Background: Procollagen needs the collagen-specific molecular chaperone Hsp47 for correct folding.
Results: Hsp47 binds to the triple-helix form of collagen model peptides in vitro and in vivo, but not to the monomer form.
Conclusion: Hsp47 functions for triple-helix collagen as a molecular chaperone in the endoplasmic reticulum.
Significance: This study provides the basis of the functional mechanism of Hsp47 in collagen molecular maturation.
Keywords: Collagen, Endoplasmic Reticulum (ER), FRET, Heat Shock Protein, Molecular Chaperone, Hsp47
Abstract
Hsp47 (heat shock protein 47), a collagen-specific molecular chaperone, is essential for the maturation of various types of procollagens. Previous studies have suggested that Hsp47 may preferentially recognize the triple-helix form of procollagen rather than unfolded procollagen chains in the endoplasmic reticulum. However, the underlying mechanism has remained unclear because of limitations in the available methods for detecting in vitro and in vivo interactions between Hsp47 and collagen. In this study, we established novel methods for this purpose by adopting a time-resolved FRET technique in vitro and a bimolecular fluorescence complementation technique in vivo. Using these methods, we provide direct evidence that Hsp47 binds to collagen triple helices but not to the monomer form in vitro. We also demonstrate that Hsp47 binds a collagen model peptide in the trimer conformation in vivo. Hsp47 did not bind collagen peptides that had been modified to block their ability to form triple helices in vivo. These results conclusively indicate that Hsp47 recognizes the triple-helix form of procollagen in vitro and in vivo.
Introduction
Collagen is the most abundant protein in mammals and, as a major component of the extracellular matrix, plays a pivotal role in tissue architecture and robustness and in cell-cell interactions. Individual collagen polypeptide chains contain large numbers of repetitive amino acid sequences, most often Gly-Xaa-Yaa, where Xaa is often Pro and Yaa is often 4-hydroxy-l-proline, the latter amino acid being produced by hydroxylation of Pro following collagen polypeptide synthesis. All types of collagen have a characteristic triple-helical structure. The presence of Gly at every third residue and the high imino acid content allow the formation of a triple-helical structure in which the three helical chains are staggered by one residue and are supercoiled in a right-handed manner. Appropriate folding of collagen into its triple helix followed by processing of N- and C-propeptides is critical for the formation of the extracellular matrix. Because the extracellular matrix is required for the formation of bones and other tissues, collagen folding defects lead to severe bone fragility and deformities such as osteogenesis imperfecta (1–3).
Several molecular chaperones and folding enzymes are involved in the molecular maturation of various types of procollagen: BiP, Grp94, and Hsp47 (heat shock protein 47) as molecular chaperones and protein-disulfide isomerase (PDI),2 prolyl 3-hydroxylase, and prolyl 4-hydroxylase as folding enzymes (4–9). Hsp47 was first identified as a collagen-binding protein residing in the endoplasmic reticulum (ER) (10, 11) and functions as a collagen-specific molecular chaperone (12, 13). Disruption of both alleles of hsp47 in mice causes aberrant procollagen folding in the ER, which results in embryonic lethality by 11.5 days post-coitus due to defects in the formation of collagen fibers and basement membranes in the embryo (12, 14, 15). Triple-helix formation, secretion, and processing of the N-terminal propeptide of type I procollagen are impaired in hsp47-disrupted fibroblasts, resulting in a failure to accumulate collagen fibers in the extracellular matrix (15, 16). These results indicate that Hsp47 is essential for the maturation of collagen.
To elucidate the mechanism of client recognition by Hsp47, in vitro binding analysis using a synthetic collagen model peptide has been used to identify a specific Hsp47-binding sequence in collagen; Arg residues at the Yaa position of the collagen Gly-Xaa-Yaa repeats are a critical minimum for Hsp47 binding (17–19). Hsp47 appeared to preferentially recognize such sequences on the triple helices of procollagen rather than on the unfolded procollagen α-chains in the ER (18–20). However, because of a lack of direct mechanistic studies of the interaction between Hsp47 and procollagen, it remains controversial as to whether Hsp47 recognizes only the triple-helix conformation or whether it also recognizes the single-chain polypeptide.
In this study, we provide direct observational evidence that Hsp47 interacts with triple-helix collagen but not with its monomer. This result was achieved using self-assembling homotrimeric collagen model peptides, separated by gel filtration chromatography, in a novel binding assay based on a time-resolved (TR) FRET technique. We also developed a versatile visualization system for detecting the interaction between Hsp47 and a collagen model peptide fused to foldon, which is derived from the C-terminal domain of T4 fibritin and is known to facilitate trimer conformation (21–23). This assay used a bimolecular fluorescence complementation (BiFC) technique (24) in living cells based on the reconstitution of two split fragments of monomeric Kusabira-Green (mKG) as a fluorescent protein (25).
EXPERIMENTAL PROCEDURES
Materials
Oligonucleotides were purchased from Hokkaido System Science Co., Ltd. (Ibaraki, Japan). Synthetic peptides were purchased from TORAY Research Center, Inc. (Kanagawa, Japan). Streptavidin (SA)-XL665 and anti-GST-europium cryptate (Eu-K) antibody were purchased from Cisbio International (Bagnols-sur-Cèze, France).
