Background: Eph kinases constitute the largest receptor tyrosine kinase family, and there is no knowledge about their function in blood pressure regulation.
Results: Ephb6 regulates vascular smooth muscle cell contraction, and its knock-out resulted in increased blood pressure in castrated male mice.
Conclusion: Ephb6 and its ligands can regulate vessel tone and blood pressure.
Significance: We have identified a new group of molecules capable of regulating blood pressure.
Keywords: Gene Knockout, Hypertension, Protein Kinases, Vascular Biology, Vascular Smooth Muscle Cells, Ephb6, Grip1, Blood Pressure, Ephrins, Reverse Signaling
Abstract
Eph kinases constitute the largest receptor tyrosine kinase family, and their ligands, ephrins (Efns), are also cell surface molecules. Our study is the first to assess the role of Ephb6 in blood pressure (BP) regulation. We observed that EphB6 and all three of its Efnb ligands were expressed on vascular smooth muscle cells (VSMC) in mice. We discovered that small arteries from castrated Ephb6 gene KO males showed increased contractility, RhoA activation, and constitutive myosin light chain phosphorylation ex vivo compared with their WT counterparts. Consistent with this finding, castrated Ephb6 KO mice presented heightened BP compared with castrated WT controls. In vitro experiments in VSMC revealed that cross-linking Efnbs but not Ephb6 resulted in reduced VSMC contractions, suggesting that reverse signaling through Efnbs was responsible for the observed BP phenotype. The reverse signaling was mediated by an adaptor protein Grip1. Additional experiments demonstrated decreased 24-h urine catecholamines in male Ephb6 KO mice, probably as a compensatory feedback mechanism to keep their BP in the normal range. After castration, however, such compensation was abolished in Ephb6 KO mice and was likely the reason why BP increased overtly in these animals. It suggests that Ephb6 has a target in the nervous/endocrine system in addition to VSMC, regulating a testosterone-dependent catecholamine compensatory mechanism. Our study discloses that Ephs and Efns, in concert with testosterone, play a critical role in regulating small artery contractility and BP.
Introduction
Primary hypertension represents a high blood pressure (BP)3 condition that is not induced by other diseases. Approximately 90–95% of hypertension cases fall into this category. As a result of numerous studies, some risk genes contributing to primary hypertension have been identified, such as genes related to the renin-angiotensin-aldosterone system (1), the sympatho-adrenal system (2), endothelial hormones (3, 4), and sex steroids (5–7), to name a few. However, the etiology of primary hypertension remains incompletely understood.
Ephs, the largest family of receptor tyrosine kinases, comprise ∼25% of known receptor tyrosine kinases (8). The ligands of Ephs, ephrins (Efns), are also cell surface molecules (8) and can transduce signals into cells (9) in a phenomenon called reverse signaling. Interactions among Ephs and Efns are promiscuous. One Eph can interface with multiple Efns and vice versa. In general, EphA members interact preferentially with EfnA members, and EphB members interact preferentially with EfnB members (10–12). Such interactions suggest that these molecules are so vital to biological systems that heavy redundancy is obligatory.
Various Eph and Efn members are expressed in various tissues and organs. They are important not only in embryonic development but also in physiological and pathophysiological conditions in adults. Most reported functions of Ephs occur in the central nervous system (10, 11). They are essential in the development of neuronal connections, circuit plasticity, and repair. Some Ephs and Efns, expressed in endothelial cells, are vital in angiogenesis during normal embryonic development as well as in tumorigenesis (12, 13). This and other studies have reported that Ephs and Efns, particularly their B family members, as well as some A family members, are expressed in thymocytes and T cells; they are capable of modulating T-cell responses and survival (14). It has been shown that Ephs/Efns are involved in intestinal epithelium self-renewal (15), urorectal development (16), pancreatic β-cell insulin secretion (17), bone development, maintenance and repair (18, 19), regulation of red blood cell production in response to hypoxia (20), clotting (21), glomerular filtration (22), and ionic homeostasis of vestibular endolymph fluid in the inner ear (23).
Efnb2 and Efna1 are expressed in vascular smooth muscle cells (VSMC) (24–27). Conditional KO of Efnb2 in pericytes and VSMC with a platelet-derived growth factor receptor β promoter leads to perinatal lethality (24). VSMC with Efnb2 deletion manifest compromised migration (24). Ogita et al. (28) showed that in long term cultures of rat and human VSMC, Efna1 triggered Epha4 signaling and actin stress fiber assembly, but whether such signaling elicited changes in VSMC contractility was not investigated. Therefore, Eph and Efn function in VSMC contractility and BP regulation has not been studied to date.
Our earlier DNA microarray analysis of Ephb6 KO mouse thymocytes indicated that the expression of some genes regulating BP seems to be altered. Based on this clue, we hypothesized that Ephb6 KO mice might have abnormal BP. This hypothesis was the focus of our study.
MATERIALS AND METHODS
EphB6 KO Mice
EphB6 KO mice were generated in our laboratory, as described previously (29). They have been backcrossed to the C57BL/6 background for more than 10 generations. Age- and gender-matched WT littermates or C57BL/6 mice were used as controls and are referred to as WT mice.
We also generated transgenic mice with human β-actin promoter-driven expression of a truncated Ephb6 (amino acids 1–667), whose intracellular domain was deleted, with a plasmid construct pAC-Ephb6Δ, as illustrated in supplemental Fig. S1. The transgenic mice were backcrossed to the C57BL/6 background for 10 generations and then crossed with Ephb6 KO. The resulting mice, called Ephb6Δ/KO mice, expressed tailless Ephb6 on the cell surface. Again, age- and gender-matched WT littermates or C57BL/6 mice were used as controls and are referred to as WT mice.
RT-qPCR
Ephn6, Efnb1, Efnb2, Efnb3, Dishevelled, PDZ-RGS3, and Grip1 mRNA levels were measured by RT-qPCR. Total RNA from VSMC or mesenteric artery endothelial cells was extracted with TRIzol® (Invitrogen) and then reverse-transcribed with Superscript IITM reverse transcriptase (Invitrogen). The primers utilized in RT-qPCR and the fragment sizes amplified are listed in supplemental Table SI. PCR conditions for the reactions were as follows: 2 min at 50 °C, 2 min at 95 °C, followed by 20–25 cycles of 10 s at 94 °C, 20 s at 58 °C, and 20 s at 72 °C. β-Actin mRNA levels served as internal controls, and the data were expressed as signal ratios of test gene mRNA/β-actin mRNA.
VSMC Isolation
Mouse VSMC were isolated as described by Golovina and Blaustein (30) with modifications. The aorta and mesenteric arteries, including their secondary branches, were digested with collagenase type II (347 units/ml) (Worthington Biochemical Corporation, Lakewood, NJ). These vessels were washed twice, and the adventitia and endothelium were removed with fine forceps and sterile cotton-tipped applicators. They were further digested with both collagenase type II (347 units/ml) and elastase type IV (6 units/ml) (Sigma-Aldrich). The dissociated cells were cultured at 37 °C in Dulbecco's modified Eagle's medium (Wisent; St-Bruno, Canada) supplemented with 15% fetal bovine serum for 4–5 days before experimentation. In the studies of sex hormones, VSMC were cultured in 15% stripped fetal bovine serum (serum reacted with active charcoal for 24 h to remove bovine sex hormones).
