Background: The cell surface, transmembrane protein neurexin may play a role in insulin secretion.
Results: Knockdown and knock-out of neurexin-1α, the predominant β-cell isoform, increased insulin secretion and impaired secretory granule docking.
Conclusion: Neurexin-1α helps mediate insulin granule docking and thereby constrains secretion.
Significance: Neurexin-1α could couple localization and functioning of the insulin docking and secretory machinery to extracellular protein interactions.
Keywords: β-Cell, Cell Surface Protein, Exocytosis, Insulin Secretion, Snare Proteins
Abstract
Neurexins are a family of transmembrane, synaptic adhesion molecules. In neurons, neurexins bind to both sub-plasma membrane and synaptic vesicle-associated constituents of the secretory machinery, play a key role in the organization and stabilization of the presynaptic active zone, and help mediate docking of synaptic vesicles. We have previously shown that neurexins, like many other protein constituents of the neurotransmitter exocytotic machinery, are expressed in pancreatic β cells. We hypothesized that the role of neurexins in β cells parallels their role in neurons, with β-cell neurexins helping to mediate insulin granule docking and secretion. Here we demonstrate that β cells express a more restricted pattern of neurexin transcripts than neurons, with a clear predominance of neurexin-1α expressed in isolated islets. Using INS-1E β cells, we found that neurexin-1α interacts with membrane-bound components of the secretory granule-docking machinery and with the granule-associated protein granuphilin. Decreased expression of neurexin-1α, like decreased expression of granuphilin, reduces granule docking at the β-cell membrane and improves insulin secretion. Perifusion of neurexin-1α KO mouse islets revealed a significant increase in second-phase insulin secretion with a trend toward increased first-phase secretion. Upon glucose stimulation, neurexin-1α protein levels decrease. This glucose-induced down-regulation may enhance glucose-stimulated insulin secretion. We conclude that neurexin-1α is a component of the β-cell secretory machinery and contributes to secretory granule docking, most likely through interactions with granuphilin. Neurexin-1α is the only transmembrane component of the docking machinery identified thus far. Our findings provide new insights into the mechanisms of insulin granule docking and exocytosis.
Introduction
β cells share many similarities with neurons, including the expression of many of the same scaffolding and exocytotic proteins (1, 2). These proteins mediate the exocytosis of synaptic vesicles in neurons and insulin granules in β cells (3). Advances in the neurobiology field regarding synaptic maturation and function thus frequently offer important clues regarding overall β-cell function and the mechanisms underlying insulin secretion.
Neurexins (NRXNs)2 are a family of synaptic adhesion molecules that function as neuronal cell-surface receptors and signaling proteins (4–6). They are encoded by three genes (NRXN1–3), each using an upstream promoter to produce the longer α-isoform (α-NRXNs) and a downstream promoter to generate a shorter β-isoform (β-NRXNs) (7). The α- and β-isoforms of each neurexin are single-pass transmembrane proteins maintaining identical transmembrane and intracellular domains but having distinct (i.e. long and short) extracellular domains (4). NRXNs in neurons localize to the presynaptic membrane and bind transsynaptically to postsynaptic adhesion molecules (8–11) and receptors (12). The intracellular, C-terminal regions of NRXNs contain PDZ-binding domains (4). Studies in neurons have demonstrated that the intracellular domains of NRXNs interact with a number of exocytotic proteins, including the scaffolding proteins Mint1 and Velis (13, 14), the Sec1/Munc18-like protein Munc18–1 (14), the t-SNARE syntaxin 1 (15), the calcium sensor synaptotagmin 1 (15, 16), and the calcium/calmodulin-dependent kinase containing membrane-associated guanylate kinase CASK (17, 18).
We previously found that β cells express NRXNs and one of their major postsynaptic binding partners, neuroligins (19). Separately, NRXN1α was one of the most abundant transcripts identified in a systematic study of human tissue mRNAs designed to identify highly expressed, membrane-associated, human islet-specific proteins (i.e. without expression in kidney, liver, or exocrine pancreas) (20).
Studies with double and triple α-NRXN KO mice have indicated that α-NRXNs in neurons are essential for the organization and stabilization of the presynaptic machinery (21–23). In vitro work suggests that NRXNs interact with the synaptic vesicle-associated protein rabphilin-3A via CASK (24) and contribute to synaptic vesicle docking (16, 25). β cells also express CASK (19) and, in addition, granuphilin, a rabphilin-3 homologue implicated in insulin granule docking (26, 27). Based on the similarities between neurotransmitter exocytosis and insulin secretion, we hypothesized that NRXNs in the β-cell are constituents of the insulin secretory machinery, perhaps helping to mediate secretory granule docking via interactions with granuphilin.
In the present study we examined the role of NRXN1α in β-cell function. Our data show that NRXN1α is an integral component of the secretory granule docking machinery. Like other proteins that contribute to the granuphilin-mediated docking of secretory granules at the β-cell membrane, NRXN1α inhibits insulin secretion (28, 29). Our findings provide new insights into the mechanisms of insulin granule exocytosis.
EXPERIMENTAL PROCEDURES
Antibodies
The following antibodies were obtained commercially: goat anti-NRXN1 (P-15), goat anti-rabbit IgG-HRP, and goat anti-mouse IgG-HRP (Santa Cruz Biotechnology); rabbit anti-granuphilin (Atlas Antibodies); mouse anti-syntaxin 1 and -GAPDH (Sigma-Aldrich); mouse anti-synaptophysin, -CASK and -Munc18 (BD Biosciences); rabbit anti-GFP/CFP and Alexa Fluor 488 donkey anti-mouse IgG (Molecular Probes); guinea pig anti-NRXN1α and donkey anti-guinea pig-Cy3 (Millipore); guinea pig anti-insulin (Dako); biotinylated goat anti-guinea pig IgG (Vector Laboratories); and IRDye 680-conjugated goat anti-mouse IgG and IRDye 800CW-conjugated goat anti-rabbit IgG (LI-COR).
To detect NRXN expression by immunoblotting, we raised a polyclonal, pan-NRXN antibody against a previously described NRXN peptide (15). Rabbits were injected with the keyhole limpet hemocyanin-conjugated peptide CAKSANKNKKNKDKEYYV, and serum was affinity purified (Open Biosystems). The antibody was validated for peptide binding by ELISA and competition assay. The antibody was also validated by Western blot and detects all six CFP-tagged NRXN isoforms (30) (NRXN1α and 2α shown in Fig. 1B, lanes 8 and 9). The identity of the bands was confirmed with an anti-GFP/CFP antibody (data not shown).
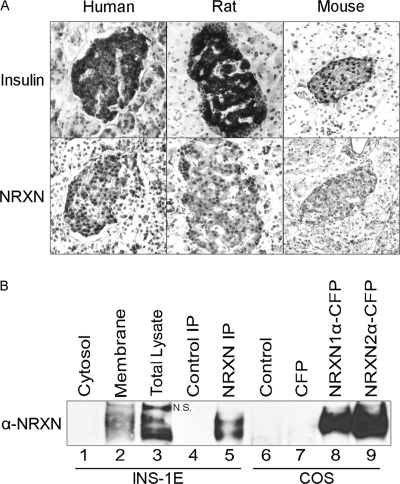
FIGURE 1.
α-NRXN protein is present in β cells. A, serial sections of human, rat, and mouse pancreas were stained for insulin (top) and NRXN (bottom). Omission of primary antibody in parallel control experiments abolished the observed islet staining (data not shown). B, cytosolic and plasma membrane fractions (10 μg each, lanes 1–2) of INS-1E cells as well as total lysate (30 μg, lane 3) were immunoblotted with a pan-NRXN antibody. Protein immunoprecipitated from INS-1E cell extracts with a NRXN1 antibody (NRXN IP, lane 5) or an isotype control antibody (Control IP, lane 4) was analyzed on the same blot. Lysates of control COS-7 cells (Control) and COS-7 cells overexpressing CFP or CFP-tagged NRXN1α and NRXN2α (lanes 6–9) were immunoblotted in parallel to further validate the pan-NRXN antibody. N.S. indicates a nonspecific band detected by the pan-NRXN antibody in the lysate (lane 3) but not in anti-NRXN1 immunoprecipitates (lane 5).