Plasmid Construction
To express target proteins in the ER, we modified the expression vectors in the CoralHue Fluo-chase kit (Amalgaam, Tokyo, Japan). A cDNA fragment containing a Kozak sequence and a sequence encoding the ER signal sequence derived from human Hsp47 (MRSLLLLSAFCLLEAAL) was subcloned into the NheI site of the phmKGN-MC and phmKGC-MC vectors, respectively. The resulting constructs were designated pER-mKGN and pER-mKGC. A cDNA encoding the collagen model peptide was made by annealing the complementary strands of oligonucleotide 5′-CCG GTA CC(CCT CCA GGT)5CCT ACA GGT CCA AGA GGT(CCT CCA GGT)2TAA CTC GAG CC. The cDNAs encoding the mature form of wild-type human Hsp47 or the CAYA mutant of Hsp47 and the collagen model peptide were subcloned into the KpnI-XhoI sites of the pER-mKGN vector. The resulting constructs were designated pER-mKGN-h47wt, pER-mKGN-h47CAYA, and pER-mKGN-CP2×9. These three cDNAs were also subcloned into the KpnI-XhoI sites of the pER-mKGC vector. The resulting constructs were designated pER-mKGC-h47wt, pER-mKGC-h47CAYA, and pER-mKGC-CP2×9. The cDNA encoding foldon (21, 22), which was made by annealing the complementary strands of oligonucleotide 5′-CCA CTC GAG ATT CCT GAA GCT CCA AGA GAT GGG CAA GCC TAC GTT CGT AAA GAT GGC GAA TGG GTA TTG CTT TCT ACC TTT TTA TGA GCG GCC GCA CC, was subcloned into the XhoI-NotI sites of the pER-mKGN-CP2×9 and the pER-mKGC-CP2×9 vectors, respectively. The stop codon of the collagen model peptide and the XhoI site were replaced with the sequence encoding Ser-Gly-Tyr (amino acid residues 1–3 of foldon) by site-directed mutagenesis using the complementary strands of oligonucleotide 5′-GGT CCT CCA GGT TCA GGC TAC ATT CCT GAA GCT CC. The resulting constructs were designated pER-mKGN-CP2×9F and pER-mKGC-CP2×9F. pER-mKGC-PPG×3F and pER-mKGC-CP2GA×9F were also similarly constructed by the site-directed mutagenesis described above. For deletion of the sequence encoding the ER signal sequence, pER-mKGN-h47wt, pER-mKGN-h47CAYA, pER-mKGC-h47wt, pER-mKGC-h47CAYA, pER-mKGC-PPG×3F, and pER-mKGC-CP2×9F were digested with NheI and self-ligated. The resulting constructs were designated pmKGN-h47wt, pmKGN-h47CAYA, pmKGC-h47wt, pmKGC-h47CAYA, pmKGC-PPG×3F, and pmKGC-CP2×9F. All constructs described above were confirmed by DNA sequencing using an ABI Prism 3130xl DNA sequencer (Applied Biosystems, Foster City, CA).
Preparation of Recombinant Hsp47 Proteins
cDNAs encoding mature Hsp47 proteins (wild-type human and chicken Hsp47 and the CAYA mutant of human Hsp47) were subcloned into a bacterial expression vector based on pET-32a (Novagen, Madison, WI), which was modified to allow the fusion of the target protein N-terminal to a polyhistidine tag and GST and to incorporate a recognition sequence for PreScission protease (GE Healthcare). Escherichia coli strain BL21(DE3) cells, expressing the rare Arg-tRNA that recognizes the similarly rare AGA codons (26), were transformed with the expression vector encoding His/GST-Hsp47 and cultured in ZB medium (1% N-Z-Amine A (Wako, Tokyo, Japan) and 0.5% NaCl) containing 100 μg/ml carbenicillin and 30 μg/ml kanamycin at 30 °C. Expression of Hsp47 was induced by adding 0.1 mm isopropyl β-d-thiogalactopyranoside and culturing at 20 °C for 16–20 h. Cells were collected by centrifugation and resuspended in PBS containing 0.5% Nonidet P-40 (Nacalai Tesque, Kyoto, Japan), 1 mm phenylmethylsulfonyl fluoride, and 0.5% protease inhibitor mixture (P8849, Sigma). Lysozyme (Wako) was added to a final concentration of 0.5 mg/ml, and the mixture was incubated for 10 min on ice. The lysate was frozen in liquid nitrogen and stored at −80 °C. After thawing, the lysate was sonicated and cleared by centrifugation at 16,000 × g for 30 min at 4 °C. Soluble proteins were purified with glutathione-Sepharose 4B (GE Healthcare) according to the manufacturer's batch protocol. GST-fused proteins were eluted with elution buffer (10 mm glutathione and 50 mm Tris-HCl, pH 8.8). The total protein concentration was determined using a Coomassie Plus protein assay reagent kit (Pierce) according to the manufacturer's instructions with BSA as a standard.
Antibodies
Mouse monoclonal antibodies against Hsp47 (Stressgen, Victoria, Canada) and mKG_C (MBL, Nagoya, Japan) and rabbit polyclonal antibodies against PDI (Stressgen) were obtained from the indicated sources. HRP-conjugated anti-mouse or rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA), Alexa Fluor 555-conjugated anti-mouse IgG (Invitrogen), and Alexa Fluor 488-conjugated anti-rabbit IgG (Invitrogen) were used as secondary antibodies.
Western Blot Analysis
Proteins were separated by SDS-PAGE using 10–20% gradient gels and transferred to either a polyvinylidene difluoride or nitrocellulose membrane. The membranes were blocked with PBS containing 2.5% skim milk, and specific binding of the antibody was detected using a chemiluminescence detection kit (ECL Plus Western blotting detection system, GE Healthcare).
