Background: Disruption of endothelial cell (EC) junction-associated proteins is a major contributing factor to inflammation-induced barrier dysfunction.
Results: Knockdown of ERG in EC led to markedly increased EC permeability and reduced expression of the tight junction protein CLDN5.
Conclusion: ERG regulates EC barrier function via transcriptional regulation of CLDN5.
Significance: ERG is a transcriptional regulator of EC barrier function.
Keywords: Endothelium, ETS Family Transcription Factor, Gene Transcription, Permeability, Vascular Biology, Junctional Protein
Abstract
ETS-related gene (ERG) is a member of the ETS transcription factor family. Our previous studies have shown that ERG expression is highly enriched in endothelial cells (EC) both in vitro and in vivo. ERG expression is markedly repressed in response to inflammatory stimuli. It has been shown that ERG is a positive regulator of several EC-restricted genes including VE-cadherin, endoglin, and von Willebrand factor, and a negative regulator of other genes such as interleukin (IL)-8 and intercellular adhesion molecule (ICAM)-1. In this study we have identified a novel role for ERG in the regulation of EC barrier function. ERG knockdown results in marked increases in EC permeability. This is associated with a significant increase of stress fiber and gap formation in EC. Furthermore, we identify CLDN5 as a downstream target of ERG in EC. Thus, our results suggest that ERG plays a pivotal role in regulating EC barrier function and that this effect is mediated in part through its regulation of CLDN5 gene expression.
Introduction
Vascular inflammation occurs in several diseases including diabetes, atherosclerosis, and rheumatoid arthritis (1–3). Increased endothelial cell (EC) permeability is a major consequence of vascular inflammation that leads to an increase in paracellular leakage of plasma fluid and protein. The paracellular route is regulated by adherens junction (AJ)2- and tight junction (TJ)-associated proteins. AJs are formed by homotypic interactions of the cadherin family of adhesion proteins, whereas TJs comprise membrane-spanning proteins, including claudin, occludin, and junctional adhesion proteins (4, 5). The claudin family consists of 24 tetraspan transmembrane proteins, each with a unique tissue distribution. Claudin 5 (CLDN5) is enriched in EC. CLDN5-knock-out mice die within 1 day after birth and demonstrate a defect in the blood-brain barrier (6).
Inflammatory stimuli such as histamine, thrombin, vascular endothelial growth factor (VEGF), and tumor necrosis factor-α (TNF-α) can cause disruption of EC cell-cell junctions as well as alterations in cytoskeletal architecture, leading to increased gap formation and stress fiber assembly. Whereas certain inflammatory stimuli, such as thrombin or histamine, lead to rapid alterations in endothelial barrier function that occur in seconds to minutes, other mediators, such as TNF-α or endotoxin, are associated with much more gradual changes that occur over several minutes to hours and that are accompanied by significant changes in gene expression.
The ETS genes are a family of transcription factors sharing a highly conserved DNA binding domain, the ETS domain, that recognize DNA with an internal conserved DNA binding sequence core motif of GGAA/T (7). We and others have shown a role for selected members of the ETS family in the regulation of inflammatory responses in EC. For example, the ETS factor ESE-1 is rapidly induced in cultured EC in response to proinflammatory cytokines, including interleukin (IL)-1β and TNF-α, and in vivo, in response to endotoxin administration (8). Target genes of ESE-1 include nitric-oxide synthase 2 (NOS2) and cyclooxygenase-2 (COX-2). Similarly, Ets-1 induction occurs in EC in response to several inflammatory stimuli including proinflammatory cytokines and angiotensin II (9). Furthermore, Ets-1 is a critical mediator of the generation of reactive oxygen species and inflammatory gene expression in EC in vivo in response to systemic infusion of angiotensin II (10).
In contrast to most other ETS factors, we have recently shown that the ETS factor ERG exhibits an EC-restricted pattern of expression in both cultured cells and in different organs in vivo (11). ERG functions as a transcriptional activator of several EC-specific genes including endoglin, von Willebrand factor, VE-cadherin, intercellular adhesion molecule (ICAM)-2, and RhoJ (12–16). Recently, a few studies have implicated a role for ERG in the regulation of EC function during inflammation. We have shown that ERG expression is down-regulated upon inflammatory stimulation both in vitro and in vivo (11). Furthermore, we and others have demonstrated that ERG can function as a transcriptional repressor of a selected number of inflammatory genes, such as IL-8 and ICAM-1, which in turn promote the recruitment and attachment of neutrophils to the activated endothelium (11, 17).
In this study, we further characterize the role of ERG in regulating EC barrier function. ERG knockdown leads to marked increases in EC permeability. This is associated with significant changes in EC cytoskeletal structure and architecture. We have identified CLDN5 as a novel ERG target in EC, which plays a pivot role in mediating EC intercellular resistance and permeability.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
Primary human umbilical vein EC (HUVEC), human coronary artery EC (HCAEC), human pulmonary artery EC (HPAEC), and human lung microvascular EC (HMVEC) were obtained from Lonza (Allendale, NJ) and grown in EBM-2 (EC Basal Medium-2) supplemented with EGM-MV SingleQuots (Lonza).
Immunofluorescent Staining
Cells were plated on 12-mm glass circular coverslips in a 24-well dish. Before immunostaining, cells were fixed in 3.7% formaldehyde for 5 min at room temperature and washed three times with PBS. After being permeabilized in 0.05% Triton X-100, cells were then incubated with phalloidin-594 (Invitrogen) for 30 min at room temperature in the dark. Samples were rinsed three times with PBS. The staining was visualized by fluorescence confocal microscopy.