Plasmid Constructs
The cDNA construct encoding CFP was previously described (31). The cDNA constructs encoding eGFP-tagged NRXN1α (32) and each of the six isoforms of CFP-tagged NRXN (30) were a gift from Dr. Markus Missler (University of Munster) and Dr. Ann Marie Craig (University of British Columbia), respectively. The NRXN1α expression vector and an empty vector control were derived from the pCMV-eGFP-NRXN1α vector by removing regions encoding eGFP and eGFP-NRXN1α, respectively. The cDNA vector pSEAP2 encoding secreted alkaline phosphatase (SEAP) was from Clontech.
Animals
NRXN1α KO mice were bred from previously described NRXN1α and 2α heterozygous KO mice (21) purchased from Jackson Laboratory. Genotyping was performed by PCR using primer pairs described on Jackson's website and was confirmed by RT-PCR using RNA isolated from brain and islets as previously described (19). Primers for RT-PCR are described in supplemental Table S1. Islets were isolated from age-matched KO and WT mice at ages 10–14 weeks as previously described (33), hand-picked and cultured overnight in 8 mm glucose before use in experiments. All procedures were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Cell Culture and Transfection
INS-1E cells (34) and 832/13 cells (35) were gifts from Dr. Pierre Maechler (Geneva University) and Dr. Christopher B. Newgard (Duke University) respectively. Both lines were cultured in RPMI 1640 medium containing 10% FBS, 2 mm l-glutamine, 10 mm HEPES, 100 units/ml penicillin, 100 μg/ml streptomycin, 1 mm sodium pyruvate, 0.05 mm 2-mercaptoethanol, and 0.25 μg/ml amphoterecin B. Islets were cultured in the same medium except without 2-mercaptoethanol. COS-7 cells were cultured in DMEM containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (36). All cells were maintained in a humidified 37 ºC incubator with 5% CO2. For transfections, cells were plated onto 12- or 24-well plates in antibiotic-free media and transfected with DNA constructs or siRNA duplexes using Lipofectamine 2000 (Invitrogen) or Pepmute (SignaGen Laboratories) according to manufacturers' protocols. Cells were stimulated or harvested 48 h or 72 h after transfection.
Histology
Pancreas tissue was obtained from adult male Sprague-Dawley rats, C57BL/6 mice, and NRXN1α KO mice, fixed for 24 h in Pen-Fix (Thermo Fisher Scientific) and embedded in paraffin. Samples of human pancreas tissue from healthy donors were obtained from the National Disease Research Interchange, fixed in 10% buffered formalin, and embedded in paraffin. Sections (6 μm) of human, rat, and mouse pancreases were prepared by the University of California, San Diego histology core. Deparaffinized sections were subjected to antigen retrieval by treating with a citrate buffer. H&E staining was performed using standard methods. Immunostaining with the Vectastain Elite ABC kit and NovaRED substrate (Vector Laboratories) was performed as previously described (37). Immunofluorescence was performed using 5% serum in PBS for blocking and antibody dilutions. Primary antibodies (4 ºC overnight) and secondary antibodies (room temperature for 1 h) were applied in a humidified chamber. Controls for immunostaining and immunofluorescence included tissue stained in parallel with secondary antibody alone. Images were captured using a Nikon Eclipse E800 microscope and SPOT RT S.E. camera at ×20.
Western Blotting
Total cell extract was prepared by lysing cells in RIPA buffer containing 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 50 mm Tris-HCl (pH 7.5), 2 mm EDTA, 1 mm PMSF, and 1% Protease Inhibitor Mixture (Sigma-Aldrich). Plasma membrane was separated from cytosolic fractions as previously described by differential centrifugation and detergent/aqueous partitioning using the Plasma Membrane Protein Extraction kit (BioVision) per the manufacturer's instructions (38). Proteins were quantified using the DC Protein Assay (Bio-Rad). NuPAGE LDS Sample Buffer and NuPAGE Reducing Agent (Invitrogen) were added to protein at a 1× final concentration. Protein was then incubated at 70 ºC for 10 min and electrophoresed on either 4–12% Bis-Tris or 3–8% Tris-acetate NuPAGE Gels (Invitrogen). Protein was transferred to PVDF membrane, blocked with 5% milk in PBS and probed with antibodies in 5% milk in PBS containing 0.1% Tween 20. Membranes probed with HRP-labeled secondary antibodies were incubated with PS-3 chemiluminescent detection reagent (Lumigen) and the chemiluminescent signal captured by HyBlot CL autoradiography film (Denville Scientific). Membranes probed with IRDye-conjugated secondary antibodies were directly imaged and bands quantified with the Odyssey Infrared Imaging System and Software (LI-COR).
Immunoprecipitation (IP)
Cells were lysed in a buffer containing 150 mm NaCl, 1% Nonidet P-40, 50 mm Tris (pH 8), 1 mm PMSF, and 1% protease inhibitor mixture. Lysate was precleared with rec-protein G-Sepharose (Invitrogen) and then incubated overnight at 4 ºC with 5 μg of goat anti-NRXN1 P-15 antibody or purified goat IgG (Millipore). The reactions were incubated with rec-protein G-Sepharose at 4 ºC for 2 h and beads were thoroughly washed with lysis buffer. Samples were combined with LDS Sample Buffer and Reducing Agent for Western blotting as described above.
Real-time Quantitative PCR (qPCR)
Brain tissue was obtained from adult male Sprague-Dawley rats. Freshly isolated islets from adult male Sprague-Dawley rats and Swiss Webster mice were provided by the University of Washington Diabetes and Endocrinology Research Center Islet Cell and Functional Analysis Core. Human islets isolated as previously described (39) were obtained from the Southern California Islet Cell Consortium and the University of Alabama at Birmingham through the National Institutes of Health-sponsored Islet Cell Resources Basic Science Islet Distribution Program and flash frozen after 1 to 3 days in culture. Pancreases were procured from heart-beating, cadaveric, non-diabetic, female donors ages 48 and 49 with body mass indices of 34 and 23, respectively. Islet preparations were deemed ≥ 80% purity and ≥ 90% viability. Total RNA was isolated and reverse transcribed as previously described (19). Gene-specific primers were used to perform qPCR with Power SYBR Green PCR Master Mix (Applied Biosystems), PerfeCTa SYBR Green FastMix (Quanta BioSciences) or 2× qPCR Master Mix (BioPioneer) on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). Each sample was analyzed in triplicate along with no-RT and no-template controls. For relative qPCR, values were normalized to 18 S RNA. For absolute qPCR, standard curves of human, rat, and mouse NRXN gene- and isoform-specific amplicons were generated by PCR and gel purified with a QIAquick gel extraction kit (Qiagen). Normalization was carried out using total RNA values and confirmed with qPCR of 18 S RNA. NRXN primers were designed to avoid major splice variant regions. All primers were designed using Primer 3 (40) or PerlPrimer (41) software and are described in supplemental Table S1.
RNAi
For NRXN1 RNAi experiments, two pools of siRNAs targeting NRXN1 (NRXN1 pools 1 and 2) and two non-targeting control pools of siRNA (non-targeting pools 1 and 2) comprised of completely different siRNAs were purchased from Dharmacon Research (Thermo Fisher Scientific). For NRXN2 RNAi experiments, a pool of siRNAs targeting NRXN2 (NRXN2 pool A) was purchased from Sigma-Aldrich and a pool of non-targeting control siRNAs (non-targeting pool A) was purchased from Dharmacon Research (Thermo Fisher Scientific). All sequences are listed in supplemental Table S2.