Immunofluorescence Microscopy
VSMC cultured for 4–5 days were fixed with paraformaldehyde (4%) for 15 min. The cells were blocked with 10% goat IgG in phosphate-buffered saline for 20 min and then incubated with various first antibodies (Abs, 2 μg/ml): goat anti-mouse Efnb1, Efnb1, Efnb3, and Ephb6 Abs (R & D Systems, Minneapolis, MN). The cells were reacted overnight at 4 °C with rhodamine-conjugated donkey anti-goat Ab (0.15 μg/ml; Jackson ImmunoResearch Laboratories, West Grove, PA). For intracellular α-actin staining, the cells were permeabilized with permeabilization buffer (BD Biosciences, San Jose, CA) for 20 min at 4 °C and then incubated with mouse anti-human α-actin mAbs (2 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) followed by FITC-conjugated goat anti-mouse IgG (0.2 μg/ml; Bethyl Laboratories, Montgomery, TX). The stained cells were examined under a Zeiss microscope.
Ex Vivo Vessel Constriction
Vessel constriction was studied ex vivo as described previously (31). Mesenteric artery segments (2 mm in length) of third-order branches (exterior diameter, 125–150 μm) were stripped off endothelium and mounted on 20-μm tungsten wires in small vessel myographs, stretched to optimal tension, and maintained in physiological saline solution (130 mm NaCl, 4.7 mm KCl, 1.18 mm KH2PO4, 1.17 mm MgSO4, 1.17 mm NaHCO3, 1.6 mm CaCl2, 0.023 mm EDTA, 10 mm glucose, aerated with 12% O2, 5% CO2, 83% N2, pH 7.4) at 37 °C. After a 40-min stabilization period, arterial segments were challenged with 40 mm KCl physiological saline solution (KCl was substituted for an equivalent concentration of NaCl). Single cumulative concentration-response curves to the α1-adrenergic receptor agonist phenylephrine (PE, 1 nm to 100 μm; Sigma) were charted. At the end of the protocol, maximal tension (Emax) was determined by changing the physiological saline solution to a solution containing 127 mm KCl. The data are expressed as percentages of Emax. Student's t tests were performed to compare concentration-response curves.
Immunoblotting of Myosin Light Chain (MLC) Phosphorylation
The aorta and mesenteric arteries of WT and KO mice were isolated, washed twice with Ca2+-free Hanks' buffered salt solution buffer, and then frozen in liquid nitrogen until they were used. The vessels were homogenized for 1 min at room temperature in 0.4 ml of radioimmunoprecipitation assay buffer, which contained Pho-stop and Protease Inhibitor Mixture (Roche Applied Science, Laval, Canada). The samples were spun at 12,000 rpm for 15 min at 4 °C, and the supernatants were collected. Twenty micrograms of proteins/sample were resolved in 12% SDS-PAGE. Proteins from the gel were transferred to PVDF membranes (Invitrogen), which were incubated in blocking buffer containing 5% (w/v) skimmed milk (for MLC, 5% BSA was used in the blocking buffer) for 1 h at room temperature and then hybridized overnight at 4 °C with mouse anti-mouse phospho-MLC mAb or rabbit anti-mouse total MLC Ab (both from Cell Signaling Technology, Danvers, MA). The Abs were used at the manufacturer's recommended dilutions or at 1:1,000. The membranes were washed three times and reacted with corresponding second Abs, i.e. horseradish peroxidase-conjugated sheep anti-mouse IgG Ab (GE Healthcare, Baie D'Urfe, Canada) or horseradish peroxidase-conjugated rabbit anti-goat IgG Ab (R & D Systems) for 90 min. The signals were detected with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Rockford, IL).
BP Measurements by Radiotelemetry
The mice were anesthetized with isoflurane and implanted surgically with TA11PA-C10 radiotelemetry sensors (Data Sciences International, St. Paul, MN) in the left carotid artery for direct measurement of arterial pressure and heart rate (HR), as described previously (32). Seven days were allowed for recovery before measurement. For castrated mice, the measurement was conducted 21 days after surgery. In some experiments, male mice were castrated one week after transmitter implantation; three weeks after the castration, mice were administered with estrogen (240 mg) daily subcutaneously for 7 days. Their BP and HR were measured by telemetry during the last 3 days of estrogen administration.
Arterial pressure and HR in conscious, free-moving mice were then recorded continuously for 3 days with the Dataquest acquisition 3.1 system (Data Sciences International). Individual 10-s waveforms of systolic pressure, diastolic pressure, mean arterial pressure, and HR were sampled every 2 min throughout the monitoring period. The raw data were processed with the Dataquest A.R.T-Analysis program (32) and are presented as the means ± S.E. Statistically significant differences between the experimental groups were evaluated by unpaired t test and repeated measures analysis of variance with Statview. Values of p < 0.05 were considered to be statistically significant.
Measurement of VSMC Contractility
Cultured primary VMSC were washed once with Hanks' buffered salt solution and cultured in the same solution. They were placed under a Zeiss microscope with environmental controls (37 °C and 5% CO2). The cells were stimulated with PE (20 μm), unless otherwise indicated, and photographed continuously for 15 min at a rate of 1 picture/min. Fifteen or more cells were selected randomly, and their length was measured at each time point with Zeiss Axiovision software. Percentage contraction was calculated as follows: % contraction = (cell length at time 0 − cell length at time X)/cell length at time 0. Student's t test was performed to assess statistically significant differences.
siRNA Transfection
siRNAs of Disheveled, PDZ-RGS3, and Grip1 as well as negative control siRNAs were synthesized by Integrated DNA Technologies (Coralville, IA), and their sequences are shown in supplemental Table SII. VSMC were cultured for 4–5 days, with the last 16 h being free of antibiotics, and then transfected with a mix of three pairs of siRNAs of a particular gene (each pair at a final concentration of 10 nm), with FuGENE HD X-tremeGENE siRNA transfection reagent (Roche Applied Science). The transfected VSMC were further cultured for 24 to 36 h, and their contractility was measured upon PE (10 μm) stimulation.
Activated RhoA Assay
GTP-associated activated RhoA levels in mesenteric artery smooth muscles were determined by G-LISK RhoA activation assay kit (Cytoskeleton Inc. Denver, CO) according to the manufacturer's instructions.
