neurotransmitter receptor systems have so far not been implicated in the regulation of Tau phosphorylation with the exception of dopamine. In SK-N-MC cells and striatal neurons, Tau was phosphorylated at Ser-199/Ser-202 in response to dopamine D1 receptor activation in a Ca2+- and PKA-dependent manner (41, 42). Interestingly, we found that GABAA receptor activators, as well as a dopamine D1/D2 receptor antagonist, increased Pin1-Tau interaction and Tau phosphorylation at Ser-199/Ser-202/Thr-205 (in case of GABAA activation; the dopamine D1/D2 antagonist was not analyzed further in this study). In addition, Tau phosphorylation was previously shown to be modulated by 3,4-methylenedioxymethamphetamine (“ecstasy”) in the hippocampus (43) and by various anesthetics in the cortex and hippocampus (28). Interestingly, reversible PHF-like Tau hyperphosphorylation has been shown to occur in hibernating mammals (44, 45). This suggests that Tau phosphorylation is not only responsive to modulation of several neurotransmitter systems and CNS-active drugs but may be more generally associated with the level of neuronal activity.
The GABAA receptors compose a family of ligand-gated Cl− channels, formed by the pentameric assembly of multiple subunits. Large number of GABAARs with different subunit composition and distinct physiological and pharmacological properties are differentially expressed throughout the brain (23). Moreover, different GABAARs can be targeted to different subcellular regions. For example, GABAARs composed of α1, α2, α3, or α5 subunits associated with β and γ subunits are benzodiazepine-sensitive, are largely synaptically located, and mediate most phasic inhibition in the brain. By contrast, those GABAARs composed of α4 or α6 subunits together with β and δ subunits form a distinct population of mostly extrasynaptic receptors that mediate tonic inhibition and are insensitive to benzodiazepine modulation (46). Our data showing that benzodiazepines can induce Tau phosphorylation in mature synaptically connected neurons suggest that these effects are mediated mostly by synaptic GABAA receptors.
Synaptic GABAAR complexes associate with the major inhibitory synapse scaffolding protein gephyrin that is directly linked to MTs (47, 48). As a MT-binding protein, Tau could participate in dynamic regulation of clustering or trafficking of GABAARs at the inhibitory synapses (49) and consequently also be a subject of modulation by GABAergic synaptic signaling. GSK3β, a major Tau kinase, was recently found to regulate GABAergic synapse formation via phosphorylation of gephyrin (50). Our data suggest that Cdk5 but not GSK3β is involved in GABAAR-induced phosphorylation of Tau. This is reminiscent of the recent data from hibernating mammals, where Cdk5 instead of GSK3β was shown to play a more important role in increased Tau phosphorylation (45).
Proline-directed phosphorylation of 14 serine-proline and threonine-proline motifs located in the proline-rich regions of Tau appears to be a central mechanism of regulation of its cellular functions. Pin1 facilitates Tau dephosphorylation by PP2A (16). Interestingly, gephyrin also undergoes proline-directed phosphorylation followed by Pin1-mediated prolyl isomerization that appears to be important for its ability to associate with glycine receptors (51). It has been proposed that prolyl cis-trans-isomerization can act as a molecular timer to help control the amplitude and duration of diverse cellular processes (52). Pin1-mediated regulation of both gephyrin scaffold and Tau could be used to regulate the timing or duration of GABAAR trafficking signals in the inhibitory synapses. Phosphorylation of GABAAR subunit β3 appears to be an important regulator of GABAAR function. PP2A associates with and dephosphorylates GABAAR-β3 (53). Interestingly, PP1 rather than PP2A seems to play a major role in gephyrin dephosphorylation (54). Our data suggest that GABAAR activation results in increased phosphorylation of Tau and increased Pin1 but reduced PP2A association with Tau. Based on our data, GABAA modulators do not directly inhibit the enzyme activity of PP2A in vitro. However, our PCA data suggest that less PP2A associates with Tau in cells exposed to GABAAR-active sedatives. In polarized cells like neurons, there may be significant compartment-specific differences in PP2A levels and activity. For example, phosphorylation of neurofilament proteins is differentially regulated in cell bodies and axons by a Pin1-PP2A-dependent mechanism (55). Moreover, increased GABAAR activity could recruit PP2A for β3 subunit dephosphorylation and receptor desensitization. It is possible that a strong and persistent GABAAR activating stimuli could result in recruitment of PP2A to the cell surface, reducing the availability of PP2A for Tau dephosphorylation.