Western Blot Analysis
After corresponding treatments, cells were washed with ice-cold PBS and lysed with radioimmuneprecipitation assay solution, containing a protease inhibitor mixture. Equal amounts of total protein extracts were subjected to 10% SDS-PAGE and transferred to 0.45-μm nitrocellulose membranes. Membranes were blocked with 5% dry milk and probed with respective primary antibodies (rabbit anti-ERG (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-CLDN5 (Invitrogen), or anti-tubulin (Sigma-Aldrich), followed by incubation with HRP-conjugated anti-rabbit or anti-mouse secondary antibody (Santa Cruz Biotechnology). Protein bands were visualized using an enhanced chemifluorescence detection system (Denville Scientific, Metuchen, NY) according to the manufacturer's protocol and chemiluminescent sensitive film.
siRNA Transfections
siRNA transfection in EC was performed as previously described (11) using Lipofectamine 2000 (Invitrogen). The sequences of siRNAs used in this study are listed in supplemental Table 1 (Dharmacon, Lafayette, CO).
Quantitative Real-time PCR (QPCR)
Total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA). Single-stranded cDNA was synthesized from total RNA using the RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). SYBR Green I-based real-time PCR was carried out on an Opticon monitor. The sequences of the primers used in this study are listed in supplemental Table 2. For each run, TBP (TATA box-binding protein) primers were used to normalize the amount of cDNA.
Luciferase Reporter Gene Constructs
Human CLDN5 promoter (−1666 to +117 bp) fragments were cloned from the human BAC clone RP11-16C10 (Invitrogen) by PCR using primers hCLDN5-P-F and hCLDN5-P-R, which contains NheI and XhoI cutting sites at the ends (sequences are shown in supplemental Table 3). This 1783-bp fragment was inserted into the NheI-XhoI site and subcloned into the pGL3 Basic luciferase reporter vector (Promega, Madison, WI).
Site-directed Mutagenesis Constructs
Site-directed mutagenesis of the ERG binding site (−116) within hCLDN5 promoter (CLDN5-mut) was generated using a QuikChange site-directed mutagenesis kit (Stratagene), following the manufacturer's instructions. In brief, pGL3-hCLDN5-WT was used as the template using primers of hCLDN5-ERG-m-F and hCLDN5-ERG-m-R, which resulted in mutated ERG binding element from TTCCT to TTTTT. Primer sequences are listed in supplemental Table 3.
Transactivation Assays
HUVEC were plated in a 12-well plate the day before transfection. CLDN5 wild-type or mutant promoter constructs were co-transfected with a mammalian expression plasmid (pCI) encoding corresponding genes or empty vector as indicated and Renilla (Promega) into HUVEC using Dharmafect I (Dharmacon). After 24 h of incubation, the cells were lysed in 200 μl of Cell Culture Lysis Reagents (Promega) and analyzed for luciferase activity by using the Luciferase Assay system (Promega) with AutoLumat LB953 (EG&G Berthold, Oak Ridge, TN).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP analysis was performed as described previously (11) using the ChIP Assay kit (Upstate Biotechnology, Waltham, MA) according to the manufacturer's protocols with minor modifications. After the DNA was extracted from the supernatant, QPCR was performed using primers hCLDN5-C-F1 and hCLDN5-C-R1 for region ChIP1 and hCLDN5-C-F2 and hCLDN5-C-R2 for region ChIP2 (DNA sequences are listed in supplemental Table 2).
Electric Cell-Substrate Impedance Sensing (ECIS) Analysis
Endothelial cell intercellular impedance across a monolayer of EC was measured using the ECIS technique (ECIS model 1600; Applied BioPhysics, Troy, NY). Briefly, 8-well ECIS arrays (8W10E+) were coated with fibronectin (Invitrogen). Treated or nontreated EC were plated at confluent density and allowed to form monolayers. The impedance of EC was acquired. Data plots are representative of triplicate experiments, with each graph showing impedance readings from a separate well, at 40 distinct electrodes per well.
FITC-Dextran Transwell Assay
EC were plated on the insert of Transwell and cultured until confluent. FITC-dextran (Invitrogen) was added to the top chamber. After 4–6 h, samples were removed from the basolateral (bottom) compartments and read in a fluorometer (FluroStar Optima, BMGLABTECH) at excitation 485 nm, emission 520 nm.
Generation of Adenovirus and Lentivirus
The human ERG cDNA was PCR- amplified using primers hERG-cDNA-F and hERG-cDNA-R. The hERG cDNA was then digested with SalI and SmaI restriction enzymes and inserted in the SalI/EcoRV sites of the pAdTrack vector pTRK CMV shuttle vector. To clone CLDN5 into adenovirus system, full-length human CLND5 cDNA was amplified from human cDNA clone (Origene, Rockville, MD) using the primers hCLDN5-Ad-F and hCLDN5-Ad-R and inserted into the BglII and SalI sites of pTRK CMV vector. The primer sequences are included in supplemental Table 3. Positive clones were confirmed by automated DNA sequencing. Recombination of the positive clones and virus production were carried out as described previously (18). The adenovirus propagation protocol used has been described previously (19). EC transfection of adenovirus was performed as described previously (15). Protein expression of ERG and CLDN5 by each of the adenoviral constructs was confirmed by Western blot analysis. ERG shRNA and control shRNA lentiviruses were generously provided by Dr. Towia Libermann, Beth Israel Deaconess Medical Center. The targeting sequence of ERG shRNA is based on the siRNA we screened with the greatest yield of reduction in ERG expression (ERG siRNA #4), as described previously (11). The infection of lentivirus was carried out as described previously (20).