RESULTS
Expression of Ephb6 and Its Ligands in VSMC
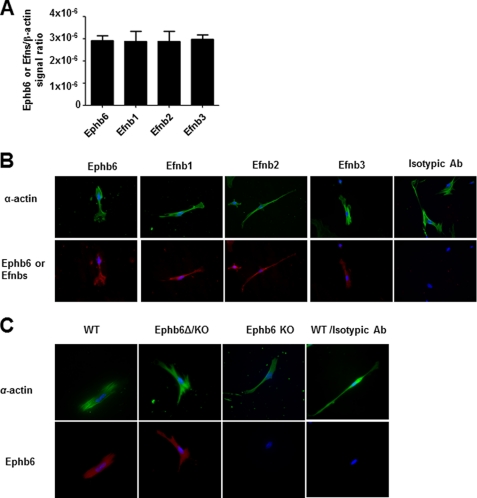
The mRNA expression of Ephb6, as well as three of its major ligands, Efnb1, Efnb2, and Efnb3, in VSMC was detectable by RT-qPCR (Fig. 1A). We assessed these four molecules in VSMC at the protein level by immunofluorescence (Fig. 1B). The expression of Ephb6 and its ligands in these cells suggests that Ephb6 might regulate VSMC function. Ephb6Δ/KO mice expressed tailless Ephb6 (Ephb6Δ) on the cell surface. Fig. 1C demonstrates the lack of Ephb6 expression in Ephb6 KO VSMC and re-expression of cell surface Ephb6Δ in Ephb6Δ/KO VSMC.
FIGURE 1.
Expression of Ephb6 and Efnbs in mouse VSMC. All of the experiments were repeated at least twice, and data from a representative experiment are reported. A, Ephb6, Efnb1, Efnb2, and Efnb3 mRNA expression in mouse VSMC. Ephb6, Efnb1, Efnb2, and Efnb3 mRNA expression in VSMC from mesenteric arteries and aorta was measured by RT-qPCR with β-actin mRNA as internal control. The means ± S.D. of Ephb6 or Efnb signal/β-actin signal ratios are shown. B, Ephb6 and Efnb expression on VSMC according to immunofluorescence. WT VSMC cultured for 4–5 days were stained with FITC goat anti-α-actin (in pseudo-green color) Ab and goat anti-mouse-Ephb6, Efnb1, Efnb2, and Efnb3 Abs, followed by PE donkey anti-goat IgG (in pseudo-red color). Cells stained with control isotypic first Ab control (normal goat IgG, last column) showed no specific signal. C, VSMC from Ephb6Δ/KO mice re-expressed cell surface Ephb6. VSMC from WT (first and last columns), Ephb6Δ/KO, and Ephb6 KO mice were stained with anti-Ephb6 Ab (first three columns) or isotypic control Ab (last column) and anti-α-actin Ab, as indicated, and two-color micrographs are presented.
Ephb6 Null Mutation Alters Blood Vessel Contraction
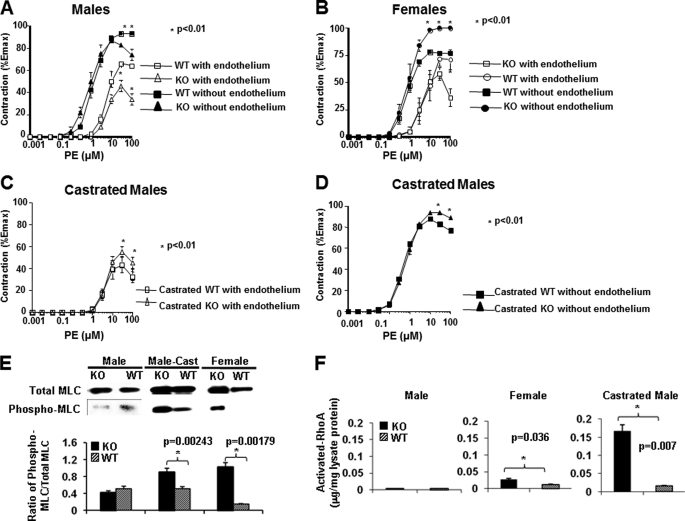
BP is controlled by cardiac output, vessel resistance, and blood volume. We did not observe any difference in cardiac output between KO and WT mice according to echocardiography (data not included). We then examined small artery contractility, a major factor contributing to BP in terms of peripheral blood flow resistance. Ex vivo contractility of the mesenteric arteries from KO and WT mice was assessed after stimulation with PE. Male KO vessels showed lower contractility than male WT vessels (Fig. 2A), whereas female KO vessels presented higher contractility than WT vessels after PE stimulation (Fig. 2B). This raised the possibility that Ephb6 might work in concert with sex hormone to regulate vasoconstriction. To test whether such is the case, we castrated male Ephb6 KO mice. Indeed, vessels from castrated KO males manifested increased contractility compared with castrated WT males (Fig. 2, C and D). It is to be noted that these observed contractility phenotypes remained unchanged in the presence or absence of endothelium (Fig. 2, A–D), indicating that the nitric oxide production by the endothelium is not involved in the altered contractility in the absence of Ephb6 and male sex hormone.
FIGURE 2.
Contractility, MLC phosphorylation, and RhoA activation of mesenteric arteries from Ephb6 KO and WT mice. A–D, contractility of mesenteric arteries from EphB6 KO and WT mice following PE stimulation. Segments (2 mm) of the third order branch of the mesenteric artery with endothelium or with endothelium removed were stimulated with PE. A single cumulative concentration-response curve to PE (1 nm to 100 μm) was obtained. The maximal tension (Emax) was determined by challenging the vessels with a physiological saline containing 127 mm KCl. Vessel contractility is expressed as a percentage of the Emax. The data from three mice per group were pooled, and the means ± S.E. are shown. *, p < 0.01 (paired Student's t test). A, vessel contractility of males; B, vessel contractility of females; C, vessel contractility with endothelium of castrated males; D, vessel contractility without endothelium of castrated males. E, constitutive MLC phosphorylation in Ephb6 KO arteries. Proteins from freshly isolated mesenteric arteries and aortas of male, castrated male (Male-Cast), and female WT and Ephb6 KO mice were analyzed for total and phosphorylated MLC by immunoblotting. The experiments were repeated three times, and data from representative immunoblotting are shown. The means ± S.D. of signal ratios of phosphorylated versus total MLC of three independent experiments were determined by densitometry and illustrated in the bar graph in the lower panel. F, activated RhoA levels in PE-stimulated smooth muscles of mesenteric arteries. Mesenteric arteries were isolated from WT and KO mice, and the epithelium of the vessels was removed. The vessels were subjected to PE stimulation (20 μm) for 5 s at room temperature and then quickly frozen in liquid nitrogen. The vessels were then homogenized, and the activated RhoA (GTP-associated RhoA) levels were determined in duplicate by the G-LISK RhoA activation assay kit. The results of two independent experiments were pooled, and the means ± S.D. are illustrated. The data were analyzed by paired Student's t tests, and the p values are indicated.
Consistent with the contractility results, at the molecular level, freshly isolated mesenteric arteries without endothelium from female KO and castrated male KO but not male KO mice presented a constitutive increase of MLC phosphorylation (Fig. 2E), reflecting the increased sensitivity of VSMC to vasoconstrictive stimuli.