BZs are widely used sedatives, hypnotics, anxiolytics, and anticonvulsants, and their potent sedative properties are routinely utilized in presurgical anesthesia. Because of their adverse effects, such as development of tolerance and addiction, BZ have not been approved for long term use. Moreover, BZ can produce anterograde amnesia, a mechanistically poorly understood adverse affect of BZ recognized more than four decades ago (reviewed in Ref. 56). The degree and duration of anterograde amnesia depends on several factors, such as the particular BZ taken, dosage, and route of administration. Interestingly, postoperative cognitive dysfunction may be partially explained by anesthesia-induced Tau hyperphosphorylation (28). Ethanol shares several pharmacological actions with barbiturates and BZs and can, among other effects, cause anterograde amnesia (57). Exposure of developing neurons to ethanol promotes Tau phosphorylation at Ser-199/Ser-202/Thr-205 (58). All of the above-mentioned agents act mostly or partially through GABAA receptor. The identification of Tau phosphorylation at the AT8 epitope, reportedly one of the priming sites (10), as a downstream target of GABAA activation raises intriguing questions about the mechanistic connections between chronic sedative or ethanol use, anterograde amnesia, and possibly risk of dementia. Our data suggest that, in cultured neurons, Tau phosphorylation at the AT8 epitope remains at an elevated level for at least 24 h after the washout of GABAA activating drugs. BZs are commonly used in clinical practice to reduce behavioral and psychological symptoms associated with dementia, such as agitation and aggression, as well as sleep disturbances. Therefore, more detailed studies are needed to address whether chronic sedative use (particularly BZs with long elimination half-lives) could accelerate the progression of dementia.
Supplementary Material
Acknowledgments
The plasmids encoding the split hGLuc fragments were a kind gift from Prof. Stephen Michnick (Université de Montréal, Montreal, Canada), and the antibodies AT8 and TG3 were from Dr. Jin-Jing Pei (Karolinska Institutet, Stockholm, Sweden).
This work was supported by grants from the Academy of Finland, Sigrid Jusélius Foundation, Biocentrum Helsinki, and Finnish Cultural Foundation. The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007-2011) under Grant Agreement 206918. H. J. H. is a cofounder, employee and shareholder of Hermo Pharma Ltd.

This article contains supplemental Tables S1 and S2.
P. Sakha, P. Tammela, and H. J. Huttunen, unpublished data.
- AD
- Alzheimer disease
- BZ
- benzodiazepine
- Cdk5
- cyclin-dependent kinase 5
- GABAAR
- γ-aminobutyric acid receptor subtype A
- hGLuc
- humanized G. princeps luciferase
- GSK3β
- glycogen synthase kinase-3β
- MT
- microtubule
- PCA
- protein-fragment complementation assay
- PPI
- protein-protein interaction
- PP2A
- protein phosphatase 2A
- SP
- serine-proline
- TP
- threonine-proline
- DIV
- day(s) in vitro
- βGal
- β-galactosidase.