Statistical Analysis
Statistical significances were assessed by a paired-samples t test. A value of p < 0.05 was significant.
RESULTS
Knockdown of ERG Results in Increased EC Permeability
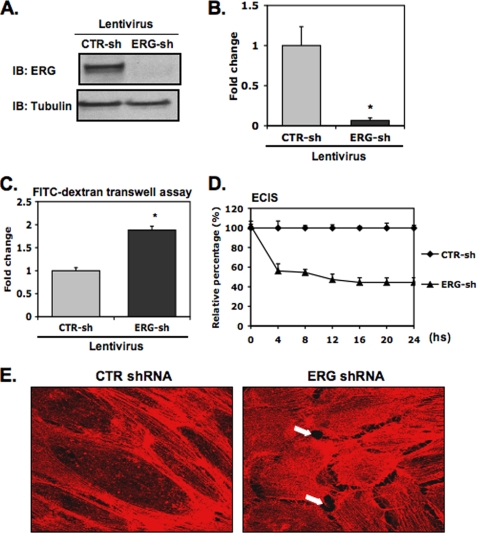
We have shown previously that ERG is markedly down-regulated in EC stimulated by TNF-α and lipopolysaccharide (LPS) (11). Stimulation of EC with proinflammatory cytokines such as TNF-α or LPS is also associated with significant changes in EC permeability. To assess the role of ERG in regulating EC permeability, we used a lentivirus expressing ERG shRNA to knock down ERG expression in EC. ERG shRNA effectively suppressed ERG protein expression by >90% in EC (Fig. 1, A and B). To evaluate the effect of suppressing ERG expression upon EC permeability, we first employed an in vitro Transwell permeability assay using FITC-dextran and demonstrated an increase in permeability of ∼2-fold in ERG shRNA-treated EC compared with control treated cells (Fig. 1C). To validate our initial findings further, we used a second approach, the ECIS system to measure the intercellular electrical resistance, which is partly determined by the integrity of junctional proteins that exist at cell-cell junctions. ERG knockdown was associated with significantly decreased electrical resistance compared with control cell monolayers (Fig. 1D), consistent with the increased permeability we observed in the in vitro Transwell permeability assay. A similar reduction of intercellular electric resistance was also observed in HMVEC (data not shown). Taken together, these results support a role for ERG in the regulation of EC barrier function.
FIGURE 1.
ERG knockdown increases EC permeability. HUVEC were infected with lentiviral ERG shRNA (ERG-sh) or a CTR shRNA (CTR-sh). A and B, cell extracts were prepared, and equal amounts of total proteins were separated on polyacrylamide gel. Protein levels of ERG and the control protein, tubulin, were determined by Western blot analysis. A, representative Western blot (IB) shows protein bands of ERG (top) and tubulin (bottom). B, densitometry analysis shows density ratios of ERG and tubulin. Three independent experiments were performed. *, p < 0.01. C, in FITC-dextran Transwell assay, ERG shRNA-treated or control shRNA-treated HUVEC were seeded onto collagen-coated inserts. When the EC monolayer became confluent, FITC-dextran was added to the top. Permeability was determining by measuring the fluorescence within the lower chamber after a 6-h incubation. The data are presented compared with control cells ± S.D. (error bars), n = 5. p < 0.01. D, in ECIS assay, the intercellular resistance was measured 24 h after cells were plated. The data represent the relative barrier resistance (Rb) values relative to control cells at the indicated time points, ± S.D., n = 3. *, p < 0.01. E, effects of ERG knockdown on stress fiber and gap formation are shown. Cells were fixed and stained with phalloidin-594 (red) to demonstrate cytoskeletal actin in either ERG or control (CTR) shRNA transfected HUVEC. Arrows indicate intercellular gaps.
Down-regulation of ERG Alters EC Cytoskeletal Structure
When we inhibited ERG expression in HUVEC using ERG shRNA and stained the cells with phalloidin-594, we observed marked changes in the structural appearance of the actin cytoskeleton. ERG shRNA-treated cells revealed increased numbers of stress fibers in the central portion and intercellular gaps, whereas control shRNA-treated demonstrated prominent cortical actin fibers, with the majority of the microfilaments tightly associated with the interendothelial junctions (Fig. 1E). A similar phenotype has been described for EC in response to inflammatory stimuli such as thrombin or TNF-α (21, 22). In addition, the ERG-knockdown EC acquired a more spindle-like morphology compared with a cobblestone pattern in the control treated cells (data not shown).