Insulin Secretion and Glucose Stimulation
INS-1E cells were switched to antibiotic-free media containing 5 mm glucose for ∼18 h and then to 2.75 mm glucose Krebs-Ringer bicarbonate buffer for 1 h. Fresh Krebs-Ringer buffer containing 2.75, 15, or 16.7 mm glucose was then applied for 1 h as previously described (19). For static studies, 10 similar-sized islets (per well in 96-well plates) were stimulated as above. Insulin in the cell lysate and media was measured using a rat insulin RIA (Millipore) or ultrasensitive rat insulin ELISA kit (Crystal Chem). For SEAP (secreted alkaline phosphatase) co-transfection studies, cell lysate and media were also assayed for SEAP as described (42). Both insulin and SEAP secretion are represented as media content normalized to total intracellular content. Total cellular insulin content in lysate was normalized to total cellular protein. Perifusion studies were performed by the University of Washington Diabetes and Endocrinology Research Center Islet Cell and Functional Analysis Core as previously described (43).
EM
INS-1E cells and isolated islets in normal culture media were fixed in 2% glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.4) for at least 4 h, postfixed in 1% osmium tetroxide in 0.1 m cacodylate buffer for 1 h and stained en bloc in 1% uranyl acetate for 1 h. Samples were dehydrated in ethanol, embedded in epoxy resin, sectioned at 60 to 70 nm and stained with uranyl acetate and lead nitrate. Grids were viewed using a transmission electron microscope (1200EX II, JEOL) and photographed using a digital camera (Gatan). Cells were chosen at random, and islet morphometry was analyzed after deidentification of experimental groups. Distances were determined by drawing a straight line from the granule center to the nearest plasma membrane. All measurements were performed in ImageJ (44).
RESULTS
NRXN Protein Is Present in β Cells
We previously demonstrated the presence of NRXN in the rat pancreatic β cell by immunofluorescence (19). β-Cell expression of NRXN protein was confirmed by immunostaining of human, rat, and mouse pancreas sections (Fig. 1A). NRXN1α protein expression in INS-1E β cells was confirmed by immunoprecipitation of NRXN1α from cell lysate followed by immunoblotting using a different, pan-NRXN antibody (Fig. 1B, lane 5). Immunoblot analysis of INS-1E cells demonstrated the presence of α-NRXN in the plasma membrane fraction, consistent with the plasma membrane localization of NRXN observed in our previous immunofluorescent staining (19). NRXN was not detected in the cytosolic fraction (Fig. 1B, lanes 1–2). As part of validation of the pan-NRXN antibody, it was also used to immunoblot CFP-tagged NRXN expressed in transfected COS cells (Fig. 1B, lanes 8–9). The expected increase in NRXN molecular weight due to the CFP tag is not obvious because of the high molecular mass of α-NRXNs (∼180 kDa) relative to CFP (∼27 kDa).
NRXN Transcript Levels in β Cells
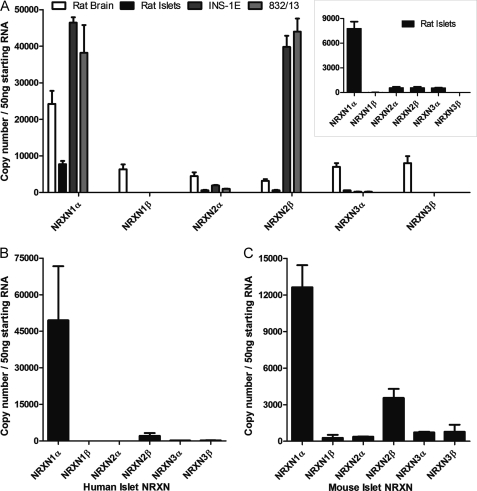
We next sought to identify the NRXN isoforms present in β cells. Because existing antibodies cannot distinguish between the three NRXN genes, we used absolute RT-qPCR to quantify transcripts of each isoform in rat islets, rat brain, as well as the rat β-cell lines INS-1E and 832/13 (Fig. 2A). Brain expresses similar levels of all NRXN isoforms except for a slight enrichment of NRXN1α. Islets and β cells demonstrated a distinctly different pattern, showing a clear predominance of one or two isoforms. In INS-1E and 832/13 β cells, NRXN 1α and 2β predominate. In rat islets, NRXN1α is the predominant transcript (Fig. 2A, inset). Human (Fig. 2B) and mouse (Fig. 2C) islets displayed a similar expression pattern, with NRXN1α transcript being most abundant, followed by lower expression of NRXN 2β. Because NRXN1α is the most abundant isoform in primary islets, it was chosen as the focus for further investigations.
FIGURE 2.
Quantification of NRXN transcripts in β cells by absolute RT-qPCR. NRXN mRNA transcript numbers in 50 ng of starting RNA from A, rat islets, the rat β-cell lines INS-1E and INS-1 832/13, and rat brain as well as B, human islets and C, mouse islets were quantified by absolute qPCR using a set of standard curves for each reaction with known NRXN copy numbers. Rat islet data are also represented as an inset in panel A. Data are represented as mean ± S.E. from three individual tissue, islet or culture preparations assayed in triplicate.
Coimmunoprecipitation of NRXN1α with Docking and Submembrane-resident Exocytotic Proteins
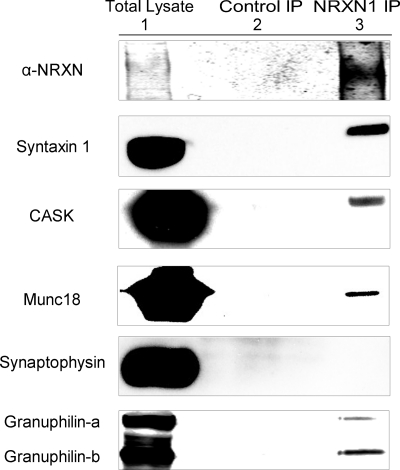
To identify NRXN-interacting proteins in INS-1E cells, we immunoprecipitated NRXN1α and analyzed coprecipitating proteins by Western blot. In neurons, NRXN seeds the assembly of the exocytotic machinery through interactions with Mint 1 (13), Velis (13, 14), CASK (17, 18, 45), syntaxin 1 (15), and Munc18 (14). Consistent with NRXN being a constituent of the insulin granule submembrane secretory apparatus in β cells, we found coprecipitation of NRXN1α with the submembrane exocytotic proteins syntaxin 1, CASK, and Munc18 (Fig. 3). In contrast, no co-precipitation with synaptophysin was detected even in overexposed immunoblots, suggesting that NRXN does not bind directly or indirectly to this vesicular protein. These results suggest NRXN is a component of the multiprotein, submembrane complex that regulates the plasma-membrane docking and exocytosis of insulin granules.
FIGURE 3.
NRXN interacts with exocytotic and docking proteins in the INS-1E β-cell line. Anti-NRXN1 immunoprecipitates from INS-1E cells were immunoblotted for the indicated proteins (NRXN1 IP, lane 3) using isotype-matched control IgG immunoprecipitates as controls (Control IP, lane 2). Total lysate is represented in lane 1. The experiments were repeated with similar results.
In neurons, NRXNs likely contribute to synaptic vesicle docking via interactions with rabphilin-3A (24). β cells express the rabphilin-3-like protein granuphilin, which associates with secretory granules (27). Granuphilin on the surface of insulin granules directly interacts with syntaxin-1 and participates in secretory granule docking and inhibition of SNARE-mediated insulin release (26, 46). Both isoforms of granuphilin, the full-length granuphilin-a and the shorter splice variant granuphilin-b, are expressed in INS-1 cells at similar levels and are reported to function in the same manner (47). Immunoprecipitation of NRXN1α revealed its interaction with both isoforms of granuphilin in β cells (Fig. 3, bottom panel).