Because activated RhoA could increase MLC phosphorylation via the RhoA-associated kinase/myosin phosphatase pathway (33), we assessed the levels of GTP-bound RhoA in mesenteric artery smooth muscles. As shown in Fig. 2F, 5 s after the activation by PE, endotheliumless mesenteric arteries from castrated and female but not uncastrated male KO mice had significantly elevated activated RhoA levels compared with their counterpart WT mice. The RhoA activity in KO mice was increased almost 15-fold after castration, much more than that of WT males after castration. Such elevation was compatible with the augmented MLC phosphorylation and constriction in female and castrated KO vessels.
Castrated KO Mice Show Increased BP
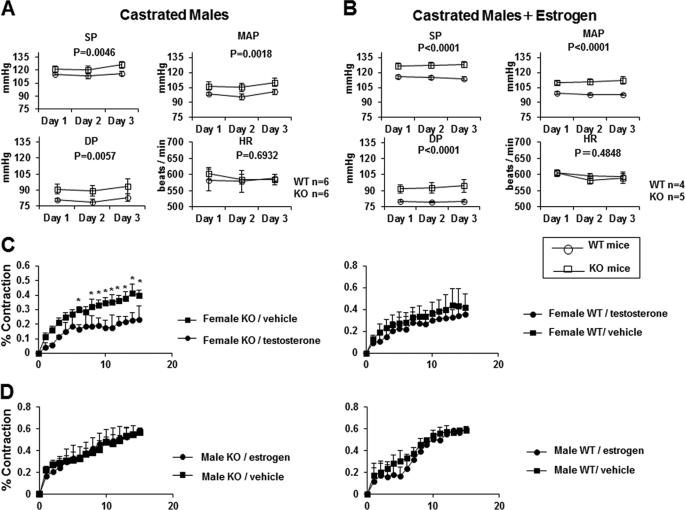
We wondered whether heightened vasoconstriction in female and castrated KO mice led to elevated BP. The BP of Ephb6 KO and WT mice was measured by radiotelemetry. The systolic pressure, diastolic pressure, mean arterial pressure, and HR of male and female KO mice presented no significant differences from their respective WT controls (supplemental Fig. S2). However, 3 weeks after castration, KO mice manifested increased systolic pressure, diastolic pressure, and mean arterial pressure compared with castrated WT mice, whereas both groups maintained similar HR (Fig. 3A).
FIGURE 3.
The effect of sex hormones on BP and VSMC contraction of castrated Ephb6 KO mice. A, increased BP in castrated Ephb6 KO mice. The mice were implanted with telemetry transmitters and then castrated. After 3 weeks, BP and HR were measured for 3 consecutive days. B, administration of estrogen to the castrated Ephb6 KO mice did not lower BP. Male mice were castrated 1 week after transmitter implantation; 3 weeks after the castration, the mice were administered with estrogen (240 μg) daily subcutaneously for 7 days. Their BP and HR were measured by telemetry during the last 3 days of estrogen administration. For A and B, mouse numbers per group are shown. The values are expressed as the means ± S.E. 24-h BP and HR for each day ± S.E. SP, systolic pressure; DP, diastolic pressure; MAP, mean arterial pressure. The data were analyzed by repeated analysis of variance, and the p values are shown. C, female KO VSMC showed higher contraction in the absence exogenous testosterone. VSMC from WT and KO female mice were cultured in 15% stripped FCS for 4 days in the presence of 10 μg/ml testosterone or vehicle. The cells were then stimulated with 20 μm PE. D, estrogen did not affect VSMC contraction in the absence of Ephb6. VSMC from WT and KO male mice were cultured in 15% stripped FCS for 4 days in the presence of 10 μg/ml estrogen or vehicle. The cells were then stimulated with 20 μm PE. For C and D, each point represents the mean ± S.E. of percentage contraction of 10 or more cells. All of the experiments were repeated at least twice, and data from a representative experiment are shown. *, significant differences compared with controls (p < 0.05; paired Student's t test).
Was the lack of BP elevation in female KO mice due to that estrogens lowered the BP? To answer this question, we administered estrogen for 7 days to the castrated KO and WT mice. As shown in Fig. 3B, BP of castrated KO did not come down after estrogen administration and remained higher compared with the castrated WT males treated with estrogen, indicating that the lack of BP elevation in female KO mice was not due to a suppressive effect of estrogen.
We next tested whether these in vivo findings could be reproduced in a defined in vitro culture system. For this purpose, female KO VSMC were cultured in medium containing 15% strip serum, in which the bovine sex hormones had been removed by active charcoal adsorption. In this system, the female KO VSMC contractility in the absence of testosterone was significantly higher than that female KO VSMC in the presence of testosterone, whereas testosterone did not affect the contractility of WT female VSMC (Fig. 3C). On the other hand, the addition of estrogen to the cultured male KO or WT VSMC did not alter their contractility (Fig. 3D). The results indicate that Ephb6 and testosterone need to be removed simultaneously to elevate VSMC contractility. These in vitro results are compatible with the ex vivo vessel contraction and in vivo BP phenotype of castrated KO mice and demonstrate that Ephb6 and testosterone are necessary to maintain normal VSMC contractility and BP, both of which will increase only when Ephb6 and testosterone are simultaneously missing.
BP-related Hormone Levels in Ephb6 KO Mice
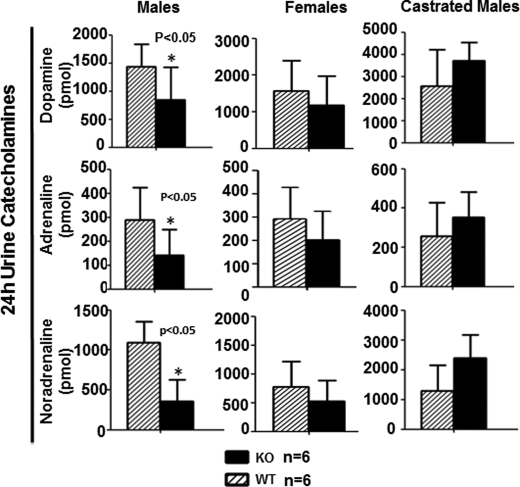
We questioned whether some key hormones involved in BP regulation were affected directly by or as a compensatory consequence of Ephb6 null mutation. As depicted in supplemental Fig. S3, plasma angiotensin II and serum aldosterone levels in male, female, and castrated KO mice were comparable with those in their WT counterparts. Twenty-four-h urine catecholamines (i.e. adrenaline, noradrenaline, and dopamine) were similar in KO females and WT females during fasting (Fig. 4, middle column). However, 24-h urine catecholamines under this condition were significantly lower in KO males than in WT males (Fig. 4, left column). After KO males were castrated, their 24-h urine catecholamines rose to levels comparable with those in castrated WT mice (Fig. 4, right column). This suggests that in male KO mice, there is a compensative reduction of ambient catecholamines, and such compensation is abolished after castration.
