Abstract
Uncle Folke inspired me to become a biochemist by demonstrating electrophoresis experiments on butterfly hemolymph in his kitchen. Glutathione became the subject for my undergraduate project in 1964 and has remained a focal point in my research owing to its multifarious roles in the cell. Since the 1960s, the multiple forms of glutathione transferase (GST), the GSTome, were isolated and characterized, some of which were discovered in our laboratory. Products of oxidative processes were found to be natural GST substrates. Examples of toxic compounds against which particular GSTs provide protection include 4-hydroxynonenal and ortho-quinones, with possible links to the etiology of Alzheimer and Parkinson diseases and other degenerative conditions. The role of thioltransferase and glutathione reductase in the cellular reduction of disulfides and other oxidized forms of thiols was clarified. Glyoxalase I catalyzes still another glutathione-dependent detoxication reaction. The unusual steady-state kinetics of this zinc-containing enzyme initiated model discrimination by regression analysis. Functional properties of the enzymes have been altered by stochastic mutations based on DNA shuffling and rationally tailored by structure-based redesign. We found it useful to represent promiscuous enzymes by vectors or points in multidimensional substrate-activity space and visualize them by multivariate analysis. Adopting the concept “molecular quasi-species,” we describe clusters of functionally related enzyme variants that may emerge in natural as well as directed evolution.
Keywords: Sulfur, Flavin, Antioxidants, Enzymes, Disulfide, Glutathione, GSTome
My Early Glimpses of Biochemistry
I learned about the scientific discipline of biochemistry as a young teenager. My uncle in Uppsala, Folke Fridén, married to my father's sister Sigrid, was the sage. Uncle Folke was an alumnus of Uppsala University but never became a member of any academic institution. Instead, he privately followed his own interests in science and arts, which he cultivated by weekly visits to the university library, Carolina Rediviva. Since childhood, Uncle Folke had a special interest in insects, and he taught me and my younger brother, Gunnar, how to find caterpillars as well as eggs of moths and butterflies on plants. The eggs were placed in cotton-plugged glass tubes, in which the hatched first-instar larvae were fed fresh leaves daily. Larger caterpillars were reared in gauze-covered glass jars, and we could watch them pupate and eventually emerge as imagos in the form of butterflies and moths.
Uncle Folke entrusted us to deliver pupae of numerous species of moths, and he stored them in his refrigerator to undergo diapause for subsequent biochemical experiments. He paid us at the rate of one Swedish crown for each large sphingid pupa and less for smaller specimens. Uncle Folke was extremely meticulous and advised us to collect the larval food from plants of the same clones and biotopes to minimize nutritional variations. Some leaves derived from bushes growing three miles away from the vacation house where we spent the summers, and we went by bicycle to the site to collect twigs several times a week.
Electrophoresis in the Kitchen
The kitchen in the small Uppsala apartment at Vaksalagatan was used as a laboratory by Uncle Folke. He showed us how amino acids in insect tissues could be separated by two-dimensional paper chromatography and be detected with ninhydrin. Shortly after publication of the new method of starch gel electrophoresis (1), Uncle Folke separated the proteins in the hemolymph of larvae and pupae. He identified pigmented proteins in all species analyzed. Some moths had several colored proteins, but the predominant component appeared to be blue-green. Inspired by the nomenclature for human globulins coined by Nobel Laureate Arne Tiselius of Uppsala University, Uncle Folke designated the main pigmented protein by the Greek letters χρ (chi rho) to indicate its colored nature (Greek, chroma). Approximately thirty years later, in a seminar at the Uppsala Biomedical Center, I witnessed with astonishment Robert Huber describe the structure of a colored bilin-binding protein from Pieris brassicae (the large white butterfly). I told Huber that, no doubt, he and his co-workers had analyzed a protein that I had seen as a distinct band in starch gels in my uncle's kitchen. Much later, I found out that the protein had also been described in the hemolymph of the sphingid moth Manduca sexta (the tobacco hornworm) and given the name “insecticyanin” (see Ref. 2).
Nurturing a Biochemical Interest
In 1958, my parents, Lisbeth and Tage Eriksson, received a thesis on the energy metabolism of caterpillars written by my uncle (3). They appreciated the gift, but, not being scientists, they could not digest its contents. However, I was intrigued by the simple but clever idea of using the defecation frequency of larvae as a measure of their food consumption, and with my drive as a 15-year-old, I read the thesis voraciously. Many scientific terms such as “cytochromes” and “endocrine secretion” were unknown to me, and I had to consult major textbooks of biochemistry in the City Library of Stockholm for enlightenment. My parents were very supportive and provided me with scientific monographs as Christmas gifts and birthday presents in the ensuing years. I read texts by Linus Pauling, Albert Szent-Györgyi, Joe Neilands, and Paul Stumpf, and other scientists, whom I got to meet personally later in my scientific life.
By 1962, I had decided to aim for a career as a biochemist and enrolled as a student majoring in chemistry at Stockholm University. During my undergraduate studies, I wanted to take advantage of the summer vacation to obtain hands-on experience of biochemical research in an academic setting. The summer of 1963 was spent in the Nobel Medical Institute of the Karolinska Institutet at the Department of Biochemistry, headed by Hugo Theorell. I got to work with Göran Eriksson, a graduate student synthesizing isotope-substituted flavin molecules to elucidate the electron spin density distribution in radicals of the cofactor. His supervisor was Anders Ehrenberg, one of the pioneers in using paramagnetic susceptibility and electron spin resonance for studies of biomolecules.
Sulfur Biochemistry
In the following summer, before enrolling in the biochemistry course at Stockholm University, I was permitted to carry out my undergraduate degree project with Bo Sörbo at the Swedish Research Institute of National Defense. Sörbo was a devoted sulfur biochemist who had obtained his Ph.D. degree with Theorell on the enzyme rhodanese, which catalyzes the formation of rhodanide (thiocyanate) from thiosulfate and cyanide. Rhodanese is the only enzyme with a name ending in “ese,” the reason being that it was considered a synthetic enzyme, in distinction from the “ase” enzymes involved in degradation of carbohydrates, proteins, and other substrates. My assignment was to prepare a new derivative of glutathione, the thiosulfonate that could potentially be formed in biological tissues subjected to irradiation. In thiosulfonates, one of the oxygens in a sulfonate is replaced by a labile bivalent sulfur (similar to that in inorganic thiosulfate). I demonstrated that rhodanese catalyzes transsulfuration from glutathione thiosulfonate to cyanide (4). The stay in Sörbo's laboratory also allowed time for additional enzymology, and I purified glutathione reductase from bovine liver for assays with my new compound. That summer, I became acquainted with the literature describing the multifarious roles of glutathione. A few years later, I read the Nature paper “Lest I Forget Thee, Glutathione … ” by Edward and Nechama Kosower (5), and I have certainly not forgotten glutathione since the summer of 1964.