Gel Filtration Chromatography
Gel filtration chromatography (GFC) was carried out using Superdex Peptide PE 7.5/300 (GE Healthcare) on an ÄKTAexplorer 10 S system (GE Healthcare) at room temperature. Aliquots (100 μl) of biotinylated collagen model peptide were loaded and separated at a flow rate of 0.28 ml/min using PBS, and 0.2-ml fractions were collected. Peptide elution was monitored by absorbance at 215 nm.
In Vitro TR-FRET Binding Assay
The assay was performed in a 384-well plate in 20 μl of binding buffer containing 10 nm recombinant GST-Hsp47 protein, 300 nm biotinylated collagen model peptide, 83.4 nm SA-XL665, 0.37 nm anti-GST-Eu-K antibody, 50 mm HEPES/NaOH, 150 mm NaCl, 1 mm EDTA, 0.01% Nonidet P-40, 0.1% BSA, and 100 mm KF. All reaction mixtures were incubated for 2 h at room temperature. Emission signals at 665 and 620 nm were measured simultaneously on an Analyst GT multimode plate reader (Molecular Devices, Sunnyvale, CA) using a 50-μs delay following an excitation pulse at 337 nm.
The 665:620 nm ratio was calculated as follows: ratio = (emission at 665 nm/emission at 620 nm) × 104. Values are means ± S.D. (n = 3).
Cell Culture and Transfection
HeLa cells, which are a human cervical epithelial carcinoma cell line, were obtained from American Type Culture Collection and maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The cells were cultured at 37 °C in an atmosphere containing 5% CO2 and transfected with each vector using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Immunofluorescence Microscopy
Cells were washed in PBS and fixed with 4% (w/v) paraformaldehyde in PBS for 20 min. After washing three times, fixed cells were permeabilized with 0.1% (v/v) Triton X-100 in PBS for 5 min. Nonspecific protein binding in permeabilized cells was blocked by incubation in PBS containing 2% BSA for 30 min. After incubation with specific antibodies in PBS containing 1% BSA, cells were incubated with Alexa Fluor-conjugated anti-mouse or anti-rabbit IgG and 1 μg/ml Hoechst 33342 (Invitrogen) in PBS containing 1% BSA. Fluorescent images were collected using an automated image acquisition system (IN Cell Analyzer 1000, GE Healthcare).
BiFC Binding Assay in Mammalian Cells
Twenty-four hours after transfection, the HeLa cells were subcultured onto BD Falcon Optilux 96-well microplates (353948, BD Biosciences), and the culture medium was replaced after 48 h with Opti-MEM I reduced serum medium (Invitrogen) containing 1 μg/ml Hoechst 33342 to avoid autofluorescence arising from the medium. Fluorescence emitted from the reconstitution of two split fragments of mKG and from Hoechst 33342 was detected using the IN Cell Analyzer 1000 system.
RESULTS
Development of Novel Hsp47 Binding Assay in Vitro
Although some in vitro methods for detecting the binding of Hsp47 to native collagen or collagen model peptides have been reported (27–29), no simple “mix and read” method has been described. To facilitate the identification of the conformation of collagen binding to Hsp47, we attempted to develop a novel binding assay using TR-FRET (Fig. 1A). GST-tagged recombinant Hsp47 proteins were readily purified by glutathione affinity chromatography from the lysates of E. coli cells harboring the corresponding expression plasmids (supplemental Fig. 1). For use as substrates, we prepared two biotinylated collagen model peptides, Bio-CP1 and Bio-CP2, which differ in the positions of key Thr and Arg residues (Fig. 1B, boldface), which are required for the interaction with Hsp47 (17, 30). As shown in Fig. 1C, an increase in FRET signals was observed when wild-type chicken Hsp47 was incubated with each of the biotinylated collagen model peptides. FRET signals were much lower for the interaction of human Hsp47 and collagen model peptides compared with that of chicken Hsp47, possibly due to the decreased protein stability of human Hsp47 (Fig. 1, C and D). On the other hand, no obvious increase in the signal was observed when the human Hsp47 CAYA mutant, in which Cys-139 and Tyr-366 were replaced by Ala, was used as a negative control. Cys-139 is important for the solubility of Hsp47, and Tyr-366 is a key residue in the interaction with collagen. The same result was obtained using the human Hsp47 CA mutant, in which only Cys-139 was replaced by Ala (data not shown). The increase in FRET signals was greater for Bio-CP2 than for Bio-CP1 (Fig. 1C). The difference in signal intensities between the two peptides would be caused by the difference in the distance between the Hsp47-binding motif (GPTGPR) and labeled biotin/SA-XL665 (Fig. 1B). Thus, we used Bio-CP2 in the following experiments. Concentration-dependent increases in the FRET signal were observed with Bio-CP2 (Fig. 1D). These results demonstrate that the binding assay based on TR-FRET technology provides a useful quantitative measurement of the binding of Hsp47 to collagen model peptides in vitro.
FIGURE 1.