Knockdown of NRXN1α in INS-1E Cells Increases Insulin Secretion, Insulin Content, and Insulin Gene Expression
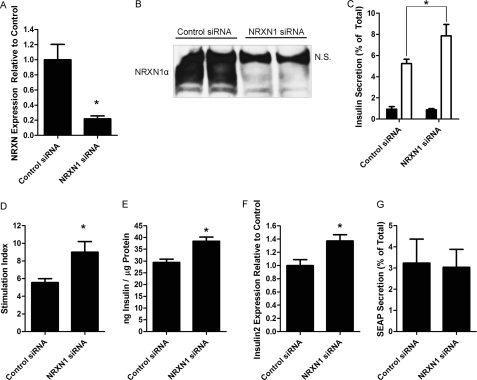
Granuphilin-mediated secretory granule docking is thought to act as a “temporal constraint” on insulin secretion, inhibiting SNARE-mediated fusion of insulin granules with the plasma membrane (26, 48). Because of this, experimental knockdown and KO of granuphilin increased insulin secretion (48, 49). If NRXN is also necessary for docking, its depletion should similarly increase insulin secretion. We thus used siRNA to knockdown NRXN1 in INS-1E cells and determined the effect on glucose-stimulated insulin secretion. siRNA treatment of INS-1E cells resulted in a 78% decrease in NRXN1α transcript levels (Fig. 4A) without increasing the levels of other α-NRXN transcripts (data not shown). A similar depletion of NRXN1α protein was also observed (Fig. 4B; the top band identified by N.S. reacted nonspecifically with the pan-NRXN antibody). NRXN1 silencing resulted in a 50% increase in insulin secretion at high glucose (Fig. 4C) and a 62% increase in the stimulation index (the ratio of insulin secretion at high versus low glucose) (Fig. 4D). Basal insulin secretion was not significantly changed. Insulin secretion experiments were repeated with similar results using a completely different pool of both control and NRXN1 siRNAs. Interestingly, the increased insulin secretion (measured as % of total cellular content) after NRXN1 knockdown was accompanied by up-regulation of cellular insulin content and insulin 2 mRNA by 31 and 37% over control, respectively (Fig. 4, E and F). Insulin content and mRNA were measured in INS-1E cells after an overnight incubation in 5 mm glucose. To determine the effect of glucose on the observed increases in cellular insulin content, NRXN1 knockdown cells were incubated for 18 h in different glucose concentrations prior to insulin assay. We found that 2.8 mm glucose, but not 11.2 mm, recapitulated the increase in insulin content (data not shown).
FIGURE 4.
siRNA-mediated knockdown of NRXN1 in INS-1E cells increases glucose-stimulated insulin secretion. INS-1E cells were transfected for 72 h with a pool of either non-targeting control or NRXN1 siRNAs. A, mRNA was isolated, and NRXN1α gene expression was determined using RT-qPCR, normalized to 18 S RNA. B, equal protein amounts of total cell lysates were immunoblotted for NRXN to confirm knockdown at the protein level. Lysates from two independent culture wells are shown for both control and NRXN1 siRNA. N.S. indicates a nonspecific band detected by the pan- NRXN antibody. C, to determine the effect of NRXN1 knockdown on insulin secretion, cells treated with control or NRXN1 siRNA were incubated for 1 h in Krebs-Ringer solution containing either 2.75 mm (black bars) or 16.7 mm glucose (white bars) and secreted insulin, shown as % of total cellular insulin-measured by RIA. D, glucose stimulation index (the ratio of insulin secretion, as % of cellular insulin, at 16.7 mm to secretion at 2.75 mm glucose) is shown for cells treated with control (left) and NRXN1 (right) siRNA. E, total lysate from siRNA-treated cells was assayed for insulin content. F, insulin 2 gene expression in siRNA-treated cells was determined by qPCR and normalized to 18 S RNA. G, INS-1E cells were transfected with a secreted alkaline phosphatase (SEAP) construct in addition to control or NRXN1 siRNA and treated with 15 mm glucose as in C. Media and cell lysates were assayed for SEAP content. The percent of total SEAP secreted is depicted. All data are represented as mean ± S.E. from six samples, and each experiment was repeated three times with similar results. Insulin secretion experiments were repeated an additional three times with similar results using a completely different pool of control and NRXN1 siRNAs. Statistical significance was determined using a Student's t test. *, p < 0.05 indicates the difference compared with non-targeting siRNA transfected controls.
To confirm that NRXN1 knockdown selectively impacts the regulated secretory pathway, we also tested the effect of NRXN1 knockdown on constitutive secretion using secreted alkaline phosphatase (SEAP). SEAP is a recombinant cargo protein commonly used to assess constitutive secretion (42, 50). We found that NRXN1 knockdown did not affect SEAP secretion at high glucose (Fig. 4G), suggesting that insulin secretion was selectively modulated by NRXN1.
To complement the knockdown study, we also overexpressed NRXN1α to investigate its effect on insulin secretion. Transient transfection of INS-1E cells with a NRXN1α overexpression vector resulted in an increase in NRXN1α protein (as in Fig. 6) but had no effect on insulin secretion (data not shown).
FIGURE 6.
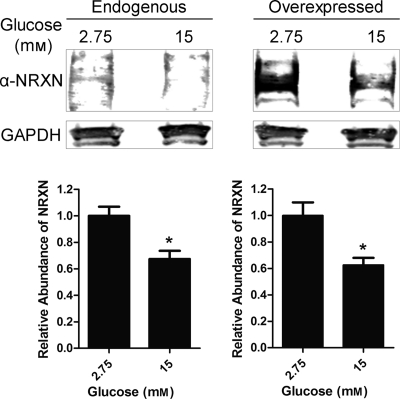
Glucose stimulation causes a decrease in NRXN protein content. INS-1E cells were either untransfected (Endogenous, left panel) or transfected for 48 h with a NRXN1α overexpression construct (Overexpressed, right panel). Cells were treated for 1 h with a Krebs-Ringer solution containing 2.75 mm (low) or 15 mm (high) glucose. Total cell lysates were immunoblotted for NRXN and the loading control GAPDH. Representative Western blot images show lysate pooled from six different untransfected (Endogenous) and NRXN1α-transfected (Overexpressed) samples treated with low or high glucose concentration. For quantitation of relative NRXN abundance, shown in the lower two panels, intensity of bands was normalized to the GAPDH loading-control band. Data in the lower two panels are represented as mean ± S.E. from seven experiments. Statistical significance was determined using a Student's t test. *, p < 0.05 indicates the difference compared with low glucose-treated controls.
To determine if NRXN isoforms other than NRXN1α also regulate insulin secretion and content, we conducted similar experiments using siRNAs targeting NRXN2β, the other abundant isoform in INS-1E cells (Fig. 2A). In INS-1E cells where NRXN2β transcript was depleted by 72% (supplemental Fig. S1A), we found a 67% increase in insulin secretion at high glucose (supplemental Fig. S1B), a 91% increase in the stimulation index (supplemental Fig. S1C), and 26% increase in cellular insulin content (supplemental Fig. S1D). Basal insulin secretion was not significantly changed.
Knockdown of NRXN1 Impairs Secretory Granule Docking
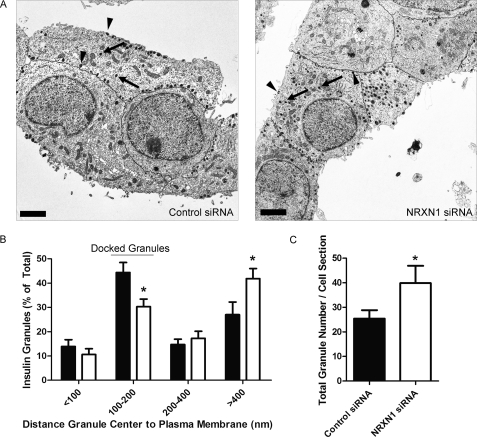
We next asked whether NRXN1, like granuphilin, contributes to secretory granule docking at the β-cell membrane. Using EM, we examined the distribution of secretory granules in INS-1E cells with and without NRXN1 siRNA treatment (Fig. 5A). Given their average diameter of ∼350 nm, secretory granules whose centers reside within 100–200 nm of the β-cell membrane are considered docked (26, 51, 52). We categorized granules into four groups based on their distance from the plasma membrane. Compared with control siRNA-treated cells, NRXN1 siRNA-treated cells had 32% fewer granules in the docked (100–200 nm) category but significantly more in the >400 nm category (Fig. 5B). Consistent with the finding of greater insulin content after NRXN1 siRNA treatment (Fig. 4G), knockdown also increased insulin granule number per cell cross section by 57% (Fig. 5C).