Graduate Student at Stockholm University
In the fall of 1964, after completing my course of biochemistry, I sought admission as a graduate student to the Department of Biochemistry of Stockholm University. I was known to Klas-Bertil Augustinsson since I had authored an article on the biochemical origin of life in one of the major daily newspapers, “Svenska Dagbladet.” My sources were books by Oparin, Calvin, Darwin, Chardin, and others, as well as the classic paper by Miller and Urey, and I attempted to put them all in perspective for a general readership. Augustinsson provided bench space in his laboratory, and I was given a teaching assistantship in the department at the beginning of 1965.
Once admitted to the laboratory, I was asked what I wanted to work on, and I suggested the flavoenzyme glutathione reductase, in line with my prior experience with flavins and glutathione. Augustinsson, himself an authority on cholinesterase, found the proposal excellent because “nobody in the department was working on oxidoreductases.” I faced the challenge, surprised that he did not want me to investigate esterases. In theory as well as in practice, I was thus left to be my own mentor, even though Augustinsson generously allowed me to use equipment and chemicals in his laboratory.
Establishing My Own Research Group
As a teaching assistant, I came in contact with students of biochemistry, and I made some of them interested in my research. In the fall of 1965, Viveka Schalén started to work with me for her degree project. I had been authorized by Karl Myrbäck to serve as a supervisor. Myrbäck was the first professor of biochemistry at Stockholm University. His mentor was Hans von Euler-Chelpin, professor of general and organic chemistry in Stockholm, actively conducting biochemical research in the neighboring building until the age of 91. Myrbäck was best known to biochemists as one of the editors of the standard treatise “The Enzymes,” with Paul Boyer and Henry Lardy, a follow-up of the first edition by James Sumner and Myrbäck. Myrbäck was also the founder and editor-in-chief of Acta Chemica Scandinavica. In this journal, I published my first paper (6) on the synthesis of the mixed disulfide of glutathione and coenzyme A. (At the time, my name was Bengt Eriksson; the surname Mannervik was adopted from my maternal grandparents by me and my brothers in 1968.)
By the time of my Ph.D. dissertation (7), I had already formed a small research group in the department. Funding for the subsequent work was obtained from the Swedish Natural Science Research Council and the Swedish Cancer Society.
Glyoxalase I
Glyoxalase I was used in an enzymatic assay specific for reduced glutathione in our research. We started to characterize the commercially available yeast enzyme and proceeded with studies of the enzyme purified from other sources. We developed an affinity gel by linking S-hexylglutathione to a matrix (8), purified the enzyme, and demonstrated that the protein was a zinc-dependent metalloenzyme (9) that was active also with several other divalent metal ions. The mammalian enzyme is a homodimer with two active sites at the interface between the subunits (10). By contrast, we subsequently found that glyoxalase I from Saccharomyces cerevisiae is a monomer with two active sites in the same polypeptide chain (11).
Discrimination by Regression Analysis
Detailed steady-state kinetic studies of glyoxalase I demonstrated unconventional rate saturation behavior. The analysis prompted the development of procedures for discrimination between alternative rate equations. We started out using algorithms published by Wallace W. Cleland (12). However, my newly recruited student, Tamas Bártfai, refined these methods in his thesis work (13) using glyoxalase I as the test case (14). Our main emphasis was the discrimination between alternative mathematical models rather than parameter estimation (15). Tamas, who graduated in 1973, became my first student to receive a Ph.D. degree.
International Conference on Glutathione
Leopold Flohé had taken notice of our research and invited me as a speaker to an international conference on glutathione in March 1973 in Tübingen, Germany. I had the unusual privilege of being asked to give three full lectures: one on glutathione reductase, one on glyoxalase I, and one on glutathione-dependent transferase reactions. At the conference, our findings of isoenzymes of what was called glutathione S-aryltransferase were first presented to an international audience (16). Enzymes present in the cytosolic fraction of rat liver were separated by isoelectric focusing, and activities were determined with distinguishing substrates in the different fractions.
Glutathionylation of Proteins and Smaller Molecules
My interest in naturally occurring mixed disulfides and their reversible thiol-disulfide interchange led to the clarification of the intermediacy of a low-Mr enzyme catalyzing the reactions. We purified the enzyme from rat liver and human placenta and demonstrated that it catalyzes the reduction of mixed disulfides of glutathione, but also disulfides in general, as well as thiosulfate esters such as S-sulfoglutathione and S-sulfocysteine (17). On the basis of its function, we named the enzyme thioltransferase (18); the corresponding Escherichia coli enzyme was later renamed as a member of the glutaredoxin family (19). We showed that thioltransferase catalyzes the reversible formation of mixed disulfides of glutathione and proteins, in addition to small substrates, and our work was summarized in a symposium in 1978 at Schloss Reisensburg (Fig. 1).
FIGURE 1.
Participants in the conference on glutathione in Schloss Reisensburg in Germany in 1978. The front row features, from left to right, Dagmar Siliprandi, Alton Meister, Sir Hans Krebs, Bengt Mannervik, Albrecht Wendel, and Leopold Flohé.
We proposed that, in addition to its central role in thiol and disulfide metabolism, thioltransferase could mediate redox regulation of cellular functions via sulfhydryl modification of proteins (20). The notion of protein-glutathione mixed disulfide formation gained increased attention in the mid-1990s under the short name of “protein thiolation.” I recommended the more accurate name “glutathionylation” to my colleague Ian Cotgreave (21), and this designation is now generally used in the steadily increasing literature on this important protein modification.