FIGURE 4.
Twenty-four-h urine catecholamine levels in Ephb6 KO mice. Male, female, and castrated Ephb6 KO and WT mice were placed in metabolic cages. Urine was collected during a 24-h fasting period. Urine catecholamines were measured by ELISA, and the means ± S.D. of hormones excreted during the 24-h period and mouse number per groups (n) are presented.
Ephb6 Modulates BP and VSMC Contractility through Efnb Reverse Signaling
Ephb6 and its major ligands, Efnb1, Efnb2, and Efnb3, are capable of bidirectional signaling, i.e. Ephb6 can be stimulated by its ligands and transmit signals into cells, and Efnbs can also be stimulated by Ephb6, transmitting signals into cells. We investigated which signaling direction was responsible for the effect of Ephb6 in BP control. This was first done in primary VSMC culture.
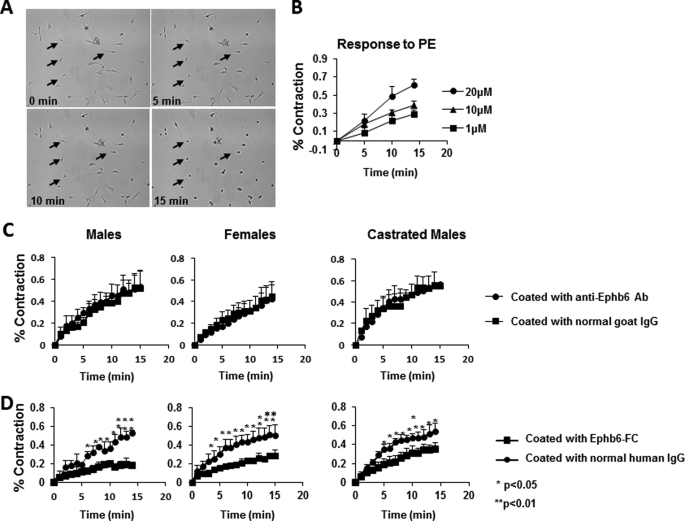
Mouse VSMC from the mesenteric arteries and aorta after 4–5 days of culture were stimulated by PE. Approximately 80% of these cells responded dose-dependently to PE stimulation (Fig. 5, A and B), indicating that they were still mainly of the contractile phenotype at that time. We monitored the speed of VSMC contraction in terms of percentages of their original length at different time points after PE stimulation. This parameter reflects the force of VSMC contraction, which dislodges adherent VSMC from the plastic well surface. WT VSMC, whether from males, females, or castrated males, were cultured in wells coated with goat anti-mouse Ephb6 Ab, so that they would receive forward signaling because of Ephb6 cross-linking by solid phase anti-Ephb6 Ab on the wells. The contraction response of these VSMC to PE was similar to that of cells cultured in wells coated with control normal goat IgG (Fig. 5C), indicating that the forward signaling received by these cells via Ephb6 did not have any effect on their contractility. Then these WT cells were cultured in wells coated with Ephb6-Fc (the Fc of recombinant protein is of human origin), which would cross-link Efnbs on VSMC and trigger reverse signaling through Efnbs (either single or multiple Efnbs, i.e. Efnb1, Efnb2, and/or Efnb3). The contractility of all these VSMC types (from WT males, females, and castrated males) after PE stimulation was significantly decreased relative to those cultured in wells coated with control normal human IgG (Fig. 5D). The results reveal that reverse signaling from Ephb6 to Efnb(s) down-regulates VSMC contractility. Possible reasons for the similarly reduced contractility of VSMC from males, females, and castrated males will be discussed later. The decrease in VSMC contraction in Ephb6-Fc-coated wells was not due to the mechanical binding of Ephb6-Fc to Efnbs expressed on VSMC, because similarly coated anti-EphB6 Ab did not have any influence on VSMC (Fig. 5C), which express Ephb6 on their surface.
FIGURE 5.
Reverse but not forward signaling between Ephb6 and Efnbs dampens VSMC contractility. Data presentation and statistical analysis are the same as described for Fig. 3 (C and D). A, micrographs of VSMC contraction after PE stimulation. VSMC were stimulated with 20 μm PE and imaged every min. Images at 0, 5, 10, and 15 min are presented. Four arrows point to the same four cells during the 15-min imaging period and show their contraction. The photos also reveal that ∼85% of the cells are capable of contraction, indicating the purity of VSMC in such cell preparations. B, dose-dependent response of VSMC contractility to PE stimulation. WT VSMC were stimulated with PE at different concentrations for 15 min at 37 °C. C, solid phase anti-Ephb6 Ab had no effect on VSMC contractility. Wells were coated with goat anti-Ephb6 Ab or normal goat IgG (2 μg/ml during coating). VSMC from WT male (left panel), WT female (middle panel), or castrated WT male (right panel) mice were cultured in these wells for 4 days and then stimulated with 20 μm PE. D, solid phase Ephb6-Fc reduced VSMC contractility. The wells were coated with recombinant Ephb6-Fc or normal human IgG (2 μg/ml during coating). VSMC from WT male (left panel), WT female (middle panel), or castrated WT mice (right panel) were cultured in these wells for 4–5 days and then stimulated with 20 μm PE.
To confirm our in vitro findings in vivo, we measured the BP of male or castrated male Ephb6Δ/KO mice, which express transgenic tailless Ephb6 in the Ephb6 KO background. Male and castrated male Ephb6Δ/KO mice showed no BP increase compared with their WT counterparts (supplemental Fig. S4), indicating that the missing intracellular Ephb6 tail, which is needed for forward signaling from ligands to Ephb6, is not important for its function in BP regulation. In other words, this result suggests that the observed BP control phenotype of Ephb6 is the consequence of a lack of reverse signaling from Ephb6 to Efnbs.
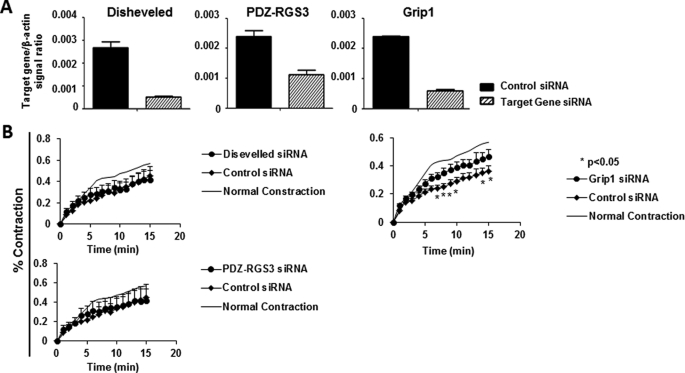
Identification of Grip1 as a Component of Efnb Reverse Signaling Pathway Related to VSMC Contraction
Efnbs have no enzymatic activity but use adaptor proteins to link Efnb intracellular tails to various signaling pathways. To identify molecules in the Efnb reverse signaling pathway that regulate VSMC contractility, siRNAs were deployed to knock down the expression of Grip1, Disheveled, and PDZ-RGS3 that are known to associate with Efnbs (34–36). The effectiveness of mRNA knockdown was verified by RT-qPCR (Fig. 6A). The solid phase EphB6-Fc-induced hyporesponsiveness of WT VSMC to PE stimulation could be partially negated by Grip1 siRNA, but not by Disheveled or PDZ-RGS3 siRNA (Fig. 6B). These data indicate that Grip1 is involved in the Ephb6 reverse signaling that dampens VSMC contractility.