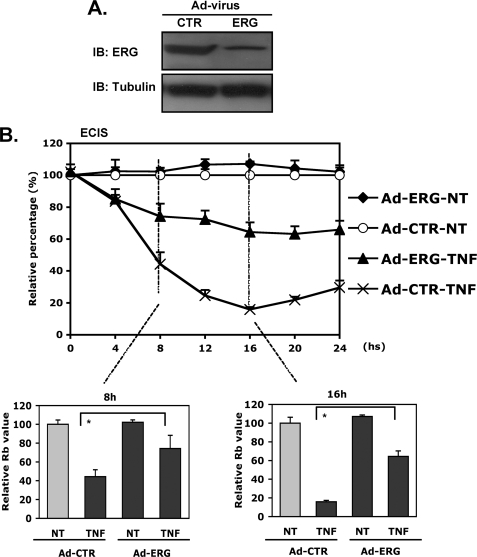
Partial Rescue of TNF-α-induced Decreased EC Intercellular Resistance by ERG Overexpression
To determine further whether ERG contributes to the alternation of permeability in response to inflammatory stimuli, we next evaluated the ability of ERG overexpression to rescue TNF-α-mediated reduction of intercellular resistance. ERG expression was enhanced using adenoviral delivery system. A GFP-expressing adenovirus was used as a control. The overexpression of ERG protein was validated by Western blotting (Fig. 2A). Importantly, the ERG adenovirus was able to rescue TNF-α-induced alteration in intercellular resistance by 50–60% (Fig. 2B), further supporting our hypothesis that ERG plays a critical role in the regulation of EC barrier function. However, enhanced ERG expression was not able to rescue the increased permeability induced by histamine (supplemental Fig. 1).
FIGURE 2.
Partial rescue of TNF-α-induced decreased EC intercellular resistance by ERG. A, HUVEC infected with adenoviruses expressing ERG or GFP control (CTR). Overexpression of ERG was determined by Western blotting (IB) using antibody against ERG. Tubulin was used as a loading control. B, ECIS assay. HUVEC infected with Ad-ERG or Ad-CTR were incubated in medium containing TNF-α (2 ng/ml) or medium alone for 24 h. The intercellular resistance was measured and collected using ECIS system over 24 h. The data are shown relative to control cells in the absence of TNF-α stimulation. The bar graphs represent the relative Rb values at the indicated time points, ± S.D. (error bars), n = 3. *, p < 0.01.
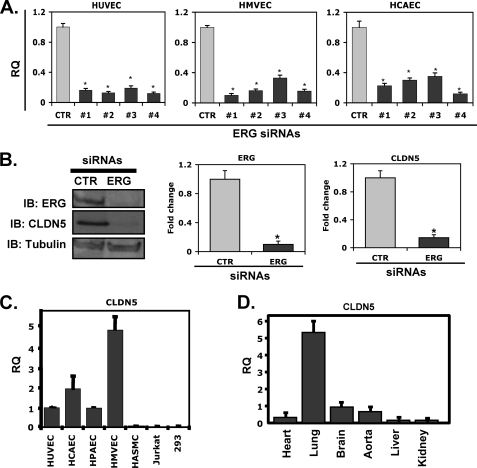
Down-regulation of ERG Is Associated with Reduced Expression of Junctional Protein CLDN5
It is well known that disruption of intercellular adhesion and tight junctions plays an important role in mediating gap formation and vascular permeability. Thus, it is possible that the effect of ERG on EC barrier function is mediated by altered expression of AJ and/or TJ-associated proteins. We have previously carried out DNA microarrays of ERG siRNA-treated HUVEC (11). Further analysis of these data revealed that expression of the TJ protein CLDN5 is reduced in ERG-deficient EC (data not shown), suggesting that it may be a downstream target gene of ERG. To validate this result, we transfected multiple EC types (HUVEC, HCAEC, and HMVEC) with four different ERG-specific siRNAs (#1–#4) and assayed the cells for CLDN5 expression. ERG knockdown was associated with markedly reduced expression of CLDN5 (85–90%) at the level of RNA (Fig. 3A). A similar reduction in CLDN5 protein expression was also demonstrated using the ERG siRNA with the greatest reduction in ERG expression (ERG siRNA #4; Fig. 3B). Previous studies have shown that CLDN5 expression is largely restricted to EC. Consistent with these reports, CLDN5 transcripts were expressed in HUVEC, HCAEC, HPAEC, and HMVEC, but not in human vascular smooth muscle cells, Jurkat cells, or 293 cells (Fig. 3C). Of note, CLDN5 is highly expressed in lung compared with other organs (Fig. 3D). This pattern contrasts with the blood-brain barrier-restricted phenotype seen in CLDN5-null mice and is very similar to what we have observed for ERG (11). In addition, both ERG and CLDN5 were repressed in the lung of the mice injected with TNF-α or LPS (supplemental Fig. 2). These results support the possibility that CLDN5 is a downstream target of ERG.
FIGURE 3.
Effect of ERG siRNA treatment of EC on CLDN5 expression. A, HUVEC, HMVEC, and HCAEC were transfected with ERG siRNAs (#1–#4) or a CTR siRNA. mRNA expression levels of CLDN5 were evaluated by QPCR using primers specific for CLDN5, normalized against TBP (TATA box-binding protein). The data are shown as relative -fold change of reduction compared with CTR siRNA-treated samples ± S.D. (error bars), n = 3. p < 0.01. B, HUVEC were transfected with either ERG siRNA (#4) or control siRNA. The protein levels of CLDN5 were assessed by Western blotting (IB) using antibody against ERG or CLDN5. Tubulin was used a loading control. Densitometry analysis shows density ratios of ERG or CLDN5 relative to tubulin. Three experiments were performed. *, p < 0.01. C, mRNA expression of CLDN5 in different cell types is shown. QPCR was used to analyze CLDN5 in different cell types. The relative quantity of expression is shown for the different cell types relative to HUVEC ± S.D., n = 3. D, mRNA expression of CLDN5 in different tissues is shown. The relative quantity of expression is shown for the different mouse tissues relative to brain ± S.D., n = 3.