Glutathione Reductase
The thioltransferase reactions generated glutathione disulfide, and my interest in glutathione reductase catalyzing the regeneration of reduced glutathione remained active. The purification of the enzyme from porcine erythrocytes bought cheaply from a local slaughterhouse was only partly successful, and my early attempts in the late 1960s to purify the flavoprotein glutathione reductase on riboflavin immobilized on cellulose did not succeed. Only after introduction of the affinity matrix 2′,5′-ADP-Sepharose, developed by Klaus Mosbach, was a facile purification procedure established (22). I formulated a novel “branching mechanism” consisting of a fusion of the well established ping-pong and sequential mechanisms for two-substrate reactions (23) and found an uncoupling of electron transfer from FAD to disulfide by nitroaromatic compounds, converting the enzyme into an oxidase (24). The flavoenzyme guru Vincent Massey in Ann Arbor became my friend in 1969 and remained a source of inspiration for many years.
Glutathione Transferases
GST activity in liver cytosol was first reported in 1961 by Eric Boyland and co-workers (25) and by Combes and Stakelum (26). About a decade later, I serendipitously became acquainted with these important enzymes, catalyzing the conjugation of glutathione with electrophiles, when new graduate students called for novel projects. With time, GSTs became a major theme in the laboratory, and the enzymes have remained a vital topic in my research for about forty years.
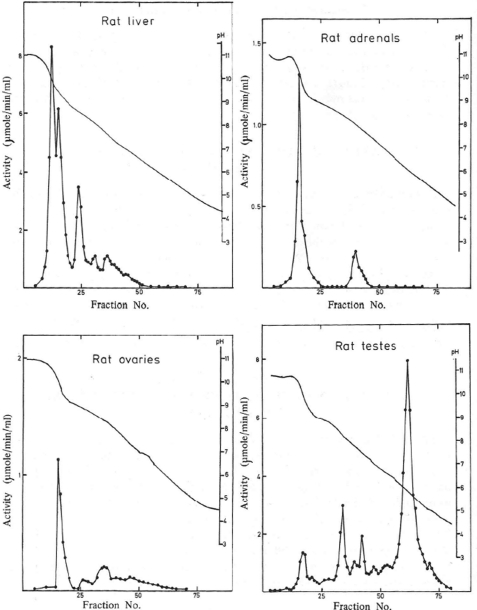
The pivotal role of GSTs in the protection of cells against electrophilic xenobiotics, including carcinogens, had been made clear by Boyland and co-workers (27), but the enzymes had not been separately identified and purified. Using high-resolution methods such as gradient elution from ion-exchange columns and isoelectric focusing, we purified and characterized several GSTs with partially overlapping substrate selectivities from rat liver (28). We subsequently purified GSTs from all major organs and found that the “isoenzyme” distribution was different among the tissues (Fig. 2) (29).
FIGURE 2.
Separation of cytosolic GSTs from rat tissues by column isoelectric focusing. Activities were measured with 1-chloro-2,4-dinitrobenzene. This figure has been reprinted from Ref. 29 with permission.
Furthermore, the multiple forms in the rat and in the mouse were shown not only to be differentially expressed in normal tissues but also to respond differentially to inducers of drug and carcinogen metabolism (30, 31). Neoplastic transformations also demonstrated major changes in GST expression such that the Pi class GST P1-1 represented 85–90% of the GST activity in ascites hepatoma cells, whereas normal hepatocytes have no detectable GST P1-1 expression (32). Our finding that human malignant melanoma cells overexpress GST P1-1 suggested a role of the enzyme in tumor drug resistance (33, 34).
GST Polymorphism in Human Liver
The significant role of GSTs in detoxication reactions made it imperative to purify and study the human complement of the enzymes. Full-term placentas were obtained from a maternity ward, and we discovered that the tissue was dominated by a single GST (35), later called GST P1-1 of the Pi class. William Jakoby and associates had previously found a GST in erythrocytes, and we concluded that it was the enzyme also found in placenta (36). The same enzyme was found in fetal (but not adult) human liver (37).
Adult human livers showed individual differences in their expression of GSTs, in particular with respect to a novel enzyme with distinctive catalytic properties (38). This enzyme, now named GST M1-1 of the Mu class, is present only in approximately half of the human population, and our discovery of the GST null phenotype appeared particularly significant in view of a high catalytic activity with carcinogenic epoxides (39). Establishment of this first GST polymorphism and the subsequent demonstration of the null allele of the corresponding gene (40) have spawned numerous epidemiological studies.
Quaternary Structure, Binary Subunit Combinations, and G- and H-sites of GSTs
The physicochemical properties indicated that the soluble GSTs found in the cytosolic fraction of mammalian cells are dimers, and inhibition studies with homologous S-alkylglutathiones demonstrated enhanced affinity with increased chain length of the alkyl group (28). Kinetic and equilibrium binding studies established high specificity for glutathione bound at a ratio of 1:1 per subunit, and the electrophilic substrates appeared to bind with similar stoichiometry at a less specific hydrophobic site. We named the two positions the “G-site” and the “H-site,” respectively (41).
In the late 1970s, data appeared suggesting that the major rat liver enzyme GST B, also known as “ligandin,” was composed of two non-identical subunits. However, SDS-PAGE analysis showed different amounts of the constituent subunits, indicating that the ligandin preparations were not homogeneous. Our characterization of the six major GSTs in rat liver showed that they could be divided into two groups of isoenzymes, each of which contains two subunits in homodimeric and heterodimeric combinations (42). The functional properties of the heterodimers could be predicted from linear combinations of the properties of the corresponding homodimers (42, 43). We proposed that the enzymes should be named in accord with their subunit composition, a principle still followed (44).
Classification of the GSTome
The GSTs occur in multiple forms in mammalian tissues, as demonstrated by several laboratories, including our own. However, no simple one-to-one correspondence could be discerned when we compared rat, mouse, and human GSTs as well as their mutually diverging tissue expressions. Our understanding of their relationships emerged from a principal component analysis of substrate selectivities and inhibition data combined with cross-reactions with antibodies as well as partial amino acid sequences (45). We concluded that the GSTome can be divided into classes encompassing enzymes from different biological species, and we named the major ones studied “Alpha,” “Mu,” and “Pi.” The number of mammalian GST classes is now known to be seven, and the human genome contains 17 GST genes encoding soluble enzymes, one or two of which may be missing in some individuals (44). In addition, there is an eighth class comprising a GST expressed in mitochondria and peroxisomes as well as a class of structurally unrelated “microsomal” GSTs. In this era of “omics,” it is time to consider the GSTome as the ensemble of all GSTs and their collective roles in an organism.