REFERENCES
- 1. Lee V. M., Goedert M., Trojanowski J. Q. (2001) Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 [DOI] [PubMed] [Google Scholar]
- 2. Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 [DOI] [PubMed] [Google Scholar]
- 3. Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Association of missense and 5′-splice-site mutations in Tau with the inherited dementia FTDP-17. Nature 393, 702–705 [DOI] [PubMed] [Google Scholar]
- 4. Morris M., Maeda S., Vossel K., Mucke L. (2011) The many faces of Tau. Neuron 70, 410–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mandelkow E. M., Stamer K., Vogel R., Thies E., Mandelkow E. (2003) Clogging of axons by Tau. Inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging 24, 1079–1085 [DOI] [PubMed] [Google Scholar]
- 6. Dixit R., Ross J. L., Goldman Y. E., Holzbaur E. L. (2008) Differential regulation of dynein and kinesin motor proteins by tau. Science 319, 1086–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoover B. R., Reed M. N., Su J., Penrod R. D., Kotilinek L. A., Grant M. K., Pitstick R., Carlson G. A., Lanier L. M., Yuan L. L., Ashe K. H., Liao D. (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ittner L. M., Ke Y. D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B. C., Christie M. J., Napier I. A., Eckert A., Staufenbiel M., Hardeman E., Götz J. (2010) Dendritic function of Tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 [DOI] [PubMed] [Google Scholar]
- 9. Luna-Muñoz J., Chávez-Macías L., García-Sierra F., Mena R. (2007) Earliest stages of Tau conformational changes are related to the appearance of a sequence of specific phospho-dependent Tau epitopes in Alzheimer's disease. J. Alzheimers Dis. 12, 365–375 [DOI] [PubMed] [Google Scholar]
- 10. Bertrand J., Plouffe V., Sénéchal P., Leclerc N. (2010) The pattern of human Tau phosphorylation is the result of priming and feedback events in primary hippocampal neurons. Neuroscience 168, 323–334 [DOI] [PubMed] [Google Scholar]
- 11. Pelech S. L. (1995) Networking with proline-directed protein kinases implicated in Tau phosphorylation. Neurobiol. Aging 16, 247–261 [DOI] [PubMed] [Google Scholar]
- 12. Steinhilb M. L., Dias-Santagata D., Fulga T. A., Felch D. L., Feany M. B. (2007) Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Mol. Biol. Cell 18, 5060–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu K. P., Zhou X. Z. (2007) The prolyl isomerase PIN1. A pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. 8, 904–916 [DOI] [PubMed] [Google Scholar]
- 14. Ranganathan R., Lu K. P., Hunter T., Noel J. P. (1997) Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell 89, 875–886 [DOI] [PubMed] [Google Scholar]
- 15. Yaffe M. B., Schutkowski M., Shen M., Zhou X. Z., Stukenberg P. T., Rahfeld J. U., Xu J., Kuang J., Kirschner M. W., Fischer G., Cantley L. C., Lu K. P. (1997) Sequence-specific and phosphorylation-dependent proline isomerization. A potential mitotic regulatory mechanism. Science 278, 1957–1960 [DOI] [PubMed] [Google Scholar]
- 16. Liou Y. C., Sun A., Ryo A., Zhou X. Z., Yu Z. X., Huang H. K., Uchida T., Bronson R., Bing G., Li X., Hunter T., Lu K. P. (2003) Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 424, 556–561 [DOI] [PubMed] [Google Scholar]
- 17. Lu P. J., Wulf G., Zhou X. Z., Davies P., Lu K. P. (1999) The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated Tau protein. Nature 399, 784–788 [DOI] [PubMed] [Google Scholar]
- 18. Remy I., Michnick S. W. (2006) A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat. Methods 3, 977–979 [DOI] [PubMed] [Google Scholar]
- 19. Hennig L., Christner C., Kipping M., Schelbert B., Rücknagel K. P., Grabley S., Küllertz G., Fischer G. (1998) Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by juglone. Biochemistry 37, 5953–5960 [DOI] [PubMed] [Google Scholar]
- 20. Noble W., Planel E., Zehr C., Olm V., Meyerson J., Suleman F., Gaynor K., Wang L., LaFrancois J., Feinstein B., Burns M., Krishnamurthy P., Wen Y., Bhat R., Lewis J., Dickson D., Duff K. (2005) Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl. Acad. Sci. U.S.A. 102, 6990–6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner U., Utton M., Gallo J. M., Miller C. C. (1996) Cellular phosphorylation of Tau by GSK-3β influences tau binding to microtubules and microtubule organisation. J. Cell Sci. 109, 1537–1543 [DOI] [PubMed] [Google Scholar]