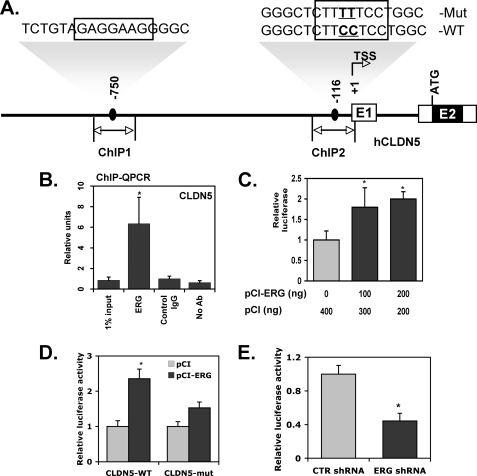
CLDN5 Is Direct Transcriptional Target of ERG
To evaluate whether CLDN5 is a direct target of ERG, we analyzed the proximal promoter of the human CLDN5 gene. Based on the known consensus binding sequence for ERG we identified two putative ERG binding sites (−750 and −116) in the proximal promoter of the CLDN5 gene (Fig. 4A). A ChIP assay was employed to determine whether or not ERG physically binds to one or both of the two putative ERG binding sites in the proximal CLDN5 promoter. ERG was shown to bind with high affinity to a region that spanned the −116 site (ChIP2 region; Fig. 4B), but not to a region spanning the −750 consensus element (ChIP1 region; data not shown). Interestingly, the ERG consensus binding site (−116) we identified within the ChIP2 region of CLDN5 promoter is highly conserved across various species (supplemental Fig. 3). We next subcloned 1.7 kb of the proximal promoter of the human CLDN5 gene into the pGL3 luciferase reporter. Co-transfections in HUVEC using the CLDN5 promoter construct and expression plasmids encoding ERG or a control plasmid demonstrated a significant increase in CLDN5 promoter activity with the ERG expression plasmid compared with the control plasmid (Fig. 4C). To evaluate the functional role of the −116 ERG binding site in the transactivation of the CLDN5 promoter by ERG, we generated a point mutation of the CLDN5 promoter (CLDN5-mut) within the ERG binding site (Fig. 4A). The resulting construct was transiently transfected into HUVEC along with the ERG expression plasmid. Mutation at the −116 ERG site resulted in significantly reduced transactivation of the CLDN5 promoter (Fig. 4D). These results suggest that this ERG binding site (−116) functions to mediate CLDN5 expression by ERG in EC. Furthermore, we demonstrated that the wild-type human CLDN5 promoter exhibited reduced activity in ERG knockdown cells (Fig. 4E).
FIGURE 4.
CLDN5 is a downstream target of ERG. A, schematic diagram of the CLDN5 promoter. Black boxes indicate the putative ERG binding sites. TSS, transcriptional start site; ATG, translational start site; E1 and E2, exon 1 and exon 2, respectively. B, ChIP assay of CLDN5 promoter using HUVEC. An ERG polyclonal antibody was used for immunoprecipitation. The data are shown as QPCR analysis using primers corresponding to the putative ERG binding site of CLDN5 promoter ChIP2 indicated in A relative to control IgG ± S.D. (error bars), n = 3. p < 0.01. C, transactivation assay. HUVEC were co-transfected with CLDN5 promoter luciferase reporter gene vector and either the empty mammalian expression plasmid (pCI) or pCI-ERG. After 24 h of incubation, cells were harvested for luciferase assay. The data are shown as -fold change of luciferase activity compared with co-transfection with empty expression plasmid ± S.D., n = 3. p < 0.01. D, HUVEC transfected with CLDN5 wild-type promoter (CLDN5-WT) or mutant at −116 (CLDN5-mut) in pGL3 vector together with pCI-ERG or pCI alone. After 24 h of incubation, cells were assessed for luciferase activities. The data are shown as relative luciferase activity compared with co-transfection with the empty expression plasmid (pCI) ± S.D., n = 3. p < 0.05. E, HUVEC infected with either ERG shRNA or control shRNA and transfected with CLDN5 promoter in reporter vector. After 24 h of incubation, cells were assayed for luciferase activity. The data are presented relative to control cells ± S.D., n = 3. p < 0.01.
CLDN5 Knockdown Is Associated with Decreased Intercellular Resistance
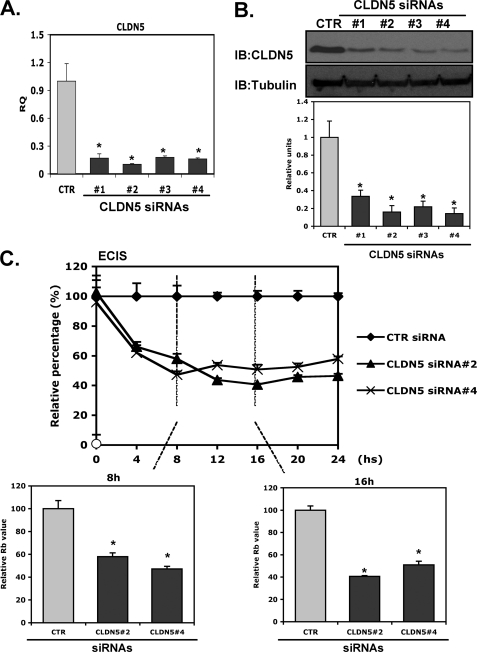
We were interested in determining whether knockdown of CLDN5 leads to phenotypic changes in intercellular resistance in EC similar to those in knockdown of ERG. The knockdown efficiency of CLDN5 siRNAs at both RNA and protein levels is shown in Fig. 5, A and B, with a reduction of approximately 80%. Interestingly, knockdown of CLDN5 using either #2 or #4 siRNA disrupted EC intercellular resistance by ∼50% (Fig. 5C).