Binding of recombinant Hsp47 to biotinylated collagen model peptides in TR-FRET binding assays in vitro. A, schematic diagram of the TR-FRET binding assays. Anti-GST-Eu-K antibody and SA-XL665 were used to generate a FRET signal when the tandem pair was in close proximity. b, biotin. B, structures of the biotinylated collagen model peptides synthesized for the TR-FRET binding assays. The single-letter abbreviation O is used for 4-hydroxy-l-proline residues. Arg and Thr residues that interact with Hsp47 are in shown in boldface. C, TR-FRET binding assays. The reaction mixtures (20-μl total volume) contained 10 nm recombinant GST-Hsp47 proteins, 300 nm biotinylated collagen model peptides, 83.4 nm SA-XL665, and 0.37 nm anti-GST-Eu-K antibody. All reaction mixtures were incubated for 2 h at room temperature. The ratio was calculated as follows: ratio = (emission at 665 nm/emission at 620 nm) × 104. Values are means ± S.D. (n = 3). D, binding of recombinant Hsp47 to Bio-CP2 in a dose-dependent manner. The reaction mixtures (20-μl total volume) contained 10 nm recombinant GST-Hsp47 proteins, Bio-CP2 at the indicated concentration, 83.4 nm SA-XL665, and 0.37 nm anti-GST-Eu-K antibody. All reaction mixtures were incubated for 2 h at room temperature. Values are means ± S.D. (n = 3).
Hsp47 Specifically Binds to Trimer Conformation of Collagen Model Peptide in Vitro
To elucidate the conformational requirements for the interaction of Hsp47 with collagen using the TR-FRET system, we separated Bio-CP2 using GFC. In the chromatographic profile of Bio-CP2, the trimer appeared as the first peak, and the monomer appeared as the second peak (Fig. 2A). The third peak was due to the presence of Nonidet P-40 in the buffer for the collagen model peptide. After collecting the first and second peaks, the binding of Hsp47 to the collagen model peptide in each fraction was analyzed. A dose-dependent increase in the FRET signal was observed for the trimer form of the collagen peptide (Fig. 2C), whereas no increase was observed for the monomer (Fig. 2B). These results conclusively demonstrate that Hsp47 interacts with the trimer (but not monomer) form of collagen peptides.
FIGURE 2.
Specific binding of Hsp47 to trimer fractions of biotinylated collagen model peptides in vitro. A, separation of the biotinylated collagen model peptide (Bio-CP2) by GFC. Separation was performed using Superdex Peptide PE 7.5/300 with PBS at room temperature. The flow rate was 0.28 ml/min, and 0.2-ml fractions were collected. Peptide elution was monitored as absorbance at 215 nm. Bio-CP2 applied to the column was 0.1 ml at a concentration of 25 μm. The first peak, eluting between 5 and 6 ml, corresponds to the trimer (8.0 kDa). The second peak, eluting between 6 and 7 ml, corresponds to the monomer (2.7 kDa). We obtained the trimer peak at a concentration of 5.4 μm as a base of the monomer (1.8 μm as a base of trimer) and the monomer peak at a concentration of 3.2 μm, which was determined by optical absorbance. The third peak was due to the presence of Nonidet P-40 included in the buffer for the collagen model peptide. mAU, milli-absorbance units. B and C, binding of recombinant Hsp47 to the monomer and trimer fractions of Bio-CP2, respectively. The reaction mixtures (20-μl total volume) contained 10 nm recombinant GST-Hsp47 proteins, each biotinylated collagen model peptide at the indicated concentration, 83.4 nm SA-XL665, and 0.37 nm anti-GST-Eu-K antibody. All reaction mixtures were incubated for 2 h at room temperature. Values are means ± S.D. (n = 3).
Next, we examined the reversibility of conformational changes in the collagen model peptides from trimer to monomer and the influence of these changes on binding to Hsp47. When Bio-CP2 was heated at 37 °C for 2 h, the trimer fraction disappeared, with a corresponding increase in the monomer peak and a complete loss of binding capacity for Hsp47 (Fig. 3, A and B). When samples heated at 37 °C were cooled to 4 °C for 4 days, the trimer peak reappeared in GFC analysis (Fig. 3F), suggesting that the conformational shift between trimer and monomer is reversible (Fig. 3, F and B). The ability to bind Hsp47 was also almost completely restored in the cooled samples (Fig. 3, C and E). These results indicate that the trimer form of collagen model peptides can reversibly convert to the monomer form in response to changes in temperature and that Hsp47 binds only to the trimer form, most possibly to the triple-helix form.
FIGURE 3.
Temperature-dependent reversibility of collagen triple-helix formation. A, binding of recombinant Hsp47 to Bio-CP2 following heat treatment. A Bio-CP2 mixture (3 μm), before separation by GFC, was heated for 2 h at 37 °C. The TR-FRET binding assays were performed as described in the legend to Fig. 2, except that the reaction mixtures were incubated for 2 h at 37 °C. B, heat treatment of the trimer fraction. The trimer fractions of the Bio-CP2 mixture was obtained by GFC and heated for 2 h at 37 °C. Using the trimer fraction, GFC analysis was performed as described in the legend to Fig. 2A. C–F, cooling of heat-treated trimer fractions. TR-FRET binding assays (C and E) and GFC (D and F), as described above, were performed using untreated (C and D) or heat-treated (E and F) trimer fractions of Bio-CP2 after cooling for 4 days at 4 °C. In B, D, and F, we applied the peptides at a concentration of 3.25 μm as the bases of trimer.