FIGURE 6.
Grip1 in the Efnb reverse signaling pathway in VSMC. The experiments in this figure were repeated more than twice, and representative data are shown. A, effective mRNA knockdown of Disheveled, PDZ-RGS3, and Grip1 by siRNAs. Cultured WT VSMC were transfected with a mixture of siRNAs of a particular gene or control siRNAs, as indicated. After additional 24-h culture, the cells were harvested, and the mRNA expression of each gene was determined by RT-qPCR. The data are expressed as the means ± S.D. of the ratios of the target gene signal versus the β-actin signal. B, Grip1 knockdown by siRNAs partially reversed the inhibitory effect of solid phase Ephb6-Fc. VSMC from WT males were cultured in wells coated with Ephb6-Fc (2 μg/ml for coating). After 2 days, the cells were transfected with siRNAs targeting Disheveled, PDZ-RGS3, or Grip1 or with control siRNA. On day 4 of culture, they were stimulated with PE (20 μm), and their percentage contraction was registered. Means ± S.D. of the percentage are shown. The thin line (indicated as Normal Contraction) represents the mean percentage contraction of VSMC cultured in wells coated with normal human IgG (2 μg/ml) without siRNA transfection (for a better visual effect, the S.D. of each time point in this control is omitted). *, p < 0.05 according to paired Student's t test, between Grip1 siRNA- and control siRNA-transfected VSMC.
DISCUSSION
This study identified a previously unknown function of Eph and Efn in vasoconstriction and BP regulation. We demonstrated that 1) Ephb6 triggered reverse signaling via Efnbs, leading to hyporesponsiveness of VSMC; 2) Ephb6, in coordination with testosterone, controlled ambient catecholamine secretion; and 3) the absence of both Ephb6 and testosterone overtly increased BP.
A few words are warranted to reconcile the differences in results on VSMC contraction, small artery contraction, and in vivo BP measurements. Increased vessel contraction (Fig. 2C), accompanied by augmented constitutive MLC phosphorylation and BP (Figs. 2D and 3), was observed consistently in castrated Ephb6 KO mice compared with castrated WT mice. On the other hand, some of the data related to VSMC contractility, small artery contraction, and BP were at odds with each other. For example, VSMC, regardless of whether they were from males, females, or castrated males, were susceptible to Ephb6-Fc-triggered reverse signaling, culminating in reduced contractility. However, in intact small artery contraction studies, only small arteries from KO females and castrated KO males, but not males, presented increased contraction. Moreover, although female KO arteries demonstrated enhanced contractility, female KO mice showed no overt BP elevation. Possible explanations are as follows.
In small arteries, VSMC are in close contact with each other, whereas under cell culture conditions, this contact is limited because cells are not confluent. In our experiments, the cells were used at ∼20% confluence (Fig. 5A), because it was difficult to conduct contraction studies if they were connected to each other. In any case, even at full confluence, VSMC were still not packed as tightly as they would be in vessels. Consequently, WT VSMC in culture lack sufficient Ephb6 stimulation from neighboring cells, and individual cells exist in a state close to Ephb6 KO cells. The lack of sufficient Ephb6 stimulation in cultured VSMC was evident from the fact that when cultured WT VSMC from males, females and castrated males were exposed to solid phase Ephb6-Fc, which gave sufficient stimulation to Efnbs in VSMC, their response to PE was drastically reduced (Fig. 5D). This suggests that the fundamental effect of reverse signaling through Efnbs in VSMC is a diminished response to vasoconstrictive stimuli. A lack of such reverse signaling translates into ex vivo increased small artery contractility and augmented constitutive MLC phosphorylation at the molecular level, as well as increased RhoA activity in female and castrated KO mice. Why then did KO females not show elevated BP? BP is a tightly controlled physiological parameter with many compensatory feedback mechanisms. In KO males, reduced ambient catecholamine release seems to be one such mechanism to balance the defect caused by Ephb6 deletion. Only when this compensation is also affected by castration is BP overtly elevated. Indeed, we have discovered that Ephb6 is highly expressed in the adrenal medulla, the major source of ambient catecholamines (supplemental Fig. S5). In females, it is possible that there is a different in vivo compensatory mechanism irrelevant to catecholamines. As a consequence, female KO mice still maintained normal BP.
A more interesting question is why male VSMC receiving Efnb reverse signaling manifested reduced contractility, but this phenotype was not reflected in intact vessels from male KO mice. In fact, mesenteric arteries from male Ephb6 KO showed even lower contractility than those from WT males (Fig. 2A), and they presented no constitutive increase of MLC phosphorylation (Fig. 2D) or increased RhoA activity (Fig. 2F), unlike the small arteries from females and castrated male KO mice. One possible explanation is that under the influence of testosterone, a compensatory mechanism in VSMC at the vascular level (in addition to the systemic change in ambient catecholamine secretion) overreaches its goal, resulting in dampened contractility. This overcompensation is not operational without testosterone, so vessels from females and castrated males showed increased but not decreased contractility. Examples of such overcompensation do exist in biological systems. For example, when ILK, a major signaling molecule in the integrin signaling pathway that controls hepatocyte differentiation, is null mutated in the liver, overexpression of multiple integrin chains and enzymes involved in the synthesis of collagens has a net result of putting ILK null-mutated hepatocytes in an overcompensated state (37). In our case, such overcompensation only occurs in intact vessels and not in isolated VSMC, because in the latter, low cell density limits cell-cell contact, and most cells, regardless of whether they are KO or WT, are all in a de facto nearly KO phenotype.
We attempted to elucidate the mechanism by which Ephb6 null mutation affects vasoconstriction and BP. We noted that neither Ephb6 null mutation nor castration affects the expression of Efnb1, Efnb2, Efnb3, and type 1a α-adrenoreceptor in VSMC at the protein level (supplemental Fig. S6A) or altered Efnb1, Efnb2, Efnb3, Ephb6, and Grip1 expression at the mRNA level (supplemental Fig. S6B). Because endothelial cells are in close contact with VSMC, their Efn and Eph expression might influence the contractility of VSMC. We confirmed that neither Ephb6 deletion nor castration altered the expression of Efnb1, Efnb2, and Efnb3 expression in the mesenteric artery endothelial cells (supplemental Fig. S6C). We further demonstrated that cultured VSMC in the presence or absence of testosterone or estrogen expressed similar levels of Efnb1, Efnb2, Efnb2, and Grip1 (supplemental Fig. S6, D and E). These data show that the mechanisms of Ephb6 in influencing VSMC contraction and BP are not via modulating the expression of their ligands or Grip1. We also examined the histology of small arteries of WT versus KO mice and uncastrated versus castrated KO mice, but no obvious difference was observed (data not shown).