FIGURE 5.
CLDN5 knockdown is associated with decreased intercellular resistance. A and B, ability of CLDN5 siRNAs to repress CLDN5 expression. HUVEC were transfected with various CLDN5 siRNAs (#1–#4; 40 nmol/liter) or a control siRNA (CTR). After incubation for 48 h, CLDN5 expression was assessed by QPCR (A) using CLDN5-specific primers or by Western blot analysis (B) using anti-CLDN5 antibody. Tubulin was used as a loading control. Quantitation was done by densitometric analysis. n = 3. *, p < 0.05. C, ECIS. EC were treated with CLDN5 #2, #4 siRNAs or a control siRNA (40 nmol/liter). After incubation for 48 h cells were then plated on the 8W10E+ to assess the intercellular resistance for a period of 24 h. Data shown are presented as values of normalized Rb value relative to control samples. The bar graphs represent the relative Rb values at indicated time points relative to control ± S.D., n = 3. *, p < 0.01.
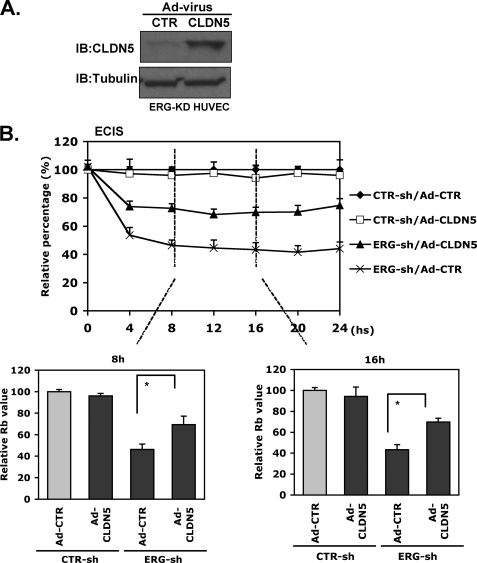
Partial Rescue of ERG Knockdown Effect on Intercellular Resistance by Adenoviral CLDN5 Expression
We next evaluated the ability of an adenovirus expressing CLDN5 to rescue EC barrier function in ERG knockdown EC. The overexpression of CLDN5 delivered in adenovirus was confirmed by Western blotting (Fig. 6A). Overexpression of CLDN5 alone had no effect on EC intercellular resistance in control cells, whereas it was able to rescue that in ERG shRNA-treated EC partially (Fig. 6B, ∼50%), supporting the concept that CLDN5 is a major downstream target of ERG that is involved in regulating EC permeability. Furthermore, adenoviral expression of CLDN5 was able to rescue partially the increased permeability induced by TNF-α stimulation (supplemental Fig. 4).
FIGURE 6.
Adenoviral delivery of CLDN5 partially rescues the effect of ERG knockdown on intercellular resistance. A, HUVEC treated with ERG shRNA and infected with either Ad-CLDN5 or Ad-GFP as a control. After incubation for 48 h, the overexpression of CLDN5 was determined by Western blotting using antibody against CLDN5. Tubulin was used as a loading control. B, ECIS assay. HUVEC infected with ERG shRNA or control shRNA were cultured and transfected with either Ad-CLDN5 or Ad-GFP as a control. After incubation for 48 h the intercellular resistance was measured and collected using ECIS system for 24 h. The data are shown relative to control cells treated with control Ad-GFP (Ad-CTR). The bar graphs represent the relative Rb values at indicated time points relative to control ± S.D., n = 3. *, p < 0.01.
DISCUSSION
CLDN5 is an EC-restricted TJ protein that promotes EC barrier function. Previous studies in mice have shown that CLDN5 is expressed in the endothelium of all segments of blood vessels in the mouse brain and lung, and in afferent and efferent arterioles of the kidney (23). Expression was also observed in EC in some segments of blood vessels in the intestine, skin, skeletal muscle, testis, and liver (23–25). In human lung, CLDN5 is strongly expressed in the endothelium of capillaries, arteries, veins, and lymphatic vessels (23). The results of our study provide further support that CLDN5 is expressed in several different tissues, with particularly high expression in the lung.
The expression of CLDN5 is dynamically regulated at transcriptional and posttranscriptional levels. In mice, acute lung injury resulted increased CLDN5 protein and mRNA expression in the lung (26). Lungs from patients with interstitial pneumonia also revealed increased CLDN5 protein expression (27). In vitro studies have shown that CLDN5 mRNA expression is induced in EC by acreolin (26), 17β-estradiol (28), and hydrocortisone (26). Conversely, mRNA and/or protein expression is suppressed by TNF-α (29, 30), IL-1 (31), and VEGF (32). Several transcription factors have been implicated in the control of CLDN5 gene expression, including SOX18 (33) and FOXO1 (34).