Visualization of Interaction between Hsp47 and Collagen Model Peptides Fused to Foldon in Living Mammalian Cells
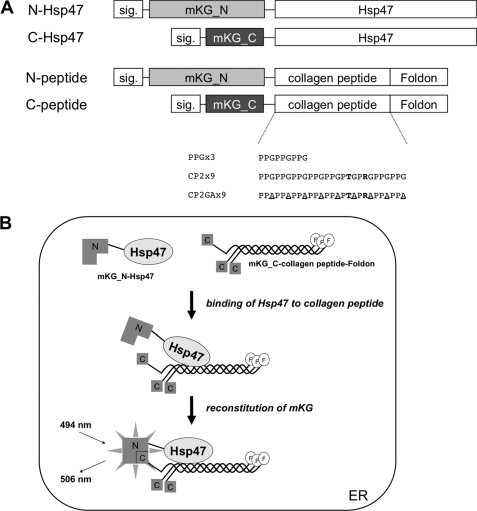
To examine the interaction of Hsp47 with the collagen model peptide in living mammalian cells, we developed a novel visualization system using a BiFC technique (24) based on the reconstitution of two split fragments of the mKG fluorescent protein. When two proteins harboring different mKG fragments interact with each other, the mKG fragments (mKG_N and mKG_C) are drawn into close proximity with one another, resulting in the emission of chromophore fluorescence (Fig. 4B). The single mKG fluorescent protein fragments do not emit fluorescence unless both fragments are brought together by the interaction of two target proteins.
FIGURE 4.
Visualization system to detect interaction between Hsp47 and collagen model peptides fused to foldon using BiFC in living cells. A, schematic representation of Hsp47 proteins and collagen model peptides used for transient expression in HeLa cells. The ER signal sequence (sig.) was derived from human Hsp47 (amino acids 1–17). Arg and Thr residues in collagen model peptides are shown in boldface. Ala residues substituted for Gly are underlined. B, visualization of the interaction between Hsp47 and collagen model peptides fused to foldon (F) in transfected cells. The principle was based on the reconstitution of two split fragments of the mKG fluorescent protein (mKG_N (N) and mKG_C (C)). Following interaction of the target proteins, mKG_N and mKG_C moved into close proximity, increasing the local concentration of both fragments. As a result, mKG fragments formed a steric structure before dividing, and the chromophore emitted fluorescence.
To express both Hsp47 and the collagen model peptide in the ER, we inserted the ER signal sequence from human Hsp47 into the mKG expression vectors (Fig. 4A). We first attempted to detect the interaction between the collagen model peptide and Hsp47 in this system by transfecting mKG_N-fused wild-type Hsp47 (N-Hsp47 wt) and mKG_C-fused model peptide (C-CP2×9 (F −)) into the cells. However, no significant interaction between the collagen peptide and Hsp47 was detected (Fig. 5B, lower row), which may be due to a failure to achieve the correct triple-helix conformation in the collagen model peptide.
FIGURE 5.
Visualization of in vivo interaction between Hsp47 and collagen model peptides. A, immunofluorescence staining of HeLa cells transfected with ER-mKG_C-Hsp47wt (C-Hsp47 wt; upper row) and ER-mKG_C-CP2×9 fused to foldon (C-CP2×9; lower row). Three days after transfection, the cells were fixed, stained with an anti-mKG_C monoclonal antibody and an anti-PDI polyclonal antibody, and visualized with Alexa Fluor 555-conjugated anti-mouse IgG and Alexa Fluor 488-conjugated anti-rabbit IgG. The nuclei were stained with Hoechst 33342. All images were acquired using the IN Cell Analyzer 1000 system. Scale bar = 10 μm. B and C, visualization of immunofluorescence staining and mKG fluorescence in HeLa cells transfected with various expression vectors with or without (E −) the ER signal sequence, respectively. Staining of PDI was performed using anti-PDI monoclonal antibody and Alexa Fluor 555-conjugated anti-mouse IgG. The nuclei were stained with Hoechst 33342. All images were acquired using the IN Cell Analyzer 1000 system. F −, without foldon. Scale bars = 10 μm.
Foldon, derived from the C-terminal domain of T4 fibritin, is known to participate in the formation of trimeric proteins (21–23, 31). To promote trimer formation, we incorporated foldon at the C terminus of the collagen model peptide (Fig. 4A). The localization of ER-mKG_C-Hsp47wt (C-Hsp47 wt) and ER-mKG_C-CP2×9 fused to foldon (C-CP2×9) in the ER was confirmed by immunofluorescence staining using an antibody specific for mKG_C together with co-staining with the ER marker PDI (Fig. 5A). Interaction between ER-mKG_C-Hsp47wt and ER-mKG_N-CP2×9 fused to foldon (N-CP2×9) was clearly observed as the emission of fluorescence (Fig. 5B, upper three rows). A similar result was also obtained when the inverse combination of fused mKG fragments (N-Hsp47 wt and C-CP2×9) was used (Fig. 5B, lower three rows). When mKG_C-Hsp47wt and mKG_N-CP2×9 fusion proteins without the ER signal sequence were cotransfected, reconstitution of mKG was also observed in the cytosol (Fig. 5C and supplemental Fig. 6B). However, the fluorescence in the cytosol was weaker than that in the ER, possibly due to decreased protein stability associated with incorrect protein localization. These results suggest that foldon is useful for facilitating trimer formation in the collagen model peptide fused to mKG in cells. These observations also clearly indicated that Hsp47 could interact with the collagen model peptide only in its trimer form.