Natural GST Substrates
Reflecting on the evolution of GSTs in living systems, it appeared obvious that the man-made industrial chemicals originally used for the characterization of the enzymes were not the natural substrates that had driven their evolution. Bruce Ames, whom I knew well since my sabbatical with Daniel Koshland at the University of California, Berkeley, in the academic year 1981–1982, had strongly expressed the view that 99.99% of all mutagens in food are naturally occurring (46). Ames estimated that thousands of oxidative hits caused damage to DNA in each cell every day. We therefore set out to assay GSTs with potential substrates that could have arisen by oxidative damage to cell constituents. The results were gratifying, and in collaboration with various colleagues who had suitable compounds available, we found that alkenals, organic hydroperoxides, epoxides, ortho-quinones, and others were indeed good substrates for GSTs (47). Particularly important was our discovery that certain GSTs displayed high catalytic efficiency with select electrophilic substrates, indicating that they had evolved for more specific functions than being generalist detoxication enzymes. We found that human GST M1-1 was particularly active with epoxides (39), even though the naturally occurring signaling molecule leukotriene A4 was found to be metabolized primarily by the specific membrane-associated enzyme leukotriene C4 synthase (48). More recently, hepoxilins and eoxins, additional physiologically important epoxides derived from arachidonic acid, were identified as GST substrates (49).
Particularly significant were the findings of the extraordinarily high activities of human GST M2-2 with ortho-quinones derived from dopamine and other catecholamines (50) and of GST A4-4 with 4-hydroxynonenal and structural homologs (51, 52). Increases in these toxic compounds are associated with the development of Parkinson and Alzheimer diseases as well as other degenerative conditions and aging.
In addition to the products of oxidative metabolism, my attention was drawn to organic isothiocyanates, which had been identified as chemoprotective agents against cancer and other diseases, in particular, by my good friend Paul Talalay. Organic isothiocyanates play various roles in the complex interplay between plants, insects, and microorganisms. These electrophilic compounds can serve as chemoattractants and cues for oviposition of Pieridae butterflies as well as chemical weapons in the defense mounted by plants against the attack of microorganisms and various other predators. Together with the Talalay group, we demonstrated that organic isothiocyanates are excellent substrates for many soluble GSTs (53). As judged by our recent enzyme engineering studies, it would appear that activity with isothiocyanates can readily emerge in the GST structural framework (54), but the biological and evolutionary significance of this notion is not entirely clear.
Finally, we identified GST A3-3 as the most efficient 3-ketosteroid isomerase in human cells (55) and demonstrated that steroid hormone production is attenuated by suppression of the enzyme activity via siRNA or chemical enzyme inhibitors (56). The steroid isomerase activity of GSTs was first described by Talalay and co-workers (57).
The Jacobsson Chair at Uppsala University
In January 1988, I was appointed to the Karin and Herbert Jacobsson Chair of Biochemistry at Uppsala University. The professorship had been created in 1938 for Arne Tiselius to become the first professor of biochemistry in Sweden. Tiselius and his successor, Jerker Porath, had held the position for 50 years, and I found it bewildering to reflect on the remarkable developments in biochemistry during this half-century. In 1938, proteins were still considered as colloidal particles lacking a well defined molecular structure, and nucleic acids had not yet been characterized as carriers of genetic information.
Tiselius and Porath were recognized for their outstanding contributions to biochemical separation methods, including electrophoresis and a variety of chromatographic techniques. Building on results from the Uppsala school, I had made some modest contributions to affinity chromatography, with the most successful application being purification of glyoxalase I (8) and glutathione transferases (58, 59) on S-alkylglutathiones, in particular, S-hexylglutathione, immobilized on agarose. However, the strengths of my laboratory were in the fields of enzymology, biochemical toxicology, and other more cellular aspects of biochemistry. We added recombinant DNA techniques, heterologous protein expression, and other molecular genetic approaches as valuable complements in teaching and in research to the well developed separation science in the department.
Beyond Naturally Occurring GSTs
Realizing that the extant GSTs in humans and several other organisms would all be characterized before long, I became interested in the possibility of generating new biocatalysts and exploring the potential of the GST scaffold for novel enzymatic functions. Such investigations became possible as a result of advances in recombinant DNA methods. In parallel, we were trying to understand how the diversity of GSTs could have arisen by natural evolution.
Philip Board, from the John Curtin School of Medical Research, Canberra, Australia, chose to spend a sabbatical in my laboratory in 1988. His expertise in recombinant DNA techniques was a great help in accelerating our work on recombinant GSTs. At the time, we had considered the possibility that exon shuffling or other recombinations of gene sequences contribute to the evolution of novel enzymes (59). This was the time before DNA manipulations were facilitated by PCR and whole-gene synthesis, but by judicious choice of restriction endonucleases, we were still able to combine sequences derived from different GST sequences (60). Several recombined GSTs, as well as a C-terminally truncated GST (61), were generated and found to be catalytically competent. Thus, the principle of joining different segments of primary structure to obtain multiple GST variants with catalytic activity was demonstrated.
We found combinatorial protein chemistry to be a useful paradigm for the redesign of novel GSTs. Interchange of amino acids or sections of primary structure was a facile means to altered functions. In some instances, the changes also involved secondary structure elements such that, for example, a helix could be added, eliminated, or exchanged. Obviously, the natural evolution of families of homologous enzymes is more complex, involving gene duplications, followed by mutations of the primary structure.
Molecular Chimeras
Our interest in making chimeras of GSTs developed beyond constructs based on suitable restriction sites in cognate DNA sequences. The next step in our forays into the molecular evolution of chimeric GSTs was to apply the stochastic approach of DNA shuffling in such experiments. Up to six different GST sequences were fragmented for recombination, and in the spirit of the mythological chimeras, portions from three different organisms were combined. The resulting GSTs contained portions from human, rat, and bovine sequences (62).
Promiscuous Enzymes Represented as Vectors
The traditional view of enzymes is that they are highly substrate-selective. However, cellular detoxication is better served by enzymes with broad substrate acceptance, as exemplified by GSTs. We became interested in the quantitative description of promiscuous catalytic activities and found it useful to describe the functions as vectors in alternative substrate-activity space (63). The direction of the vector of an enzyme specifies its substrate selectivity, and vectors with similar directions can be regarded as showing variants of the same enzyme. In evolution, as well as in our recombinant chimeragenesis, vectors significantly diverging from one another indicate distinct enzymes.