Our additional mechanistic studies established that reverse signaling from Ephb6 to Efnbs, but not forward signaling transduced by Ephb6 intracellular domains, was responsible for the observed phenotype, according to in vivo BP measurements in Ephb6Δ/KO mice and in vitro VSMC contraction with solid phase Ephb6-Fc. This conclusion on the critical role of Efnb reverse signaling in BP regulation is corroborated by our finding that Efnb1 smooth muscle-specific conditional KO mice presented similar, although not identical, abnormal BP regulation, like Ephb6 KO mice (to be reported elsewhere).
EphB6 null mutation and Efnb reverse signaling did not modulate Ca2+ flux (supplemental Fig. S7). Thus, parameters upstream of Ca2+ flux regulating VSMC contractility seem to be normal. We discovered that constitutive VSMC MLC phosphorylation, a critical event controlling VSMC responsiveness to Ca2+ influx, was increased in female KO mice and castrated male KO mice. Therefore, although there was no change in Ca2+ flux, these VSMC became more responsive to Ca2+ because of their increased MLC phosphorylation, and this translated into increased vessel constriction upon PE stimulation.
Although Efnbs only have short intracytoplasmic tails with no enzymatic activity, there are five conserved tyrosine phosphorylation sites in the tails in addition to a PDZ-binding motif at the C terminus. The former can dock SH2 domain-containing proteins, such as Tiam1 (37) and Disheveled (33), and the latter, PDZ domain-containing signaling proteins, such as Grip (34) and PDZ-RGS3 (35). We discovered that knocking down Grip1 but not Disheveled or PDZ-RGS3 expression in VSMC by siRNAs partially reversed the hyporesponsiveness induced by solid phase Ephb6-Fc. Therefore, Grip1 is located in the Efnb reverse signaling pathway that leads to the reduced response of VSMC. We also discovered that RhoA activation in the form of GTP-associated RhoA was augmented in the mesenteric artery smooth muscles of KO females and castrated KO males but not unmanipulated KO males within 5 s after PE activation, compared with their WT counterparts. Activated RhoA can activate RhoA-associated kinase, which phosphorylates myosin phosphatase. The phosphorylation of the phosphatase will reduce its activity, which will then prolong the phosphorylation of MLC (33). This might be a mechanism to increase the responsiveness of MLC to Ca2+ flux in the VSMC of castrated and female KO small arteries. How the reverse signaling through Efnb in VSMC leads to a reduction of RhoA activity is not clear, and we can only speculate at this point. Grip1 contains seven PDZ domains (38). It is possible that multiple PDZ domains in Grip1 can bridge Efnbs and modulators of RhoA activity, such as GDP dissociation inhibitors or GDP exchange factors, and influence the function of these modulators, which will in turn regulate RhoA activity. Indeed, Grip1 can associate with a Rho GDP dissociation inhibitor (39) and a Ras GDP exchange factor (40) through its PDZ domains. Validation of such a hypotheses is in progress.
Based on the observations described above, we propose the following unified scheme regarding the newly discovered functions of Ephb6 and Efnbs in BP regulation. Under physiological conditions, Ephb6 on neighboring cells provides VSMC with a negative signal to dampen their contractility. Because interactions between Ephb6 and Efnbs are constant, such a dampening effect is not regulated rapidly and likely controls constitutive vascular tone rather than fast responses to neuroendocrine stimulation. The dampening signal is transmitted reversely through Efnbs into VSMC via an Efnb-associating protein, Grip1. Through a so-far unknown signaling pathway that leads to the activation of RhoA, the reverse signaling modulates constitutive MLC phosphorylation, which is increased in the absence of reverse signaling and results in enhanced contraction responses to Ca2+ flux in VSMC. Although Efnb reverse signaling can alter VSMC contractility at the cellular level regardless of gender or castration, such a modulation is not always reflected in BP because of various in vivo compensatory mechanisms. In male KO mice, one of these mechanisms is a negative feedback loop that decreases ambient catecholamine secretion by the nervous/endocrine system. This loop depends on both Ephb6 and testosterone. When both are absent, the loop is no longer functional, and catecholamine secretion is no longer reduced. In combination with heightened VSMC responsiveness, it leads to overtly increased BP. Conceivably, defects in Ephb6 and Efnb interactions or in the Efnb reverse signaling pathway, in combination with declining testosterone levels in a subpopulation of aging males, might result in elevated BP.
Supplementary Material
This work was supported by Canadian Institutes of Health Research Grants MOP57697 and MOP69089 (to J. W.), MOP97829 (to H. L.), MOP14496 (to J. T. and E. T.), and CMI72323 (to G. C.). It was also financed by a grant from the Heart and Stroke Foundation of Quebec, Quebec Ministry of Economic Development, Innovation and Exportation Grant PSR-SIIRI-069, and funds from the J.-Louis Levesque Foundation (to J. W.). This study was also made possible by a group grant from Fonds de la Recherche en Santé du Québec for Transfusional and Hemovigilance Medical Research (to J. W.). T. W. was supported in part by grants from the Zhejiang Provincial Natural Science Foundation of China (Grant Y2080374) and the National Natural Sciences Foundation of China (Projects for Young Scientists 30800999).

This article contains supplemental text, Tables SI and SII, and Figs. S1–S7.
- BP
- blood pressure
- VSMC
- vascular smooth muscle cell(s)
- qPCR
- quantitative PCR
- Ab
- antibody
- PE
- phenylephrine
- MLC
- myosin light chain
- HR
- heart rate.