The mouse CLDN5 promoter was previously cloned and characterized (29). The promoter does not have a classical TATA box. DNA sequence analysis of the promoter revealed six putative glucocorticoid-response elements, two NF-κB sites, three Sp1 sites, one Ap2 site, and three E-box elements. High basal activity of the CLDN5 promoter could be repressed by TNF-α, providing further support for a transcriptional effect of this cytokine. However, the precise transcriptional mechanism by which TNF-α repressed promoter activity was not elucidated.
Our findings point to CLDN5 as a direct transcriptional target of ERG. The human promoter contains two ERG consensus sites (at −750 and −116). Using ChIP, we found that ERG binds to a region spanning the −116 ERG element. A point mutation of this site resulted in a significant reduction in ERG-mediated activation of the CLDN5 promoter, suggesting a functional role for the −116 ERG binding motif. These data, together with our previous observation that TNF-α suppresses ERG expression (11) are consistent with a role for this transcription factor in mediating cytokine repression of CLDN5.
Several lines of evidence support a biologically relevant link between TNF-α, ERG, CLDN5 and EC barrier function. First, both ERG and CLDN5 knockdown promoted barrier dysfunction. Second, ERG overexpression partially rescued the pro-permeability effect of TNF-α. Finally, CLDN5 overexpression partially rescued the negative effect of ERG knockdown on barrier properties. Together, these findings support a model in which TNF-α (and perhaps other cytokines) promotes increased permeability in part by inhibiting ERG expression and its downstream target gene, CLDN5. Finally, we recognize that there may be additional downstream targets of ERG that have yet to be identified and that also play an important role in the regulation of EC barrier function. Future studies will be directed at trying to identify these additional downstream targets of ERG and to define further the role of ERG in the regulation of EC barrier function in vivo in diseases such as bacterial sepsis, atherosclerosis, that are associated with significant endothelial dysfunction.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 HL76540 (to P. O. and W. C. A.) and R01 HL04006 (to C. V. C.). This work was also supported by American Heart Association Established Investigator Award EIA0740012 (to P. O.).

This article contains supplemental Tables 1–3 and Figs. 1–4.
- AJ
- adherens junction
- CLDN5
- claudin 5
- ECIS
- electric cell-substrate impedance sensing
- ERG
- ETS-related gene
- HCAEC
- human coronary artery EC
- HMVEC
- human lung microvascular EC
- HUVEC
- human umbilical vein EC
- ICAM
- intercellular adhesion molecule
- QPCR
- quantitative PCR
- Rb
- barrier resistance
- TJ
- tight junction.
REFERENCES
- 1. Hansson G. K., Hermansson A. (2011) The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 [DOI] [PubMed] [Google Scholar]
- 2. Garcia C., Feve B., Ferré P., Halimi S., Baizri H., Bordier L., Guiu G., Dupuy O., Bauduceau B., Mayaudon H. (2010) Diabetes and inflammation: fundamental aspects and clinical implications. Diabetes Metab. 36, 327–338 [DOI] [PubMed] [Google Scholar]
- 3. Khan F., Galarraga B., Belch J. J. (2010) The role of endothelial function and its assessment in rheumatoid arthritis. Nat. Rev. Rheumatol. 6, 253–261 [DOI] [PubMed] [Google Scholar]
- 4. Aghajanian A., Wittchen E. S., Allingham M. J., Garrett T. A., Burridge K. (2008) Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J. Thromb. Haemost. 6, 1453–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vandenbroucke E., Mehta D., Minshall R., Malik A. B. (2008) Regulation of endothelial junctional permeability. Ann. N.Y. Acad. Sci. 1123, 134–145 [DOI] [PubMed] [Google Scholar]
- 6. Nitta T., Hata M., Gotoh S., Seo Y., Sasaki H., Hashimoto N., Furuse M., Tsukita S. (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 161, 653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wasylyk B., Hagman J., Gutierrez-Hartmann A. (1998) Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem. Sci. 23, 213–216 [DOI] [PubMed] [Google Scholar]
- 8. Rudders S., Gaspar J., Madore R., Voland C., Grall F., Patel A., Pellacani A., Perrella M. A., Libermann T. A., Oettgen P. (2001) ESE-1 is a novel transcriptional mediator of inflammation that interacts with NF-κB to regulate the inducible nitric-oxide synthase gene. J. Biol. Chem. 276, 3302–3309 [DOI] [PubMed] [Google Scholar]
- 9. Zhan Y., Brown C., Maynard E., Anshelevich A., Ni W., Ho I. C., Oettgen P. (2005) Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J. Clin. Invest. 115, 2508–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ni W., Kitamoto S., Ishibashi M., Usui M., Inoue S., Hiasa K., Zhao Q., Nishida K., Takeshita A., Egashira K. (2004) Monocyte chemoattractant protein-1 is an essential inflammatory mediator in angiotensin II-induced progression of established atherosclerosis in hypercholesterolemic mice. Arterioscler. Thromb. Vasc. Biol. 24, 534–539 [DOI] [PubMed] [Google Scholar]