- 22. Charney D. S., Mihic S. J., Harris R. A. (2005) in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton L. L., Lazo J. S., Parker K. L., eds) pp. 401–428, McGraw-Hill, New York [Google Scholar]
- 23. Farrant M., Kaila K. (2007) The cellular, molecular and ionic basis of GABAA receptor signalling. Prog. Brain Res. 160, 59–87 [DOI] [PubMed] [Google Scholar]
- 24. Baraldi M., Guidotti A., Schwartz J. P., Costa E. (1979) GABA receptors in clonal cell lines. A model for study of benzodiazepine action at molecular level. Science 205, 821–823 [DOI] [PubMed] [Google Scholar]
- 25. Sontag J. M., Nunbhakdi-Craig V., Montgomery L., Arning E., Bottiglieri T., Sontag E. (2008) Folate deficiency induces in vitro and mouse brain region-specific downregulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A Bα subunit expression that correlate with enhanced tau phosphorylation. J. Neurosci. 28, 11477–11487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lesuisse C., Martin L. J. (2002) Long-term culture of mouse cortical neurons as a model for neuronal development, aging, and death. J. Neurobiol. 51, 9–23 [DOI] [PubMed] [Google Scholar]
- 27. Kato-Negishi M., Muramoto K., Kawahara M., Kuroda Y., Ichikawa M. (2004) Developmental changes of GABAergic synapses formed between primary cultured cortical neurons. Brain Res. Dev. Brain Res. 152, 99–108 [DOI] [PubMed] [Google Scholar]
- 28. Planel E., Richter K. E., Nolan C. E., Finley J. E., Liu L., Wen Y., Krishnamurthy P., Herman M., Wang L., Schachter J. B., Nelson R. B., Lau L. F., Duff K. E. (2007) Anesthesia leads to Tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci. 27, 3090–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Humar M., Pischke S. E., Loop T., Hoetzel A., Schmidt R., Klaas C., Pahl H. L., Geiger K. K., Pannen B. H. (2004) Barbiturates directly inhibit the calmodulin/calcineurin complex. A novel mechanism of inhibition of nuclear factor of activated T cells. Mol. Pharmacol. 65, 350–361 [DOI] [PubMed] [Google Scholar]
- 30. Zhou X. Z., Kops O., Werner A., Lu P. J., Shen M., Stoller G., Küllertz G., Stark M., Fischer G., Lu K. P. (2000) Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and Tau proteins. Mol. Cell 6, 873–883 [DOI] [PubMed] [Google Scholar]
- 31. Galas M. C., Dourlen P., Bégard S., Ando K., Blum D., Hamdane M., Buée L. (2006) The peptidylprolyl cis/trans-isomerase Pin1 modulates stress-induced dephosphorylation of Tau in neurons. Implication in a pathological mechanism related to Alzheimer disease. J. Biol. Chem. 281, 19296–19304 [DOI] [PubMed] [Google Scholar]
- 32. Landrieu I., Smet-Nocca C., Amniai L., Louis J. V., Wieruszeski J. M., Goris J., Janssens V., Lippens G. (2011) Molecular implication of PP2A and Pin1 in the Alzheimer's disease specific hyperphosphorylation of Tau. PLoS One 6, e21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Plattner F., Angelo M., Giese K. P. (2006) The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in Tau hyperphosphorylation. J. Biol. Chem. 281, 25457–25465 [DOI] [PubMed] [Google Scholar]
- 34. Louis J. V., Martens E., Borghgraef P., Lambrecht C., Sents W., Longin S., Zwaenepoel K., Pijnenborg R., Landrieu I., Lippens G. (2011) Mice lacking phosphatase PP2A subunit PR61/B′δ (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3β. Proc. Natl. Acad. Sci. U.S.A. 108, 6957–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lew J., Huang Q. Q., Qi Z., Winkfein R. J., Aebersold R., Hunt T., Wang J. H. (1994) A brain-specific activator of cyclin-dependent kinase 5. Nature 371, 423–426 [DOI] [PubMed] [Google Scholar]
- 36. Tsai L. H., Delalle I., Caviness V. S., Jr., Chae T., Harlow E. (1994) p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371, 419–423 [DOI] [PubMed] [Google Scholar]
- 37. Lim J., Balastik M., Lee T. H., Nakamura K., Liou Y. C., Sun A., Finn G., Pastorino L., Lee V. M., Lu K. P. (2008) Pin1 has opposite effects on wild-type and P301L Tau stability and tauopathy. J. Clin. Invest. 118, 1877–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sontag E., Nunbhakdi-Craig V., Sontag J. M., Diaz-Arrastia R., Ogris E., Dayal S., Lentz S. R., Arning E., Bottiglieri T. (2007) Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J. Neurosci. 27, 2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luchsinger J. A., Tang M. X., Miller J., Green R., Mayeux R. (2007) Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch. Neurol. 64, 86–92 [DOI] [PubMed] [Google Scholar]