To confirm that the fluorescence of reconstituted mKG observed in Fig. 5B was due to a specific interaction between Hsp47 and the collagen model peptide, we tested the activity of an Hsp47 mutant and model peptides with GPP repeats of varying length in the experimental system. When the mKG_N-Hsp47 CAYA mutant and mKG_C-CP2×9 peptide were cotransfected, no reconstituted mKG fluorescence was observed (Fig. 6A and supplemental Fig. 6A), even though these proteins were expressed at the same level as the wild-type proteins (Fig. 6, B and C). This result is consistent with our in vitro data (Fig. 3). Cotransfection of mKG_N-Hsp47wt and mKG_C-PPG×3 peptide, which has only three PPG repeats, also did not result in mKG fluorescence (Fig. 6A and supplemental Fig. 6A). The same result was obtained using the PPG×5 peptide, with five PPG repeats (data not shown). Considering that these peptides had foldon at the C terminus, the failure to reconstitute mKG might be due to a failure to achieve triple-helix conformation because of the short GPP repeat region, which is consistent with a previous report on the interaction of recombinant Hsp47 with collagen model peptides with various lengths of PPG repeats in vitro (29). Similarly, no specific reconstitution of mKG was observed following the cotransfection of mKG_N-Hsp47wt and mKG_C-CP2GA×9, in which all Gly residues in CP2×9 were replaced by Ala (see Fig. 4A), leading to a failure to form a triple helix (Fig. 6A and supplemental Fig. 6A). These results clearly establish that Hsp47 recognizes the triple-helix form of collagen not only in vitro but also in vivo.
FIGURE 6.
Specific interaction of Hsp47 with triple helices of collagen model peptides in vivo. A, fluorescent images of HeLa cells coexpressing (i) various collagen model peptides fused to foldon and mKG_C and (ii) wild-type or CAYA mutant Hsp47 fused to mKG_N. All constructs also had an ER signal sequence. Three days after transfection, fluorescence from reconstituted mKG, together with Hoechst 33342 staining, was measured using the IN Cell Analyzer 1000 system. Scale bar = 10 μm. B and C, expression levels of Hsp47 and foldon-fused collagen model peptides in transfected HeLa cells, respectively. Whole cell lysates were extracted and analyzed by Western blotting using specific antibodies against Hsp47 (B) and the mKG_C fragment (C). The asterisk indicates a nonspecific band. The molecular sizes are shown in kilodaltons. w, wild-type; C, CAYA mutant; Hsp47(endo), endogenous Hsp47.
DISCUSSION
We have developed an in vitro novel binding assay in which the interaction between purified recombinant Hsp47 and synthetic collagen model peptides was analyzed by TR-FRET signals, without the need for washing. This technique provides a tremendous advantage over existing techniques to measure protein-protein interactions, including immunoprecipitation, surface plasmon resonance, and pulldown techniques (27–29). For example, indirect interaction via other proteins cannot be excluded using immunoprecipitation or pulldown techniques, and steric effects can potentially interfere with analysis by surface plasmon resonance. Additional advantages of our new technique include the shorter overall incubation time, greater throughput capacity, stability of the readout signal, and ease of automation for high-throughput screening. Recently, Okano-Kosugi et al. (32) reported a turbidimetric assay to screen inhibitors of collagen-binding proteins, including Hsp47. The assay is based on changes in absorbance at 313 nm due to collagen fibril formation in vitro. However, this interesting method is not suitable for drug screening because of the large number of compounds with absorption close to 313 nm, which could cause false positive or negative signals. Meanwhile, the TR-FRET technique that we have employed in this assay may be an effective tool for drug screening due to the large Stokes shift of the assay and the long lifetime of the emitted light.
Several lines of circumstantial evidence suggest that Hsp47 recognizes triple helices of collagen preferentially rather than unfolded collagen chains in the ER (18–20). Using a novel TR-FRET assay, we have now conclusively demonstrated in vitro that Hsp47 can interact with the trimer form of a collagen model peptide containing GPP triplets and GPTGPR, although it cannot interact with the monomer form (Fig. 2). Collagen model peptides with GPP triplet repeats form a triple helix at 25 °C when the number of repeats is greater than nine, which was judged by CD spectroscopic analysis (30, 33), and our results therefore strongly suggest that Hsp47 binds to the triple-helix form of collagen.
The trimer fraction of the collagen model peptide was converted to the monomer form by heat treatment at 37 °C (Fig. 3B) but reverted to the trimer form after cooling at 4 °C (Fig. 3F). By referring to supplemental Fig. 2A, the transition from trimer to monomer did not occur during gel filtration. Interestingly, the monomer form separated by GFC did not revert to the trimer after cooling at 4 °C (supplemental Fig. 5F). The data presented in supplemental Fig. 3 suggest that the formation of the triple helix is concentration-dependent. The concentration of the monomer fraction separated by GFC was 4.9 μm, which was substantially higher than the concentration of 1 μm at which trimer formation can take place (supplemental Fig. 3A). Some unidentified difference might cause such a difference in the triple-helix formation.
We also successfully developed a novel visualization system for detecting the interaction of Hsp47 with collagen model peptides within the ER of mammalian cells using a BiFC technique (24) based on the reconstitution of two split fragments of the fluorescent protein mKG (25). We first attempted to detect the interaction of Hsp47 with the collagen model peptide fused to the C terminus of mKG together with the ER signal sequence. However, no significant interaction was detected (Fig. 5B, lower row). Detection of the interacting signals was dramatically improved by incorporating the foldon sequence into the collagen model peptide, suggesting that collagen model peptides do not form trimers without foldon and that Hsp47 cannot interact with non-trimeric model peptides within the cells. Collagen model peptides without foldon may be unable to form trimers because of conformational restrictions or poor prolyl hydroxylation by prolyl 4-hydroxylase due to the fusion with mKG or to decreased triple-helix formation at the incubation temperature of 37 °C, as described above. Du et al. (31) reported that the thermostability of recombinant collagen-like proteins was significantly improved when fused with the foldon sequence. Thus, the promotion of trimer formation in the collagen model peptides by foldon was necessary for the interaction with Hsp47 in this in vivo visualization system.