FIGURE 5.
NRXN1 knockdown in INS-1E cells reduces the percentage of insulin granules docked at the plasma membrane. INS-1E cells were treated with NRXN1 or control siRNAs. Cells were fixed, pelleted, and sectioned for EM. A, representative electron micrographs of control siRNA- and NRXN1 siRNA-treated cells are shown. Cell borders are traced in black. Scale bars indicate 2 μm. Arrows point to examples of undocked granules. Arrowheads point to examples of docked granules. B, in cells treated with control siRNA (black bars) and NRXN1 siRNA (white bars), granules were classified into four groups based on the distance from the center of each granule to the plasma membrane. Data are represented as the distribution of granules among the four groups. C, average total granule number per cell cross section after control or NRXN1 siRNA treatment is depicted. All data are represented as mean ± S.E. from 20 cells. Statistical significance was determined using a Student's t test. *, p < 0.05 indicates the comparison of control siRNA and NRXN1 siRNA-transfected cells.
NRXN Protein Levels Decrease at High Glucose
We next asked whether glucose affects NRXN1α protein levels. We found that after a 1 h treatment with high glucose, endogenous NRXN1α protein levels were decreased by 33% compared with low-glucose-treated controls (Fig. 6, left panel). High glucose also decreased the expression of transfected NRXN1α protein by a similar 40% (Fig. 6, right panel). Though this decrease in NRXN1α was unexpected, it is noteworthy that glucose transporter 2, another β-cell membrane protein, has been reported to undergo internalization and degradation upon glucose stimulation (53).
Loss of NRXN1α Increases Insulin Secretion in Isolated Mouse Islets
To further examine the role of NRXN1α in insulin secretion, islets were isolated from NRXN1α KO mice and age-matched WT controls. RT-PCR was performed using RNA from the isolated islets to confirm the absence of NRXN1α transcript and the presence of both NRXN2α and 2β transcripts (supplemental Fig. S2A). Immunohistochemistry of pancreas sections from these mice revealed normal islet architecture despite loss of NRXN1α (supplemental Fig. S2B).
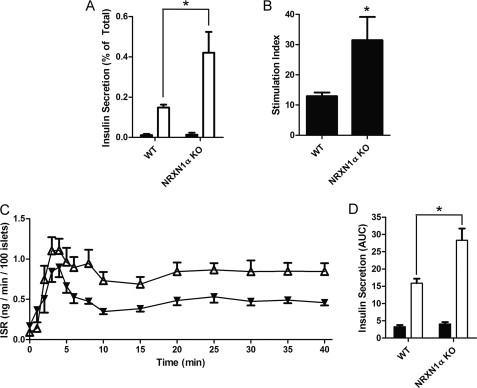
Assessment of insulin secretion from isolated islets was performed to investigate the effect of NRXN1α KO on glucose-stimulated insulin secretion. Islets from KO mice had a 184% increase in insulin secretion at high glucose (Fig. 7A) and a 143% increase in the stimulation index (Fig. 7B). Basal secretion was not significantly changed. Isolated islets were next perifused to compare insulin secretion rates over time after high-glucose stimulation. Overall, the insulin secretion rate (ISR) of NRXN1α KO islets was higher than that of WT controls after glucose stimulation (Fig. 7C). Although the increase in the area under the curve (AUC) of first-phase (0–5 min) secretion by KO islets was not statistically significant, the AUC of second-phase secretion (10–40 min) by the KO islets was significantly (79%) higher than with WT islets (Fig. 7D). A separate, post-hoc analysis of insulin secretion during the peak of first phase secretion revealed that the ISR of KO islets was significantly greater than the ISR of WT islets (mean 40% increase, p = 0.02) during the interval consisting of the 3 to 5 min time points.
FIGURE 7.
Islets from NRXN1α KO mice have increased glucose-stimulated insulin secretion. Islets were isolated from WT or NRXN1α KO mice and allowed to recover overnight in culture. A, islets were incubated for 1 h in Krebs-Ringer solution containing either 2.75 mm (black bars) or 16.7 mm glucose (white bars). Insulin secreted by WT (left) and KO (right) islets is depicted as percent of total insulin content. B, glucose stimulation index for WT and KO islets was derived from the experiment shown in panel A. The stimulation index is the ratio of insulin secretion (% of total) at 16.7 mm to secretion at 2.75 mm glucose. C, islets were perifused in Krebs-Ringer solution containing 3 mm glucose and then switched at time 0 to 20 mm glucose. The insulin secretion rate (ISR) of WT (black triangles) and NRXN1α KO islets (white triangles) was measured every minute for the first 6 min, every 2 min until minute 10 and then every 5 min until minute 40. D, area under the curve (AUC) was calculated for the first 5 min to determine first-phase insulin secretion (black bars) and then from minute 10 to 40 to determine second-phase insulin secretion (white bars). The ISR at the peak of first-phase secretion (3 to 5 min) was on average 40% greater in KO islets (p = 0.02). Data from static cultures are represented as mean ± S.E. from three samples, and each experiment was repeated three times with similar results. Perifusion data are represented as mean ± S.E. from six islet preparations of each type. Statistical significance was determined using a Student's t test. *, p < 0.05 indicates the comparison of secretion from WT and NRXN1α KO islets.
Islets from NRXN1α KO Mice Have Reduced Secretory Granule Docking
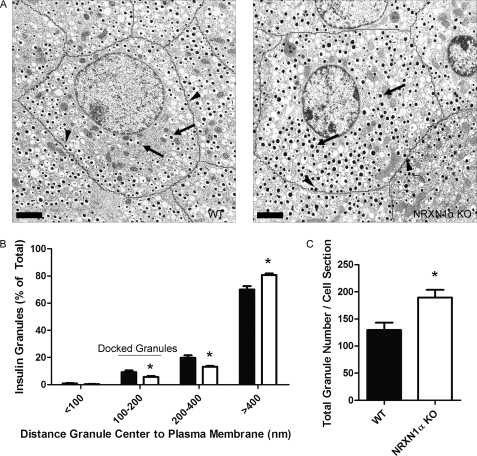
We next sought to determine if, as was the case in siRNA-treated INS-1E cells, docking is reduced in NRXN1α KO mouse islets. Analysis of granule distrubtion by EM revealed that, compared with WT, KO islets had 39% fewer granules in the 100–200 nm distance category (Fig. 8). Consistent with the finding of greater insulin content (Fig. 4G) and granule number (Fig. 5C) after NRXN1 knockdown in INS-1E cells, NRXN1α KO islets also had a 46% increase in insulin granule number per cell cross section compared with WT controls (Fig. 8C).
FIGURE 8.
Islets from NRXN1α KO mice have a reduced percentage of insulin granules docked at the plasma membrane. Islets isolated from WT or NRXN1α KO mice were allowed to recover overnight in culture. The next day, islets were fixed, pelleted, and sectioned for EM. A, representative electron micrographs of WT and NRXN1α KO islets are shown. Cell borders are traced in black. Scale bars indicate 2 μm. Arrows point to examples of undocked granules. Arrowheads point to examples of docked granules. B, distance from the center of each granule to the plasma membrane was measured for WT (black bars) and KO (white bars) cells and used to assign granules to one of four distance groups. Data is represented as the distribution of granules among the four groups. C, average total granule number per cell cross section is depicted. All data are represented as mean ± S.E. from 20 cells. Statistical significance was determined using a Student's t test. *, p < 0.05 indicates the comparison of cells from WT and NRXN1α KO islets.
DISCUSSION
The present study establishes a role for NRXN1 in insulin secretion and specifically in insulin granule docking at the β-cell membrane. Consistent with our prior analysis of transcripts from INS-1 β cells and human islets (19), with the more in-depth qPCR analysis reported here, and with our prior immunostaining of rat pancreas (19), we found that NRXN protein is present in INS-1E and human, rat, and mouse islet β cells. NRXN transcripts in β cells have a very different, more restricted expression profile than in the brain. In rat, human, and mouse islets, NRXN1α transcript expression predominates. NRXN1α message is also highly expressed in INS-1E cells. There are also substantial levels of NRXN2β transcripts in INS-1E cells and, to a lesser degree, in mouse and human islets.