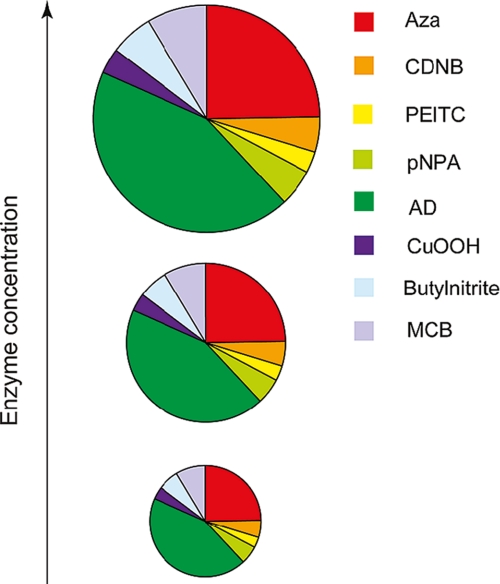
For illustration of the differences in substrate selectivities, pie charts or doughnut diagrams show the relative components of the vectors, i.e. the proportions of the different activities with the alternative substrates (Fig. 3). These diagrams depict the most important functional property in terms of substrate discrimination because the proportions are independent of enzyme concentration and because poor discrimination cannot be overcome by supplying more of the enzyme.
FIGURE 3.
Pie chart representation of the substrate-activity profile of a GST variant picked from the library and assayed with eight alternative substrates (cf. Ref. 62). The segments in different colors indicate the activity of a given substrate as a fraction of the sum of all the specific activities. The area of the chart represents the total catalytic capacity of the GST, and an increased amount of the enzyme will result in a proportionate increase in the catalytic capacity for all alternative substrates. Aza, azathioprine; CDNB, 1-chloro-2,4-dinitrobenzene; PEITC, phenethyl isothiocyanate; pNPA, p-nitrophenyl acetate; AD, 5-androstene-3,17-dione; CuOOH, cumene hydroperoxide; MCB, monochlorobimane. This figure has been reprinted from Ref. 63 with permission.
Evolving Molecular Quasi-species
In connection with theoretical studies of molecular evolution, Manfred Eigen developed the concept of molecular quasi-species (64), which was successfully applied to experimental systems of evolving RNA ensembles. Encouraged by Eigen, we introduced the notion of molecular quasi-species in enzyme evolution (65). The quasi-species is an abstraction that captures the salient features of related enzyme variants. From the evolutionary perspective, it can also be thought of as a focused cloud at an optimum in the multidimensional fitness landscape.
We decided to explore the concept of GST quasi-species by functional studies of molecular variants in multidimensional factor space. Randomly picked mutants from a library obtained by DNA shuffling of two Mu class GSTs were assayed with eight alternative substrates, thereby providing the substrate-activity profile for every enzyme variant examined (65). Each mutant was thus represented by a point or vector in eight-dimensional substrate-activity space. Principal component analysis showed that the data points formed three clusters, two of which clustered near the coordinates for the two parental GSTs, respectively. The third cluster had reduced activities with all signature substrates of the parental enzymes (65). Apparently, the clusters represented three divergent quasi-species, and the third was distinct from the parental ones. A second round of mutagenesis via DNA shuffling of the members of the third quasi-species, followed by screening, identified a new cluster characterized by high activity with organic isothiocyanates (54).
In directed enzyme evolution, it may seem self-evident that the enzyme variant featuring the highest expression of the targeted trait should be chosen as the progenitor of the following generation of mutants. However, in the crossing of plants and animals, it is well known that a limited genetic background can lead to inbreeding and loss of viability. By the same token, enzyme evolution can benefit from a broadened genetic background. Our experiments demonstrated that the concept of the molecular quasi-species is helpful both in the understanding of enzyme evolution and for the design of enzymes with novel functional properties.
The Students
Over the years, I have been fortunate to receive students in my laboratory from all continents of the world. So far, more than fifty Ph.D. theses have been completed under my supervision. Undergraduate students and postdoctoral fellows score a three-digit number. I have come to appreciate that there comes a time in your career when the success of your former students takes precedence over accounts of your own accomplishments.
Funding and Visits by the Swedish Government
Reflecting on the conditions when my research group was formed in the 1960s compared with present day conditions, I realize that it would be much more difficult to accrue and finance a corresponding group of graduate students. A few decades ago, Ph.D. students could be supported by modest, but tax-free, stipends or by employment as teaching assistants and be provided with an adequate salary for a relatively limited period of teaching time. Today, Ph.D. students in Sweden expect to receive full-time employment with all social benefits, making the financing of students 2–3-fold more expensive. In addition, the cost of consumables per capita has increased by an order of magnitude such that running a laboratory requires a sizeable budget.
From my perspective, I strongly disagreed when Thomas Östros, Minister for Education and Science, in 2001 wrote an article in a major newspaper stating that Swedish research funding was steadily increasing and was in parity with top-ranking nations. My newspaper rebuttal led to a call from the Minister for an official visit to my laboratory in Uppsala. Our three-hour conversation gave me the chance to express my concerns directly to him.
A second visit by the government (now representing the opposite political block) occurred in August 2008, when Prime Minister Fredrik Reinfeldt and three additional ministers, all heads of the four ruling political parties, came from Stockholm to learn about our research. Thus, I got an additional opportunity to explain the problems of funding and careers in research face-to-face with the politicians in charge (Fig. 4). Their request for a visit to my laboratory was directly linked to a press conference in which a five-billion SEK (Swedish kronor) increase in Swedish research funding was announced. I assume that the background I had provided accentuated the significance of the government's proposition of improvements.
FIGURE 4.
Swedish ministers visiting the Mannervik laboratory at Uppsala University in 2008. Shown, from left to right, are Bengt Mannervik, Emil Hamnevik (student at the spectrophotometer), Minister for Education Jan Björklund (behind), Deputy Prime Minister and Minister for Enterprise and Energy Maud Olofsson, and Prime Minister Fredrik Reinfeldt. Following the tour of the laboratory, the Prime Minister and his colleagues held a press conference announcing a five-billion SEK increase in Swedish governmental funding of research.