No significant interaction was detected after the cotransfection of Hsp47 with (i) the PPG×3 peptide, which has only three Pro-Pro-Gly triplet repeats (Fig. 6A and supplemental Fig. 6A); (ii) the PPG×5 peptide, which has five Pro-Pro-Gly triplet repeats (data not shown); and (iii) the CP2GA×9 peptide, in which all of the Gly residues in CP2×9 were replaced by Ala (Fig. 6A and supplemental Fig. 6A). These results are attributed to the failure to form triple helices because of the small number of the triplet repeats (i and ii) or steric hindrance due to the substitution of Gly with Ala at the innermost position of the triple-helical structure (iii). Notably, the data using PPG×3 and PPG×5 peptides correlate well with previous work by Koide et al. (29), in which Hsp47 did not interact with collagen model peptides containing fewer than six GPP triplet repeats. From these results, we conclude that the novel visualization system described here provides an efficient and specific methodology for detecting interactions between collagen-binding proteins and collagen model peptides within the ER of mammalian cells. To our knowledge, this is the first study demonstrating the real-time interaction of Hsp47 with collagen model peptides in living cells.
In summary, we have conclusively demonstrated that Hsp47 recognizes and binds the triple-helix form (but not the monomer form) of collagen model peptides in vitro and in vivo using two newly developed methods. These findings suggest an important role for Hsp47 as a collagen-specific molecular chaperone in stabilizing the triple helices of procollagen in the ER. Our newly developed in vitro and in vivo analysis systems may also provide useful tools for screening small molecule inhibitors of the interaction between Hsp47 and collagen.
Supplementary Material
Acknowledgment
We thank Rina Isobe for technical assistance.

This article contains supplemental Figs. 1–6.
- PDI
- protein-disulfide isomerase
- ER
- endoplasmic reticulum
- TR
- time-resolved
- BiFC
- bimolecular fluorescence complementation
- mKG
- monomeric Kusabira-Green
- SA
- streptavidin
- Eu-K
- europium cryptate
- GFC
- gel filtration chromatography.
REFERENCES
- 1. Prockop D. J., Kivirikko K. I. (1995) Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 64, 403–434 [DOI] [PubMed] [Google Scholar]
- 2. Byers P. H. (2001) Folding defects in fibrillar collagens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marini J. C., Forlino A., Cabral W. A., Barnes A. M., San Antonio J. D., Milgrom S., Hyland J. C., Körkkö J., Prockop D. J., De Paepe A., Coucke P., Symoens S., Glorieux F. H., Roughley P. J., Lund A. M., Kuurila-Svahn K., Hartikka H., Cohn D. H., Krakow D., Mottes M., Schwarze U., Chen D., Yang K., Kuslich C., Troendle J., Dalgleish R., Byers P. H. (2007) Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen-binding sites for integrins and proteoglycans. Hum. Mutat. 28, 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chessler S. D., Byers P. H. (1993) BiP binds type I procollagen pro-α-chains with mutations in the carboxyl-terminal propeptide synthesized by cells from patients with osteogenesis imperfecta. J. Biol. Chem. 268, 18226–18233 [PubMed] [Google Scholar]
- 5. Ferreira L. R., Norris K., Smith T., Hebert C., Sauk J. J. (1994) Association of Hsp47, Grp78, and Grp94 with procollagen supports the successive or coupled action of molecular chaperones. J. Cell Biochem. 56, 518–526 [DOI] [PubMed] [Google Scholar]
- 6. Wilson R., Lees J. F., Bulleid N. J. (1998) Protein-disulfide isomerase acts as a molecular chaperone during the assembly of procollagen. J. Biol. Chem. 273, 9637–9643 [DOI] [PubMed] [Google Scholar]
- 7. Walmsley A. R., Batten M. R., Lad U., Bulleid N. J. (1999) Intracellular retention of procollagen within the endoplasmic reticulum is mediated by prolyl 4-hydroxylase. J. Biol. Chem. 274, 14884–14892 [DOI] [PubMed] [Google Scholar]
- 8. Nagata K. (2003) HSP47 as a collagen-specific molecular chaperone: function and expression in normal mouse development. Semin. Cell Dev. Biol. 14, 275–282 [DOI] [PubMed] [Google Scholar]
- 9. Vranka J. A., Sakai L. Y., Bächinger H. P. (2004) Prolyl 3-hydroxylase 1, enzyme characterization and identification of a novel family of enzymes. J. Biol. Chem. 279, 23615–23621 [DOI] [PubMed] [Google Scholar]
- 10. Nagata K., Saga S., Yamada K. M. (1986) A major collagen-binding protein of chick embryo fibroblasts is a novel heat shock protein. J. Cell Biol. 103, 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saga S., Nagata K., Chen W. T., Yamada K. M. (1987) pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J. Cell Biol. 105, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagai N., Hosokawa M., Itohara S., Adachi E., Matsushita T., Hosokawa N., Nagata K. (2000) Embryonic lethality of molecular chaperone Hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 150, 1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tasab M., Batten M. R., Bulleid N. J. (2000) Hsp47: a molecular chaperone that interacts with and stabilizes correctly folded procollagen. EMBO J. 19, 2204–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marutani T., Yamamoto A., Nagai N., Kubota H., Nagata K. (2004) Accumulation of type IV collagen in dilated ER leads to apoptosis in Hsp47 knockout mouse embryos via induction of CHOP. J. Cell Sci. 117, 5913–5922 [DOI] [PubMed] [Google Scholar]