- 40. Ittner L. M., Gotz J. (2011) Nat. Rev. Neurosci. 12, 65–72 [DOI] [PubMed] [Google Scholar]
- 41. Lebel M., Cyr M. (2011) Molecular and cellular events of dopamine D1 receptor-mediated Tau phosphorylation in SK-N-MC cells. Synapse 65, 69–76 [DOI] [PubMed] [Google Scholar]
- 42. Lebel M., Patenaude C., Allyson J., Massicotte G., Cyr M. (2009) Dopamine D1 receptor activation induces Tau phosphorylation via Cdk5 and GSK3 signaling pathways. Neuropharmacology 57, 392–402 [DOI] [PubMed] [Google Scholar]
- 43. Busceti C. L., Biagioni F., Riozzi B., Battaglia G., Storto M., Cinque C., Molinaro G., Gradini R., Caricasole A., Canudas A. M., Bruno V., Nicoletti F., Fornai F. (2008) Enhanced Tau phosphorylation in the hippocampus of mice treated with 3,4-methylenedioxymethamphetamine (“ecstasy”). J. Neurosci. 28, 3234–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arendt T., Stieler J., Strijkstra A. M., Hut R. A., Rüdiger J., Van der Zee E. A., Harkany T., Holzer M., Härtig W. (2003) Reversible paired helical filament-like phosphorylation of Tau is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 23, 6972–6981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stieler J. T., Bullmann T., Kohl F., Tøien Ø., Brückner M. K., Härtig W., Barnes B. M., Arendt T. (2011) The physiological link between metabolic rate depression and Tau phosphorylation in mammalian hibernation. PLoS One 6, e14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brünig I., Scotti E., Sidler C., Fritschy J. M. (2002) Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 443, 43–55 [DOI] [PubMed] [Google Scholar]
- 47. Prior P., Schmitt B., Grenningloh G., Pribilla I., Multhaup G., Beyreuther K., Maulet Y., Werner P., Langosch D., Kirsch J. (1992) Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron 8, 1161–1170 [DOI] [PubMed] [Google Scholar]
- 48. Essrich C., Lorez M., Benson J. A., Fritschy J. M., Lüscher B. (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat. Neurosci. 1, 563–571 [DOI] [PubMed] [Google Scholar]
- 49. Jacob T. C., Moss S. J., Jurd R. (2008) GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tyagarajan S. K., Ghosh H., Yévenes G. E., Nikonenko I., Ebeling C., Schwerdel C., Sidler C., Zeilhofer H. U., Gerrits B., Muller D., Fritschy J. M. (2011) Regulation of GABAergic synapse formation and plasticity by GSK3β-dependent phosphorylation of gephyrin. Proc. Natl. Acad. Sci. U.S.A. 108, 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zita M. M., Marchionni I., Bottos E., Righi M., Del Sal G., Cherubini E., Zacchi P. (2007) Post-phosphorylation prolyl isomerization of gephyrin represents a mechanism to modulate glycine receptors function. EMBO J. 26, 1761–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lu K. P., Finn G., Lee T. H., Nicholson L. K. (2007) Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 3, 619–629 [DOI] [PubMed] [Google Scholar]
- 53. Jovanovic J. N., Thomas P., Kittler J. T., Smart T. G., Moss S. J. (2004) Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABAA receptor phosphorylation, activity, and cell-surface stability. J. Neurosci. 24, 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bausen M., Weltzien F., Betz H., O'Sullivan G. A. (2010) Regulation of postsynaptic gephyrin cluster size by protein phosphatase 1. Mol. Cell Neurosci. 44, 201–209 [DOI] [PubMed] [Google Scholar]
- 55. Rudrabhatla P., Albers W., Pant H. C. (2009) Peptidyl-prolyl isomerase 1 regulates protein phosphatase 2A-mediated topographic phosphorylation of neurofilament proteins. J. Neurosci. 29, 14869–14880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Curran H. V. (1991) Benzodiazepines, memory and mood. A review. Psychopharmacology 105, 1–8 [DOI] [PubMed] [Google Scholar]
- 57. Fadda F., Rossetti Z. L. (1998) Chronic ethanol consumption. From neuroadaptation to neurodegeneration. Prog. Neurobiol. 56, 385–431 [DOI] [PubMed] [Google Scholar]
- 58. Saito M., Chakraborty G., Mao R. F., Paik S. M., Vadasz C., Saito M. (2010) Tau phosphorylation and cleavage in ethanol-induced neurodegeneration in the developing mouse brain. Neurochem. Res. 35, 651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.