Glutathione and Gastronomy
In the period of half a century, the research on glutathione and GSTs has diversified and steadily opened new doors in enzymology as well as other branches of molecular life sciences. There are certain similarities to the Greek tale about the Hydra; when a knotty issue has been disentangled, new challenges appear in an endless series. Recently, gastronomy has received its share of the subject matter. GST-catalyzed glutathione conjugation of endogenous compounds in grapes leads to varietal aromas in wines such as sauvignon blanc (66), suggesting that the enzymology of GSTs could find valuable applications in viniculture as well as in other areas of applied biotechnology. Furthermore, it has been reported that the presence in food of glutathione itself makes a meal more delicious because it confers “kokumi,” the Japanese word for the feeling of enhanced richness, mouthfulness, and continuity (Fig. 5) (67).
FIGURE 5.
Entertaining guests in the Mannervik country house in 2010. Shown, from left to right, are Judith Klinman (President of the American Society for Biochemistry and Molecular Biology in 1998), Anne-Charlotte Mannervik, and Mordechai Mitnick (Klinman's husband).
The GSTome and Aging
At this stage in my scientific career, it may be appropriate to consider the biochemical mechanisms of aging. I was intrigued by the finding that overexpression of certain GSTs is associated with longevity assurance (68). Transcriptome analysis of worms (Caenorhabditis elegans), flies (Drosophila melanogaster), and mice (Mus musculus) demonstrated that long-lived strains of each species overexpressed a limited number of the genes of the GSTome. A reasonable explanation of extended life time is that the corresponding GSTs confer an elevated detoxication capacity toward toxic compounds produced by oxidative processes, in line with our earlier findings. Overexpression of certain GSTs may have similar effects in humans, and regulation of GST expression could possibly influence longevity. However, more importantly, it is well established that lower levels of glutathione and GSTs in elderly individuals raise their susceptibility to oxidative stress and degenerative conditions such as Parkinson and Alzheimer diseases, atherosclerosis, cataracts, and diabetes. A challenge is to explore if preventive medicine could restore the juvenile levels, not necessarily to increase longevity but to promote the quality of life in the aging human population. This would be a worthy task for the next 50 years of GST research.
Acknowledgments
My wife, Anne-Charlotte, has always been supportive of my scientific activities, particularly in preparing dinner parties with delicious food and entertaining guests from all parts of the world (Fig. 5). More than fifty graduate students, numerous postdoctoral fellows, and an unknown number of undergraduates have implemented and further developed my research plans. I have greatly appreciated their willingness to spend important years of their lives engaged in my research activities, and most of all, I treasure their warm friendship. Kerstin Larson (Stockholm University) and Birgit Olin (Uppsala University) are the co-workers who have spent the longest periods of time in my laboratory, and their dedicated efforts over decades have been invaluable. Major funding for the research has been provided by the Swedish Research Council, the Swedish Cancer Society, and the Herbert and Karin Jacobsson Fund.
REFERENCES
- 1. Smithies O. (1955) Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem. J. 61, 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holden H. M., Rypniewski W. R., Law J. H., Rayment I. (1987) The molecular structure of insecticyanin from the tobacco hornworm Mandica sexta L. at 2.6 Å resolution. EMBO J. 6, 1565–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fridén F. (1958) Frass-Drop Frequency in Lepidoptera, Almqvist & Wiksell, Uppsala [Google Scholar]
- 4. Eriksson B., Sörbo B. (1967) The synthesis and some properties of the thiosulfonate analogue of glutathione (γ-l-glutamyl-l-3-thiosulfoalanylglycine). Acta Chem. Scand. 21, 958–960 [Google Scholar]
- 5. Kosower E. M., Kosower N. S. (1969) Lest I forget thee, glutathione … Nature 224, 117–120 [DOI] [PubMed] [Google Scholar]
- 6. Eriksson B. (1966) On the synthesis and enzymatic reduction of the coenzyme A-glutathione mixed disulfide. Acta Chem. Scand. 20, 1178–1179 [DOI] [PubMed] [Google Scholar]
- 7. Mannervik B. (1969) Syntheses, Quantitative Analyses, and Enzymatic Reactions of Some Naturally Occurring Glutathione Sulfenyl Derivatives. Ph.D. dissertation, Stockholm University [Google Scholar]
- 8. Aronsson A. C., Mannervik B. (1977) Characterization of glyoxalase I purified from pig erythrocytes by affinity chromatography. Biochem. J. 165, 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aronsson A. C., Marmstål E., Mannervik B. (1978) Glyoxalase I, a zinc metalloenzyme of mammals and yeast. Biochem. Biophys. Res. Commun. 81, 1235–1240 [DOI] [PubMed] [Google Scholar]
- 10. Cameron A. D., Olin B., Ridderström M., Mannervik B., Jones T. A. (1997) Crystal structure of human glyoxalase I: evidence for gene duplication and three-dimensional domain swapping. EMBO J. 16, 3386–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frickel E. M., Jemth P., Widersten M., Mannervik B. (2001) Yeast glyoxalase I is a monomeric enzyme with two active sites. J. Biol. Chem. 276, 1845–1849 [DOI] [PubMed] [Google Scholar]
- 12. Cleland W. W. (1967) Statistical analysis of enzyme kinetic data. Adv. Enzymol. 29, 1–32 [DOI] [PubMed] [Google Scholar]
- 13. Bártfai T., Mannervik B. (1972) A procedure based on statistical criteria for discrimination between steady-state kinetic models. FEBS Lett. 26, 252–256 [DOI] [PubMed] [Google Scholar]
- 14. Mannervik B., Górna-Hall B., Bártfai T. (1973) The steady-state kinetics of glyoxalase I from porcine erythrocytes. Evidence for a random-pathway mechanism involving one- and two-substrate branches. Eur. J. Biochem. 37, 270–281 [DOI] [PubMed] [Google Scholar]