- 15. Matsuoka Y., Kubota H., Adachi E., Nagai N., Marutani T., Hosokawa N., Nagata K. (2004) Insufficient folding of type IV collagen and formation of abnormal basement membrane-like structure in embryoid bodies derived from Hsp47-null embryonic stem cells. Mol. Biol. Cell 15, 4467–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishida Y., Kubota H., Yamamoto A., Kitamura A., Bächinger H. P., Nagata K. (2006) Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol. Biol. Cell 17, 2346–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koide T., Takahara Y., Asada S., Nagata K. (2002) Xaa-Arg-Gly triplets in the collagen triple helix are dominant binding sites for the molecular chaperone HSP47. J. Biol. Chem. 277, 6178–6182 [DOI] [PubMed] [Google Scholar]
- 18. Tasab M., Jenkinson L., Bulleid N. J. (2002) Sequence-specific recognition of collagen triple helices by the collagen-specific molecular chaperone HSP47. J. Biol. Chem. 277, 35007–35012 [DOI] [PubMed] [Google Scholar]
- 19. Koide T., Asada S., Takahara Y., Nishikawa Y., Nagata K., Kitagawa K. (2006) Specific recognition of the collagen triple helix by chaperone HSP47: minimal structural requirement and spatial molecular orientation. J. Biol. Chem. 281, 3432–3438 [DOI] [PubMed] [Google Scholar]
- 20. Thomson C. A., Tenni R., Ananthanarayanan V. S. (2003) Mapping Hsp47-binding site(s) using CNBr peptides derived from type I and type II collagen. Protein Sci. 12, 1792–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frank S., Kammerer R. A., Mechling D., Schulthess T., Landwehr R., Bann J., Guo Y., Lustig A., Bächinger H. P., Engel J. (2001) Stabilization of short collagen-like triple helices by protein engineering. J. Mol. Biol. 308, 1081–1089 [DOI] [PubMed] [Google Scholar]
- 22. Stetefeld J., Frank S., Jenny M., Schulthess T., Kammerer R. A., Boudko S., Landwehr R., Okuyama K., Engel J. (2003) Collagen stabilization at atomic level: crystal structure of designed (GlyProPro)10foldon. Structure 11, 339–346 [DOI] [PubMed] [Google Scholar]
- 23. Meier S., Güthe S., Kiefhaber T., Grzesiek S. (2004) Foldon, the natural trimerization domain of T4 fibritin, dissociates into a monomeric A-state form containing a stable β-hairpin: atomic details of trimer dissociation and local β-hairpin stability from residual dipolar couplings. J. Mol. Biol. 344, 1051–1069 [DOI] [PubMed] [Google Scholar]
- 24. Kerppola T. K. (2006) Visualization of molecular interactions by fluorescence complementation. Nat. Rev. Mol. Cell Biol. 7, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ueyama T., Kusakabe T., Karasawa S., Kawasaki T., Shimizu A., Son J., Leto T. L., Miyawaki A., Saito N. (2008) Sequential binding of cytosolic Phox complex to phagosomes through regulated adaptor proteins: evaluation using the novel monomeric Kusabira-Green system and live imaging of phagocytosis. J. Immunol. 181, 629–640 [DOI] [PubMed] [Google Scholar]
- 26. Saxena P., Walker J. R. (1992) Expression of argU, the Escherichia coli gene coding for a rare arginine tRNA. J. Bacteriol. 174, 1956–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakai A., Satoh M., Hirayoshi K., Nagata K. (1992) Involvement of the stress protein HSP47 in procollagen processing in the endoplasmic reticulum. J. Cell Biol. 117, 903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Natsume T., Koide T., Yokota S., Hirayoshi K., Nagata K. (1994) Interactions between collagen-binding stress protein HSP47 and collagen. Analysis of kinetic parameters by surface plasmon resonance biosensor. J. Biol. Chem. 269, 31224–31228 [PubMed] [Google Scholar]
- 29. Koide T., Asada S., Nagata K. (1999) Substrate recognition of collagen-specific molecular chaperone HSP47. Structural requirements and binding regulation. J. Biol. Chem. 274, 34523–34526 [DOI] [PubMed] [Google Scholar]
- 30. Koide T., Nishikawa Y., Asada S., Yamazaki C. M., Takahara Y., Homma D. L., Otaka A., Ohtani K., Wakamiya N., Nagata K., Kitagawa K. (2006) Specific recognition of the collagen triple helix by chaperone HSP47. II. The HSP47-binding structural motif in collagens and related proteins. J. Biol. Chem. 281, 11177–11185 [DOI] [PubMed] [Google Scholar]
- 31. Du C., Wang M., Liu J., Pan M., Cai Y., Yao J. (2008) Improvement of thermostability of recombinant collagen-like protein by incorporating a foldon sequence. Appl. Microbiol. Biotechnol. 79, 195–202 [DOI] [PubMed] [Google Scholar]
- 32. Okano-Kosugi H., Matsushita O., Asada S., Herr A. B., Kitagawa K., Koide T. (2009) Development of a high-throughput screening system for the compounds that inhibit collagen-protein interactions. Anal. Biochem. 394, 125–131 [DOI] [PubMed] [Google Scholar]
- 33. Nishikawa Y., Takahara Y., Asada S., Shigenaga A., Otaka A., Kitagawa K., Koide T. (2010) A structure-activity relationship study elucidating the mechanism of sequence-specific collagen recognition by the chaperone HSP47. Bioorg. Med. Chem. 18, 3767–3775 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.