As we hypothesized, the proteins that interact with NRXN1α in β cells seem to mirror those in the neuronal presynaptic active zone. This is further evidence that β cells and neurons employ highly conserved machineries to mediate regulated secretion. As in neurons, NRXN1α in β cells binds to components of the submembrane exocytotic machinery. NRXN1α also associates with granuphilin, a granule-associated protein that plays a key role in secretory granule docking (26). This, together with the insulin secretion and EM results presented herein, indicate that NRXN is a component of the plasma membrane-associated protein complex that mediates insulin granule docking.
Granuphilin is associated with the insulin granule surface and is essential for mediating docking to the submembrane, syntaxin 1A/Munc18-containing docking site (26, 48). Granuphilin-mediated docking is inhibitory to insulin secretion (26, 46, 49). Although this finding is at odds with studies suggesting that docking precedes or facilitates insulin exocytosis (52, 54, 55), it is in line with a recent study showing attenuation of insulin secretion by α-synuclein, another β-cell protein that promotes secretory granule association with the membrane (56). By providing another example of a constituent of the docking apparatus that inhibits insulin secretion, our results support the idea that docking serves to constrain insulin exocytosis, halting secretory granules en route to SNARE complex formation and subsequent insulin release.
To clarify the role of NRXN1 in exocytosis, we used siRNA to deplete NRXN1 in INS-1E cells. Loss of NRXN1, as was previously observed with loss of granuphilin (48, 49), increased glucose-stimulated insulin secretion (Fig. 4D). Also as in the case with granuphilin (47), NRXN1 knockdown did not impact basal insulin secretion, nor did it affect constitutive secretion, indicating that the knockdown did not cause a general impairment of β-cell secretory function. These findings were confirmed by studies of islets isolated from NRXN1α KO mice in which loss of NRXN1α also resulted in increased glucose-stimulated insulin secretion. Results from perifusion of KO and control mouse islets revealed a significant effect on second-phase secretion as well as evidence of increased first-phase secretion. These results resemble those observed with granuphilin KO mice, in which both first and second phase secretion were increased (57). It is possible that the continued expression of other NRXN isoforms (supplemental Figs. S1 and S2A) moderated the effect observed here of NRXN1α KO on first (and second) phase secretion.
Overexpression of NRXN1α had no effect on insulin secretion, suggesting that the maximal effect of NRXN1α is already achieved at endogenous levels, perhaps, for example, due to limited availability of key binding partners at supraphysiological expression levels. In any case, this lack of significant effect on insulin secretion would be expected after increasing expression of a protein, such as we now believe NRXN1α to be- normally expressed at levels not rate-limiting for insulin exocytosis. An example of this is glucose transporter 2, knockdown of which impairs glucose-stimulated insulin secretion (58) but overexpression of which has no effect (59).
The parallel between the effects of NRXN1α and granuphilin levels on insulin secretion is consistent with NRXN, like granuphilin, being essential to the docking mechanism. Interestingly, NRXN1 knockdown caused an increase in insulin content and transcript levels. Electron micrographs also show increased numbers of secretory granules in NRXN1 siRNA-treated cells and islets from NRXN1α KO mice. The increase in insulin levels was most likely not due to increased insulin secretion caused by NRXN knockdown: insulin content increased in INS-1E cells treated with NRXN1 siRNA even at non-stimulatory glucose levels at which NRXN1 silencing did not increase insulin secretion. The mechanism, then, whereby NRXNs influence β-cell insulin content remains to be uncovered. In NRXN1 siRNA-treated INS-1E cells and islets from NRXN1α KO mice, the increases in insulin secretion cannot be explained by increased insulin content since insulin secretion in our static culture studies was normalized to total cellular insulin content and also the magnitudes of the increases in secretion resulting from loss of NRXN1α exceeded those of the increases in content.
It is thought that insulin granule docking occurs via interactions of granuphilin with syntaxin 1 beneath the β-cell plasma membrane (46). However, loss of syntaxin 1 had no effect on secretory granule docking (57), suggesting the presence of an alternative submembrane receptor for granuphilin. The findings presented here suggest that NRXN1α may be the membrane-associated protein that anchors the docking complex at a subplasmalemmal site. First, NRXN1α associates, either directly or indirectly, with granuphilin (Fig. 3). Second, NRXN1 knockdown in INS-E cells and KO in mouse islets impairs secretory granule docking. Third, given the extensive resemblance between regulated secretion in neurons and in the β cell, the known importance of interactions involving NRXN in synaptic vesicle docking at the presynaptic membrane suggests a similar role for NRXN in β cells (16, 24, 25). In neurons, the interaction between NRXN and the granuphilin-related protein rabphilin-3 is mediated by the intermediary protein CASK (24), a known NRXN binding partner (17, 18, 45). We have found that NRXN in β cells also interacts with CASK. Overall, as would be predicted by extrapolation of findings in the synapse, our results suggest that secretory granule docking in β cells is mediated by the interaction of granuphilin with NRXN1, either directly or indirectly (perhaps via CASK).
While it seems likely that NRXNs are essential for docking of secretory granule docking, granule docking was not completely abolished in INS-1E cells treated with NRXN1 siRNA and in islets from NRXN1α KO mice. Possible explanations for the persistence of docked granules in these studies include incomplete NRXN1 gene silencing in the siRNA-treated cells and functional redundancy with NRXN2β or other NRXN isoforms (supplemental Figs. S1 and S2A). Alternatively, the remaining granules that appear to be docked by morphological criteria may be stochastically located close to the plasma membrane without being molecularly docked, as has been proposed to account for the ∼30% of granules that remain in the 100–200 nm distance category in granuphilin-null mice (26).
NRXN1 is different from most established mediators of docking and exocytosis in that it is a transmembrane protein with a large extracellular domain that binds to multiple partners. Some of these binding partners, such as neuroligins and α-dystroglycan, are expressed on the β-cell surface and perhaps also by the islet vasculature (19, 60–62). NRXN1 thus provides a direct link between the exocytotic machinery and the β-cell surface and could influence the localization of the exocytotic microdomains beneath the plasma membrane (63). It is also possible that trans interactions of NRXN with extracellular binding partners contribute to the functional architecture of the islet by juxtaposing β cells with endothelial cells to promote cell β-cell development, secretory function (64) and polarized orientation (65) as well as maintaining cell-cell contacts important for signaling and regulated secretion (66). Because NRXNs help initiate and guide synaptic differentiation (67), it is also possible that NRXN1, in addition to its role in exocytosis, contributes to the development and functional maturation of the β-cell. We also found that glucose stimulation causes a reduction in NRXN protein. Because NRXN1 down-regulation increases glucose-stimulated insulin secretion, NRXN degradation may enhance the insulin secretory response to elevated glucose levels.
In summary, our data demonstrate that NRXN1 has a functional role in β cells as a component of the machinery for regulated exocytosis and, more specifically, is essential for insulin granule docking. NRXN1α interacts with constituents of the submembrane exocytotic and docking protein machinery and with the secretory granule-associated protein granuphilin. Decreased expression of NRXN1 in β cells increases glucose-stimulated insulin secretion, most likely by decreasing secretory-granule docking. This increase in insulin secretion after NRXN knockdown provides further evidence that docking acts, as has been proposed by others, as a “temporal constraint” on insulin secretion (48). Under stimulatory conditions, NRXN1α protein levels are decreased, and this glucose-dependent regulation of NRXN could allow the β cell an additional level of control over insulin secretion. Further work is needed to determine whether NRXN1α has a role in β-cell maturation, whether its extracellular interactions determine the docking sites of insulin granules, and whether it is a potential therapeutic target for modulating β-cell function in diabetes.