- 15. Mannervik B. (2009) in Contemporary Enzyme Kinetics and Mechanism (Purich D., ed) pp. 73–94, Elsevier, Amsterdam [Google Scholar]
- 16. Mannervik B., Eriksson S. A. (1974) in Glutathione (Flohé L., Benöhr H. C., Sies H., Waller H. D., Wendel A., eds) pp. 120–131, Georg Thieme Publishers, Stuttgart, Germany [Google Scholar]
- 17. Axelsson K., Eriksson S., Mannervik B. (1978) Purification and characterization of cytoplasmic thioltransferase (glutathione:disulfide oxidoreductase) from rat liver. Biochemistry 17, 2978–2984 [DOI] [PubMed] [Google Scholar]
- 18. Askelöf P., Axelsson K., Eriksson S., Mannervik B. (1974) Mechanism of action of enzymes catalyzing thiol-disulfide interchange. Thioltransferases rather than transhydrogenases. FEBS Lett. 38, 263–267 [DOI] [PubMed] [Google Scholar]
- 19. Lillig C. H., Berndt C., Holmgren A. (2008) Glutaredoxin systems. Biochim. Biophys. Acta 1780, 1304–1317 [DOI] [PubMed] [Google Scholar]
- 20. Mannervik B., Axelsson K. (1980) Role of cytoplasmic thioltransferase in cellular regulation by thiol-disulfide interchange. Biochem. J. 190, 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lind C., Gerdes R., Schuppe-Koistinen I., Cotgreave I. A. (1998) Studies on the mechanism of oxidative modification of human glyceraldehyde-3-phosphate dehydrogenase by glutathione: catalysis by glutaredoxin. Biochem. Biophys. Res. Commun. 247, 481–486 [DOI] [PubMed] [Google Scholar]
- 22. Mannervik B., Jacobsson K., Boggaram V. (1976) Purification of glutathione reductase from erythrocytes by the use of affinity chromatography on 2′,5′-ADP-Sepharose 4B. FEBS Lett. 66, 221–224 [DOI] [PubMed] [Google Scholar]
- 23. Mannervik B. (1973) A branching reaction mechanism of glutathione reductase. Biochem. Biophys. Res. Commun. 53, 1151–1158 [DOI] [PubMed] [Google Scholar]
- 24. Carlberg I., Mannervik B. (1986) Reduction of 2,4,6-trinitrobenzenesulfonate by glutathione reductase and the effect of NADP+ on the electron transfer. J. Biol. Chem. 261, 1629–1635 [PubMed] [Google Scholar]
- 25. Booth J., Boyland E., Sims P. (1961) An enzyme from rat liver catalyzing conjugations with glutathione. Biochem. J. 79, 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Combes B., Stakelum G. S. (1961) A liver enzyme that conjugates sulfobromophthalein sodium with glutathione. J. Clin. Invest. 40, 981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boyland E., Chasseaud L. F. (1969) Role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. 32, 173–219 [DOI] [PubMed] [Google Scholar]
- 28. Askelöf P., Guthenberg C., Jakobson I., Mannervik B. (1975) Purification and characterization of two glutathione S-aryltransferase activities from rat liver. Biochem. J. 147, 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mannervik B., Guthenberg C., Jensson H., Warholm M., Ålin P. (1983) in Functions of Glutathione: Biochemical, Physiological, Toxicological, and Clinical Aspects (Larsson A., Orrenius S., Holmgren A., Mannervik B. eds) pp. 75–88, Raven Press, New York [Google Scholar]
- 30. Guthenberg C., Morgenstern R., DePierre J. W., Mannervik B. (1980) Induction of glutathione S-transferases A, B, and C in rat liver cytosol by trans-stilbene oxide. Biochim. Biophys. Acta 631, 1–10 [DOI] [PubMed] [Google Scholar]
- 31. Di Simplicio P., Jensson H., Mannervik B. (1989) Effects of inducers of drug metabolism on basic hepatic forms of mouse glutathione transferase. Biochem. J. 263, 679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tahir M. K., Guthenberg C., Mannervik B. (1989) Glutathione transferases in rat hepatoma cells. Effects of ascites cells on the isoenzyme pattern in liver and induction of glutathione transferases in the tumor cells. Biochem. J. 257, 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mannervik B., Castro V. M., Danielson U. H., Tahir M. K., Hansson J., Ringborg U. (1987) Expression of class Pi glutathione transferase in human malignant melanoma cells. Carcinogenesis 8, 1929–1932 [DOI] [PubMed] [Google Scholar]
- 34. Hansson J., Berhane K., Castro V. M., Jungnelius U., Mannervik B., Ringborg U. (1991) Sensitization of human melanoma cells to the cytotoxic effect of melphalan by the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 51, 94–98 [PubMed] [Google Scholar]
- 35. Guthenberg C., Åkerfeldt K., Mannervik B. (1979) Purification of glutathione S-transferase from human placenta. Acta Chem. Scand. B33, 595–596 [DOI] [PubMed] [Google Scholar]
- 36. Guthenberg C., Mannervik B. (1981) Glutathione S-transferase (transferase π) from human placenta is identical or closely related to glutathione S-transferase (transferase ρ) from erythrocytes. Biochim. Biophys. Acta 661, 255–260 [DOI] [PubMed] [Google Scholar]
- 37. Guthenberg C., Warholm M., Rane A., Mannervik B. (1986) Two distinct forms of glutathione transferase from human fetal liver. Purification and comparison with isoenzymes isolated from adult liver and placenta. Biochem. J. 235, 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Warholm M., Guthenberg C., Mannervik B., von Bahr C., Glaumann H. (1980) Identification of a new glutathione S-transferase in human liver. Acta Chem. Scand. B34, 607–610 [DOI] [PubMed] [Google Scholar]
- 39. Warholm M., Guthenberg C., Mannervik B., von Bahr C. (1981) Purification of a new glutathione S-transferase (transferase μ) from human liver having high activity with benzo(a)pyrene 4,5-oxide. Biochem. Biophys. Res. Commun. 98, 512–519 [DOI] [PubMed] [Google Scholar]
- 40. Seidegård J., Vorachek W. R., Pero R. W., Pearson W. R. (1988) Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc. Natl. Acad. Sci. U.S.A. 85, 7293–7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mannervik B., Guthenberg C., Jakobson I., Warholm M. (1978) in Conjugation Reactions in Drug Biotransformation (Aitio A., ed) pp. 101–110, Elsevier North-Holland, Amsterdam [Google Scholar]
- 42. Mannervik B., Jensson H. (1982) Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J. Biol. Chem. 257, 9909–9912 [PubMed] [Google Scholar]