REFERENCES
- 1. Weir M. R., Bakris G. L. (2008) Combination therapy with renin-angiotensin-aldosterone receptor blockers for hypertension. How far have we come? J. Clin. Hypertens. 10, 146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Victor R. G., Shafiq M. M. (2008) Sympathetic neural mechanisms in human hypertension. Curr. Hypertens. Rep. 10, 241–247 [DOI] [PubMed] [Google Scholar]
- 3. Versari D., Daghini E., Virdis A., Ghiadoni L., Taddei S. (2009) Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br. J. Pharmacol. 157, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iglarz M., Schiffrin E. L. (2003) Role of endothelin-1 in hypertension. Curr. Hypertens. Rep. 5, 144–148 [DOI] [PubMed] [Google Scholar]
- 5. Qiao X., McConnell K. R., Khalil R. A. (2008) Sex steroids and vascular responses in hypertension and aging. Gend. Med. 5, S46–S64 [DOI] [PubMed] [Google Scholar]
- 6. Kienitz T., Quinkler M. (2008) Testosterone and blood pressure regulation. Kidney Blood Press Res. 31, 71–79 [DOI] [PubMed] [Google Scholar]
- 7. Ashraf M. S., Vongpatanasin W. (2006) Estrogen and hypertension. Curr. Hypertens. Rep. 8, 368–376 [DOI] [PubMed] [Google Scholar]
- 8. Eph Nomenclature Committee (1997) Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 90, 403–404 [DOI] [PubMed] [Google Scholar]
- 9. Pasquale E. B. (2008) Eph-ephrin bidirectional signalling in physiology and disease. Cell 133, 38–52 [DOI] [PubMed] [Google Scholar]
- 10. Wilkinson D. G. (2000) Eph receptors and ephrins. Regulators of guidance and assembly. Int. Rev. Cytol. 196, 177–244 [DOI] [PubMed] [Google Scholar]
- 11. Flanagan J. G., Vanderhaeghen P. (1998) The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21, 309–345 [DOI] [PubMed] [Google Scholar]
- 12. Wang H. U., Chen Z. F., Anderson D. J. (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741–753 [DOI] [PubMed] [Google Scholar]
- 13. Noren N. K., Pasquale E. B. (2007) Paradoxes of the EphB4 receptor in cancer. Cancer Res. 67, 3994–3997 [DOI] [PubMed] [Google Scholar]
- 14. Wu J., Luo H. (2005) Recent advances on T-cell regulation by receptor tyrosine kinases. Curr. Opin. Hematol. 12, 292–297 [DOI] [PubMed] [Google Scholar]
- 15. Batlle E., Henderson J. T., Beghtel H., van den Born M. M., Sancho E., Huls G., Meeldijk J., Robertson J., van de Wetering M., Pawson T., Clevers H. (2002) β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111, 251–263 [DOI] [PubMed] [Google Scholar]
- 16. Dravis C., Yokoyama N., Chumley M. J., Cowan C. A., Silvany R. E., Shay J., Baker L. A., Henkemeyer M. (2004) Bidirectional signalling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev. Biol. 271, 272–290 [DOI] [PubMed] [Google Scholar]
- 17. Konstantinova I., Nikolova G., Ohara-Imaizumi M., Meda P., Kucera T., Zarbalis K., Wurst W., Nagamatsu S., Lammert E. (2007) EphA-Ephrin-A-mediated β cell communication regulates insulin secretion from pancreatic islets. Cell 129, 359–370 [DOI] [PubMed] [Google Scholar]
- 18. Davy A., Bush J. O., Soriano P. (2006) Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 4, e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao C., Irie N., Takada Y., Shimoda K., Miyamoto T., Nishiwaki T., Suda T., Matsuo K. (2006) Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 4, 111–121 [DOI] [PubMed] [Google Scholar]
- 20. Pasquale E. B. (2005) Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 6, 462–475 [DOI] [PubMed] [Google Scholar]
- 21. Arvanitis D., Davy A. (2008) Eph/ephrin signaling. Networks. Genes Dev. 22, 416–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hashimoto T., Karasawa T., Saito A., Miyauchi N., Han G. D., Hayasaka K., Shimizu F., Kawachi H. (2007) Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int. 72, 954–964 [DOI] [PubMed] [Google Scholar]
- 23. Dravis C., Wu T., Chumley M. J., Yokoyama N., Wei S., Wu D. K., Marcus D. C., Henkemeyer M. (2007) EphB2 and ephrin-B2 regulate the ionic homeostasis of vestibular endolymph. Hear. Res. 223, 93–104 [DOI] [PubMed] [Google Scholar]
- 24. Foo S. S., Turner C. J., Adams S., Compagni A., Aubyn D., Kogata N., Lindblom P., Shani M., Zicha D., Adams R. H. (2006) Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161–173 [DOI] [PubMed] [Google Scholar]
- 25. Shin D., Garcia-Cardena G., Hayashi S., Gerety S., Asahara T., Stavrakis G., Isner J., Folkman J., Gimbrone M. A., Jr., Anderson D. J. (2001) Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev. Biol. 230, 139–150 [DOI] [PubMed] [Google Scholar]
- 26. Gale N. W., Baluk P., Pan L., Kwan M., Holash J., DeChiara T. M., McDonald D. M., Yancopoulos G. D. (2001) Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev. Biol. 230, 151–160 [DOI] [PubMed] [Google Scholar]
- 27. Deroanne C., Vouret-Craviari V., Wang B., Pouysségur J. (2003) EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J. Cell Sci. 116, 1367–1376 [DOI] [PubMed] [Google Scholar]
- 28. Ogita H., Kunimoto S., Kamioka Y. (2003) EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ. Res. 93, 23–31 [DOI] [PubMed] [Google Scholar]
- 29. Luo H., Yu G., Tremblay J., Wu J. (2004) EphB6-null mutation results in compromised T cell function. J. Clin. Invest. 114, 1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Golovina V. A., Blaustein M. P. (2006) Preparation of primary cultured mesenteric artery smooth muscle cells for fluorescent imaging and physiological studies. Nat. Protoc. 1, 2681–2687 [DOI] [PubMed] [Google Scholar]
- 31. Thorin E., Huang P. L., Fishman M. C., Bevan J. A. (1998) Nitric oxide inhibits α2-adrenoceptor-mediated endothelium-dependent vasodilation. Circ. Res. 82, 1323–1329 [DOI] [PubMed] [Google Scholar]
- 32. Lavoie J. L., Lake-Bruse K. D., Sigmund C. D. (2004) Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am. J. Physiol. Renal Physiol 286, F965–F971 [DOI] [PubMed] [Google Scholar]
- 33. Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science 273, 245–248 [DOI] [PubMed] [Google Scholar]
- 34. Tanaka M., Kamo T., Ota S., Sugimura H. (2003) Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J. 22, 847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brückner K., Pablo Labrador J., Scheiffele P., Herb A., Seeburg P. H., Klein R. (1999) EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22, 511–524 [DOI] [PubMed] [Google Scholar]
- 36. Lu Q., Sun E. E., Klein R. S., Flanagan J. G. (2001) Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell 105, 69–79 [DOI] [PubMed] [Google Scholar]
- 37. Gkretsi V., Apte U., Mars W. M., Bowen W. C., Luo J. H., Yang Y., Yu Y. P., Orr A., St-Arnaud R., Dedhar S., Kaestner K. H., Wu C., Michalopoulos G. K. (2008) Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 48, 1932–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dong H., O'Brien R. J., Fung E. T., Lanahan A. A., Worley P. F., Huganir R. L. (1997) GRIP. A synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386, 279–284 [DOI] [PubMed] [Google Scholar]
- 39. Su L. F., Wang Z., Garabedian M. J. (2002) Regulation of GRIP1 and CBP Coactivator activity by Rho GDI modulates estrogen receptor transcriptional enhancement. J. Biol. Chem. 277, 37037–37044 [DOI] [PubMed] [Google Scholar]
- 40. Ye B., Liao D., Zhang X., Zhang P., Dong H., Huganir R. L. (2000) GRASP-1. A neuronal RasGEF associated with the AMPA receptor/GRIP complex. Neuron 26, 603–617 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.