Supplementary Material
Acknowledgments
We thank Ying Jones for electron microscopy preparation and Dr. M. Farquhar for use of the Cellular and Molecular Medicine Electron Microscopy Facility. S. D. C. also thanks the anonymous NIH study section reviewer who suggested the SEAP experiment.
This work was supported, in whole or in part, by National Institutes of Health Grant R01DK080971 (to S. D. C.), Juvenile, Diabetes Research Foundation Grant 37-2009-44 (to S. D. C.), and the University of California, San Diego, Graduate Training Program in Cellular and Molecular Pharmacology through National Institutes of Health Institutional Training Grant T32 GM007752. The University of Washington DERC is supported by National Institutes of Health Grant DK17047.

This article contains supplemental Figs. S1 and S2 and Tables S1 and S2.
- NRXN
- neurexin
- SEAP
- secreted alkaline phosphatase
- IP
- immunoprecipitation
- qPCR
- real-time quantitative PCR
- ISR
- insulin secretion rate
- AUC
- area under the curve.
REFERENCES
- 1. Abderrahmani A., Niederhauser G., Plaisance V., Haefliger J. A., Regazzi R., Waeber G. (2004) Neuronal traits are required for glucose-induced insulin secretion. FEBS Lett. 565, 133–138 [DOI] [PubMed] [Google Scholar]
- 2. Lang J. (1999) Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur. J. Biochem. 259, 3–17 [DOI] [PubMed] [Google Scholar]
- 3. Burgoyne R. D., Morgan A. (2003) Secretory granule exocytosis. Physiol. Rev. 83, 581–632 [DOI] [PubMed] [Google Scholar]
- 4. Missler M., Fernandez-Chacon R., Südhof T. C. (1998) The making of neurexins. J. Neurochem. 71, 1339–1347 [DOI] [PubMed] [Google Scholar]
- 5. Lisé M. F., El-Husseini A. (2006) The neuroligin and neurexin families: from structure to function at the synapse. Cell Mol. Life Sci. 63, 1833–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Craig A. M., Kang Y. (2007) Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol 17, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tabuchi K., Südhof T. C. (2002) Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics 79, 849–859 [DOI] [PubMed] [Google Scholar]
- 8. Siddiqui T. J., Pancaroglu R., Kang Y., Rooyakkers A., Craig A. M. (2010) LRRTMs and neuroligins bind neurexins with a different code to cooperate in glutamate synapse development. J. Neurosci. 30, 7495–7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berninghausen O., Rahman M. A., Silva J. P., Davletov B., Hopkins C., Ushkaryov Y. A. (2007) Neurexin Iβ and neuroligin are localized on opposite membranes in mature central synapses. J. Neurochem. 103, 1855–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottmann K. (2008) Transsynaptic modulation of the synaptic vesicle cycle by cell-adhesion molecules. J. Neurosci. Res. 86, 223–232 [DOI] [PubMed] [Google Scholar]
- 11. Araç D., Boucard A. A., Ozkan E., Strop P., Newell E., Südhof T. C., Brunger A. T. (2007) Structures of neuroligin-1 and the neuroligin-1/neurexin-1 β complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron 56, 992–1003 [DOI] [PubMed] [Google Scholar]
- 12. Uemura T., Lee S. J., Yasumura M., Takeuchi T., Yoshida T., Ra M., Taguchi R., Sakimura K., Mishina M. (2010) Trans-synaptic interaction of GluRδ2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 141, 1068–1079 [DOI] [PubMed] [Google Scholar]
- 13. Butz S., Okamoto M., Südhof T. C. (1998) A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell 94, 773–782 [DOI] [PubMed] [Google Scholar]
- 14. Biederer T., Südhof T. C. (2000) Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J. Biol. Chem. 275, 39803–39806 [DOI] [PubMed] [Google Scholar]
- 15. O'Connor V. M., Shamotienko O., Grishin E., Betz H. (1993) On the structure of the 'synaptosecretosome'. Evidence for a neurexin/synaptotagmin/syntaxin/Ca2+ channel complex. FEBS Lett. 326, 255–260 [DOI] [PubMed] [Google Scholar]
- 16. Perin M. (1994) The COOH terminus of synaptotagmin mediates interaction with the neurexins. J. Biol. Chem. 269, 8576–8581 [PubMed] [Google Scholar]
- 17. Mukherjee K., Sharma M., Urlaub H., Bourenkov G. P., Jahn R., Südhof T. C., Wahl M. C. (2008) CASK Functions as a Mg2+-independent neurexin kinase. Cell 133, 328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hata Y., Butz S., Südhof T. C. (1996) CASK: A novel d1g/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J. Neurosci. 16, 2488–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suckow A. T., Comoletti D., Waldrop M. A., Mosedale M., Egodage S., Taylor P., Chessler S. D. (2008) Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic β cells and the involvement of neuroligin in insulin secretion. Endocrinology 149, 6006–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maffei A., Liu Z., Witkowski P., Moschella F., Del Pozzo G., Liu E., Herold K., Winchester R. J., Hardy M. A., Harris P. E. (2004) Identification of tissue-restricted transcripts in human islets. Endocrinology 145, 4513–4521 [DOI] [PubMed] [Google Scholar]
- 21. Missler M., Zhang W., Rohlmann A., Kattenstroth G., Hammer R. E., Gottmann K., Südhof T. C. (2003) α-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423, 939–948 [DOI] [PubMed] [Google Scholar]
- 22. Dudanova I., Sedej S., Ahmad M., Masius H., Sargsyan V., Zhang W., Riedel D., Angenstein F., Schild D., Rupnik M., Missler M. (2006) Important contribution of α-neurexins to Ca2+-triggered exocytosis of secretory granules. J. Neurosci. 26, 10599–10613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dudanova I., Tabuchi K., Rohlmann A., Südhof T. C., Missler M. (2007) Deletion of α-neurexins does not cause a major impairment of axonal pathfinding or synapse formation. J. Comp. Neurol 502, 261–274 [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y., Luan Z., Liu A., Hu G. (2001) The scaffolding protein CASK mediates the interaction between rabphilin3a and β-neurexins. FEBS Lett. 497, 99–102 [DOI] [PubMed] [Google Scholar]
- 25. Hata Y., Davletov B., Petrenko A. G., Jahn R., Südhof T. C. (1993) Interaction of synaptotagmin with the cytoplasmic domains of neurexins. Neuron 10, 307–315 [DOI] [PubMed] [Google Scholar]
- 26. Gomi H., Mizutani S., Kasai K., Itohara S., Izumi T. (2005) Granuphilin molecularly docks insulin granules to the fusion machinery. J. Cell Biol. 171, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang J., Takeuchi T., Yokota H., Izumi T. (1999) Novel rabphilin-3-like protein associates with insulin-containing granules in pancreatic β cells. J. Biol. Chem. 274, 28542–28548 [DOI] [PubMed] [Google Scholar]
- 28. Regazzi R., Ravazzola M., Iezzi M., Lang J., Zahraoui A., Andereggen E., Morel P., Takai Y., Wollheim C. B. (1996) Expression, localization, and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J. Cell Sci. 109, 2265–2273 [DOI] [PubMed] [Google Scholar]
- 29. Iezzi M., Escher G., Meda P., Charollais A., Baldini G., Darchen F., Wollheim C. B., Regazzi R. (1999) Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol. Endocrinol. 13, 202–212 [DOI] [PubMed] [Google Scholar]
- 30. Kang Y., Zhang X., Dobie F., Wu H., Craig A. M. (2008) Induction of GABAergic postsynaptic differentiation by α-neurexins. J. Biol. Chem. 283, 2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Violin J. D., Zhang J., Tsien R. Y., Newton A. C. (2003) A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J. Cell Biol. 161, 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fairless R., Masius H., Rohlmann A., Heupel K., Ahmad M., Reissner C., Dresbach T., Missler M. (2008) Polarized targeting of neurexins to synapses is regulated by their C-terminal sequences. J. Neurosci. 28, 12969–12981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li D. S., Yuan Y. H., Tu H. J., Liang Q. L., Dai L. J. (2009) A protocol for islet isolation from mouse pancreas. Nat. Protoc. 4, 1649–1652 [DOI] [PubMed] [Google Scholar]