- 43. Tahir M. K., Mannervik B. (1986) Simple inhibition studies for distinction between homodimeric and heterodimeric isoenzymes of glutathione transferase. J. Biol. Chem. 261, 1048–1051 [PubMed] [Google Scholar]
- 44. Mannervik B., Board P. G., Hayes J. D., Listowsky I., Pearson W. R. (2005) Nomenclature for mammalian soluble glutathione transferases. Methods Enzymol. 401, 1–8 [DOI] [PubMed] [Google Scholar]
- 45. Mannervik B., Ålin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. (1985) Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc. Natl. Acad. Sci. U.S.A. 82, 7202–7206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ames B. N. (1983) Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 221, 1256–1264 [DOI] [PubMed] [Google Scholar]
- 47. Mannervik B. (1986) Glutathione and the evolution of enzymes for detoxication of products of oxygen metabolism. Chem. Scripta 26B, 281–284 [Google Scholar]
- 48. Söderström M., Hammarström S., Mannervik B. (1988) Leukotriene C synthase in mouse mastocytoma cells. An enzyme distinct from cytosolic and microsomal glutathione transferases. Biochem. J. 250, 713–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brunnström A., Hamberg M., Griffiths W. J., Mannervik B., Claesson H. E. (2011) Biosynthesis of 14,15-hepoxilins in human L1236 Hodgkin lymphoma cells and eosinophils. Lipids 46, 69–79 [DOI] [PubMed] [Google Scholar]
- 50. Segura-Aguilar J., Baez S., Widersten M., Welch C. J., Mannervik B. (1997) Human class Mu glutathione transferases, in particular, isoenzyme M2-2, catalyze detoxication of the dopamine metabolite aminochrome. J. Biol. Chem. 272, 5727–5731 [DOI] [PubMed] [Google Scholar]
- 51. Stenberg G., Ridderström M., Engström A., Pemble S. E., Mannervik B. (1992) Cloning and heterologous expression of cDNA encoding class Alpha rat glutathione transferase 8-8, an enzyme with high catalytic activity toward genotoxic α,β-unsaturated carbonyl compounds. Biochem. J. 284, 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hubatsch I., Ridderström M., Mannervik B. (1998) Human glutathione transferase A4-4: an Alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem. J. 330, 175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kolm R. H., Danielson U. H., Zhang Y., Talalay P., Mannervik B. (1995) Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem. J. 311, 453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Runarsdottir A., Mannervik B. (2010) A novel quasi-species of glutathione transferase with high activity toward naturally occurring isothiocyanates evolves from promiscuous low-activity variants. J. Mol. Biol. 401, 451–464 [DOI] [PubMed] [Google Scholar]
- 55. Johansson A. S., Mannervik B. (2001) Human glutathione transferase A3-3, a highly efficient catalyst of double-bond isomerization in the biosynthetic pathway of steroid hormones. J. Biol. Chem. 276, 33061–33065 [DOI] [PubMed] [Google Scholar]
- 56. Raffalli-Mathieu F., Orre C., Stridsberg M., Hansson Edalat M., Mannervik B. (2008) Targeting human glutathione transferase A3-3 attenuates progesterone production in human steroidogenic cells. Biochem. J. 414, 103–109 [DOI] [PubMed] [Google Scholar]
- 57. Benson A. M., Talalay P., Keen J. H., Jakoby W. B. (1977) Relationship between the soluble glutathione-dependent Δ5-3-ketosteroid isomerase and the glutathione S-transferases of the liver. Proc. Natl. Acad. Sci. U.S.A. 74, 158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guthenberg C., Mannervik B. (1979) Purification of glutathione S-transferases from rat lung by affinity chromatography. Evidence for an enzyme form absent in rat liver. Biochem. Biophys. Res. Commun. 86, 1304–1310 [DOI] [PubMed] [Google Scholar]
- 59. Mannervik B. (1985) The isoenzymes of glutathione transferase. Adv. Enzymol. Relat. Areas Mol. Biol. 57, 357–417 [DOI] [PubMed] [Google Scholar]
- 60. Björnestedt R., Widersten M., Board P. G., Mannervik B. (1992) Design of two chimeric human-rat class Alpha glutathione transferases for probing the contribution of C-terminal segments of protein structure to the catalytic properties. Biochem. J. 282, 505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Board P. G., Mannervik B. (1991) The contribution of the C-terminal sequence to the catalytic activity of GST2, a human Alpha class glutathione transferase. Biochem. J. 275, 171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kurtovic S., Modén O., Shokeer A., Mannervik B. (2008) Structural determinants of glutathione transferases with azathioprine activity identified by DNA shuffling of Alpha class members. J. Mol. Biol. 375, 1365–1379 [DOI] [PubMed] [Google Scholar]
- 63. Kurtovic S., Mannervik B. (2009) Identification of emerging quasi-species in directed enzyme evolution. Biochemistry 48, 9330–9339 [DOI] [PubMed] [Google Scholar]
- 64. Eigen M., McCaskill J., Schuster P. (1988) Molecular quasi-species. J. Phys. Chem. 92, 6881–6891 [Google Scholar]
- 65. Emrén L. O., Kurtovic S., Runarsdottir A., Larsson A. K., Mannervik B. (2006) Functionally diverging molecular quasi-species evolve by crossing two enzymes. Proc. Natl. Acad. Sci. U.S.A. 103, 10866–10870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kobayashi H., Takase H., Suzuki Y., Tanzawa F., Takata R., Fujita K., Kohno M., Mochizuki M., Suzuki S., Konno T. (2011) Environmental stress enhances biosynthesis of flavor precursors, S-3-(hexan-1-ol)-glutathione and S-3-(hexan-1-ol)-l-cysteine, in grapevine through glutathione S-transferase activation. J. Exp. Bot. 62, 1325–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ohsu T., Amino Y., Nagasaki H., Yamanaka T., Takeshita S., Hatanaka T., Maruyama Y., Miyamura N., Eto Y. (2010) Involvement of the calcium-sensing receptor in human taste perception. J. Biol. Chem. 285, 1016–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McElwee J. J., Schuster E., Blanc E., Piper M. D., Thomas J. H., Patel D. S., Selman C., Withers D. J., Thornton J. M., Partridge L., Gems D. (2007) Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biology 8, R132. [DOI] [PMC free article] [PubMed] [Google Scholar]