- 11. Yuan L., Nikolova-Krstevski V., Zhan Y., Kondo M., Bhasin M., Varghese L., Yano K., Carman C. V., Aird W. C., Oettgen P. (2009) Antiinflammatory effects of the ETS factor ERG in endothelial cells are mediated through transcriptional repression of the interleukin-8 gene. Circ. Res. 104, 1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pimanda J. E., Chan W. Y., Donaldson I. J., Bowen M., Green A. R., Göttgens B. (2006) Endoglin expression in the endothelium is regulated by Fli-1, Erg, and Elf-1 acting on the promoter and a −8-kb enhancer. Blood 107, 4737–4745 [DOI] [PubMed] [Google Scholar]
- 13. Hasegawa Y., Abe M., Yamazaki T., Niizeki O., Shiiba K., Sasaki I., Sato Y. (2004) Transcriptional regulation of human angiopoietin-2 by transcription factor Ets-1. Biochem. Biophys. Res. Commun. 316, 52–58 [DOI] [PubMed] [Google Scholar]
- 14. Birdsey G. M., Dryden N. H., Amsellem V., Gebhardt F., Sahnan K., Haskard D. O., Dejana E., Mason J. C., Randi A. M. (2008) Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood 111, 3498–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yuan L., Sacharidou A., Stratman A. N., Le Bras A., Zwiers P. J., Spokes K., Bhasin M., Shih S. C., Nagy J. A., Molema G., Aird W. C., Davis G. E., Oettgen P. (2011) RhoJ is an endothelial cell-restricted Rho GTPase that mediates vascular morphogenesis and is regulated by the transcription factor ERG. Blood 118, 1145–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gory S., Dalmon J., Prandini M. H., Kortulewski T., de Launoit Y., Huber P. (1998) Requirement of a GT box (Sp1 site) and two Ets binding sites for vascular endothelial cadherin gene transcription. J. Biol. Chem. 273, 6750–6755 [DOI] [PubMed] [Google Scholar]
- 17. Sperone A., Dryden N. H., Birdsey G. M., Madden L., Johns M., Evans P. C., Mason J. C., Haskard D. O., Boyle J. J., Paleolog E. M., Randi A. M. (2011) The transcription factor Erg inhibits vascular inflammation by repressing NF-κB activation and proinflammatory gene expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 31, 142–150 [DOI] [PubMed] [Google Scholar]
- 18. Bayless K. J., Davis G. E. (2002) The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J. Cell Sci. 115, 1123–1136 [DOI] [PubMed] [Google Scholar]
- 19. He X., Kuijpers G. A., Goping G., Kulakusky J. A., Zheng C., Delporte C., Tse C. M., Redman R. S., Donowitz M., Pollard H. B., Baum B. J. (1998) A polarized salivary cell monolayer useful for studying transepithelial fluid movement in vitro. Pflugers Arch. 435, 375–381 [DOI] [PubMed] [Google Scholar]
- 20. Melero-Martin J. M., De Obaldia M. E., Kang S. Y., Khan Z. A., Yuan L., Oettgen P., Bischoff J. (2008) Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 103, 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vouret-Craviari V., Boquet P., Pouysségur J., Van Obberghen-Schilling E. (1998) Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol. Biol. Cell 9, 2639–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petrache I., Verin A. D., Crow M. T., Birukova A., Liu F., Garcia J. G. (2001) Differential effect of MLC kinase in TNF-α-induced endothelial cell apoptosis and barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 280, L1168–1178 [DOI] [PubMed] [Google Scholar]
- 23. Morita K., Sasaki H., Furuse M., Tsukita S. (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 147, 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morita K., Sasaki H., Furuse K., Furuse M., Tsukita S., Miyachi Y. (2003) Expression of claudin-5 in dermal vascular endothelia. Exp. Dermatol. 12, 289–295 [DOI] [PubMed] [Google Scholar]
- 25. Sasaki H., Matsui C., Furuse K., Mimori-Kiyosue Y., Furuse M., Tsukita S. (2003) Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 100, 3971–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jang A. S., Concel V. J., Bein K., Brant K. A., Liu S., Pope-Varsalona H., Dopico R. A., Jr., Di Y. P., Knoell D. L., Barchowsky A., Leikauf G. D. (2011) Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am. J. Respir. Cell. Mol. Biol. 44, 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaarteenaho-Wiik R., Soini Y. (2009) Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J. Histochem. Cytochem. 57, 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burek M., Arias-Loza P. A., Roewer N., Förster C. Y. (2010) Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 30, 298–304 [DOI] [PubMed] [Google Scholar]
- 29. Förster C., Burek M., Romero I. A., Weksler B., Couraud P. O., Drenckhahn D. (2008) Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood-brain barrier. J. Physiol. 586, 1937–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burek M., Förster C. Y. (2009) Cloning and characterization of the murine claudin-5 promoter. Mol. Cell. Endocrinol. 298, 19–24 [DOI] [PubMed] [Google Scholar]
- 31. Williams M. R., Kataoka N., Sakurai Y., Powers C. M., Eskin S. G., McIntire L. V. (2008) Gene expression of endothelial cells due to interleukin-1β stimulation and neutrophil transmigration. Endothelium 15, 73–165 [DOI] [PubMed] [Google Scholar]
- 32. Argaw A. T., Gurfein B. T., Zhang Y., Zameer A., John G. R. (2009) VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. U.S.A. 106, 1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fontijn R. D., Volger O. L., Fledderus J. O., Reijerkerk A., de Vries H. E., Horrevoets A. J. (2008) SOX-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am. J. Physiol. Heart Circ. Physiol. 294, H891–900 [DOI] [PubMed] [Google Scholar]
- 34. Dejana E., Taddei A., Randi A. M. (2007) Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim. Biophys. Acta 1775, 298–312 [DOI] [PubMed] [Google Scholar]