- 34. Merglen A., Theander S., Rubi B., Chaffard G., Wollheim C. B., Maechler P. (2004) Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145, 667–678 [DOI] [PubMed] [Google Scholar]
- 35. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 36. Gluzman Y. (1981) SV40-transformed simian cells support the replication of early SV40 mutants. Cell 23, 175–182 [DOI] [PubMed] [Google Scholar]
- 37. Suckow A. T., Sweet I. R., Van Yserloo B., Rutledge E. A., Hall T. R., Waldrop M., Chessler S. D. (2006) Identification and characterization of a novel isoform of the vesicular γ-aminobutyric acid transporter with glucose-related expression in rat islets. J. Mol. Endocrinol. 36, 187–199 [DOI] [PubMed] [Google Scholar]
- 38. Zatyka M., Ricketts C., da Silva Xavier G., Minton J., Fenton S., Hofmann-Thiel S., Rutter G. A., Barrett T. G. (2008) Sodium-potassium ATPase 1 subunit is a molecular partner of Wolframin, an endoplasmic reticulum protein involved in ER stress. Hum. Mol. Genet. 17, 190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Todorov I., Omori K., Pascual M., Rawson J., Nair I., Valiente L., Vuong T., Matsuda T., Orr C., Ferreri K., Smith C. V., Kandeel F., Mullen Y. (2006) Generation of human islets through expansion and differentiation of non-islet pancreatic cells discarded (pancreatic discard) after islet isolation. Pancreas 32, 130–138 [DOI] [PubMed] [Google Scholar]
- 40. Skaletsky S. R. (2000) in Bioinformatics Methods and Protocols: Methods in Molecular Biology. (Krawetz S. M. S., ed), pp. 365–386, Humana Press, Totowa, NJ [Google Scholar]
- 41. Marshall O. J. (2004) PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20, 2471–2472 [DOI] [PubMed] [Google Scholar]
- 42. Molinete M., Lilla V., Jain R., Joyce P. B., Gorr S. U., Ravazzola M., Halban P. A. (2000) Trafficking of non-regulated secretory proteins in insulin secreting (INS-1) cells. Diabetologia 43, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 43. Sweet I. R., Gilbert M. (2006) Contribution of calcium influx in mediating glucose-stimulated oxygen consumption in pancreatic islets. Diabetes 55, 3509–3519 [DOI] [PubMed] [Google Scholar]
- 44. Abramoff M. D., Magelhaes P. J., Ram S. J. (2004) Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 45. Biederer T., Sudhof T. C. (2001) CASK and protein 4.1 support F-actin nucleation on neurexins. J. Biol. Chem. 276, 47869–47876 [DOI] [PubMed] [Google Scholar]
- 46. Torii S., Zhao S., Yi Z., Takeuchi T., Izumi T. (2002) Granuphilin modulates the exocytosis of secretory granules through interaction with syntaxin 1a. Mol. Cell Biol. 22, 5518–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coppola T., Frantz C., Perret-Menoud V., Gattesco S., Hirling H., Regazzi R. (2002) Pancreatic β-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol. Biol. Cell 13, 1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kasai K., Fujita T., Gomi H., Izumi T. (2008) Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic 9, 1191–1203 [DOI] [PubMed] [Google Scholar]
- 49. Tomas A., Meda P., Regazzi R., Pessin J. E., Halban P. A. (2008) Munc 18–1 and granuphilin collaborate during insulin granule exocytosis. Traffic 9, 813–832 [DOI] [PubMed] [Google Scholar]
- 50. Hays L. B., Wicksteed B., Wang Y., McCuaig J. F., Philipson L. H., Edwardson J. M., Rhodes C. J. (2005) J. Endocrinol. 185, 57–67 [DOI] [PubMed] [Google Scholar]
- 51. Kasai K., Ohara-Imaizumi M., Takahashi N., Mizutani S., Zhao S., Kikuta T., Kasai H., Nagamatsu S., Gomi H., Izumi T. (2005) Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J. Clin. Invest. 115, 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olofsson C. S., Göpel S. O., Barg S., Galvanovskis J., Ma X., Salehi A., Rorsman P., Eliasson L. (2002) Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic β cells. Pflugers Arch 444, 43–51 [DOI] [PubMed] [Google Scholar]
- 53. Hou J. C., Williams D., Vicogne J., Pessin J. E. (2009) The glucose transporter 2 undergoes plasma membrane endocytosis and lysosomal degradation in a secretagogue-dependent manner. Endocrinology 150, 4056–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barg S. (2003) Mechanisms of exocytosis in insulin-secreting β cells and glucagon-secreting α cells. Pharmacol. Toxicol 92, 3–13 [DOI] [PubMed] [Google Scholar]
- 55. Barg S., Lindqvist A., Obermüller S. (2008) Granule docking and cargo release in pancreatic β cells. Biochem. Soc. Trans. 36, 294–299 [DOI] [PubMed] [Google Scholar]
- 56. Geng X., Lou H., Wang J., Li L., Swanson A. L., Sun M., Beers-Stolz D., Watkins S., Perez R. G., Drain P. (2011) α-Synuclein binds the K(ATP) channel at insulin-secretory granules and inhibits insulin secretion. Am. J. Physiol. Endocrinol. Metab. 300, E276–E286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang H., Ishizaki R., Kobayashi E., Fujiwara T., Akagawa K., Izumi T. (2011) Loss of granuphilin and loss of Syntaxin-1A cause differential effects on insulin granule docking and fusion. J. Biol. Chem. 286, 32244–32250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Valera A., Solanes G., Fernández-Alvarez J., Pujol A., Ferrer J., Asins G., Gomis R., Bosch F. (1994) Expression of GLUT-2 antisense RNA in β cells of transgenic mice leads to diabetes. J. Biol. Chem. 269, 28543–28546 [PubMed] [Google Scholar]
- 59. Ishihara H., Asano T., Tsukuda K., Katagiri H., Inukai K., Anai M., Yazaki Y., Miyazaki J., Kikuchi M., Oka Y. (1995) Human GLUT-2 overexpression does not affect glucose-stimulated insulin secretion in MIN6 cells. Am. J. Physiol. 269, E897–E902 [DOI] [PubMed] [Google Scholar]
- 60. Bottos A., Destro E., Rissone A., Graziano S., Cordara G., Assenzio B., Cera M. R., Mascia L., Bussolino F., Arese M. (2009) The synaptic proteins, neurexins, and neuroligins are widely expressed in the vascular system and contribute to its functions. Proc. Natl. Acad. Sci. 106, 20782–20787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang F. X., Georges-Labouesse E., Harrison L. C. (2001) Regulation of laminin 1-induced pancreatic β-cell differentiation by α6 integrin and α-dystroglycan. Mol. Med. 7, 107–114 [PMC free article] [PubMed] [Google Scholar]
- 62. Jiang X., Rieder S., Giese N. A., Friess H., Michalski C. W., Kleeff J. (2011) Reduced α-dystroglycan expression correlates with shortened patient survival in pancreatic cancer. J. Surg. Res. 171, 120–126 [DOI] [PubMed] [Google Scholar]
- 63. Rutter G. A., Tsuboi T., Ravier M. A. (2006) Ca2+ microdomains and the control of insulin secretion. Cell Calcium 40, 539–551 [DOI] [PubMed] [Google Scholar]
- 64. Konstantinova I., Lammert E. (2004) Microvascular development: learning from pancreatic islets. BioEssays 26, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 65. Bonner-Weir S. (1988) Morphological evidence for pancreatic polarity of β-cell within islets of Langerhans. Diabetes 37, 616–621 [DOI] [PubMed] [Google Scholar]
- 66. Benninger R. K., Head W. S., Zhang M., Satin L. S., Piston D. W. (2011) Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J. Physiol. 589, 5453–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dean C., Dresbach T. (2006) Neuroligins and neurexins: Linking cell adhesion, synapse formation, and cognitive function. Trends Neurosci. 29, 21–29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.