Background: Glutathione S-transferase Omega has been shown to be associated with Parkinson disease.
Results: Drosophila GSTO1 regulates mitochondrial ATP synthase activity in parkin mutants.
Conclusion: Drosophila GSTO1 plays a protective role in a Drosophila model of Parkinson disease.
Significance: These findings may lead to a better understanding of the molecular mechanism of neuroprotection due to GSTO in Parkinson disease.
Keywords: ATP Synthase, Drosophila Genetics, Enzymes, Mitochondria, Parkinson Disease, Glutathione S-Transferase
Abstract
A loss-of-function mutation in the gene parkin causes a common neurodegenerative disease that may be caused by mitochondrial dysfunction. Glutathione S-transferase Omega (GSTO) is involved in cell defense mechanisms, but little is known about the role of GSTO in the progression of Parkinson disease. Here, we report that restoration of Drosophila GSTO1 (DmGSTO1), which is down-regulated in parkin mutants, alleviates some of the parkin pathogenic phenotypes and that the loss of DmGSTO1 function enhances parkin mutant phenotypes. We further identified the ATP synthase β subunit as a novel in vivo target of DmGSTO1. We found that glutathionylation of the ATP synthase β subunit is rescued by DmGSTO1 and that the expression of DmGSTO1 partially restores the activity and assembly of the mitochondrial F1F0-ATP synthase in parkin mutants. Our results suggest a novel mechanism for the protective role of DmGSTO1 in parkin mutants, through the regulation of ATP synthase activity, and provide insight into potential therapies for Parkinson disease neurodegeneration.
Introduction
Parkinson disease (PD)3 is a progressive neurodegenerative disorder characterized by the degeneration of dopaminergic (DA) neurons. Previous reports suggested that mitochondrial dysfunction, oxidative stress, and ER stress induced by the aggregation of abnormal proteins can contribute to the pathogenesis of PD (1, 2). However, the molecular mechanisms of PD pathogenesis have not yet been fully elucidated. A loss-of-function mutation in the parkin gene is a major cause of autosomal recessive, early onset PD.
Glutathione S-transferases (GSTs) constitute a superfamily of enzymes that are grouped into at least 10 classes; some superfamily members are known to be involved in cell defense mechanisms (3, 4). The active sites of GST Omega (GSTOs) have a unique cysteine residue that can form a disulfide bond with GSH, whereas other eukaryotic GSTs have tyrosine or serine residues in their active sites (4). The biological functions of several GSTOs have been determined. Human GSTO1-1 modulates calcium channels (5), has a role in the activation of interleukin-1β (6), and interacts with a serine protease inhibitor (7). Recently, our group reported that one of the four GSTO genes in Drosophila, CG6781, is the structural gene of the Drosophila eye color mutant, sepia, which encodes pyrimidodiazepine synthase, a key enzyme in the drosopterin biosynthetic pathway (8). In humans, GSTOs are mapped to the linkage region correlated with the age at onset of Alzheimer disease (9). Variations in human GSTO1 genes that modify the age at onset of Alzheimer and Parkinson diseases have been reported (10). However, the in vivo function of GSTOs and their target proteins have not yet been fully identified.
Recently, Pallanck's group (11–13) reported that expression of Drosophila melanogaster GST Sigma 1 (DmGSTS1), which is another member of the GST family in Drosophila, suppresses the phenotypes of parkin mutants and α-synuclein-expressing mutants. To elucidate the protective role of GSTO in neurodegenerative diseases, we investigated the biological function of D. melanogaster GSTO (DmGSTO) in a Drosophila model of PD. We found that one of the DmGSTOs, DmGSTO1, is able to rescue some phenotypes of parkin mutants, including the degeneration of DA neurons and muscle. The ability of DmGSTO1 to rescue these phenotypes was dependent on the catalytic activity of DmGSTO1. Furthermore, tubulin accumulation and ER stress caused by the parkin mutation were also significantly reduced by ectopic expression of DmGSTO1. We discovered that the Drosophila ATP synthase β subunit (14) is a novel target of DmGSTO1, and up-regulation of DmGSTO1 restored glutathionylation levels of the ATP synthase β subunit in parkin mutants. The mitochondrial F1F0-ATP synthase is a membrane protein complex that couples the synthesis of ATP to the hydrogen ion gradient generated by the respiratory chain (15, 16). The up-regulation of DmGSTO1 also restored ATP synthase activity, complex assembly, and ATP levels in parkin mutants. Our findings suggest that DmGSTO1 plays a protective role in parkin mutants by regulating mitochondrial ATP synthase activity.
EXPERIMENTAL PROCEDURES
Drosophila Stocks
To generate DmGSTO1 mutants, we obtained the GE26508 P-element insertion line from the GenExel Drosophila library (KAIST, Daejeon, Korea). The P-element was mobilized using transposase, and GE26508 was imprecisely excised to generate DmGSTO1null (supplemental Fig. S1A). The UAS-mitGFP line was a gift from H. J. Bellen (Baylor College of Medicine, Houston, TX) (17). We generated four transgenic lines: DmGSTO1A, DmGSTO1B, DmGSTO1AC31A, and CG6662. The coding sequences for these four genes were amplified by PCR. The four PCR products were ligated into the pUAST expression vector and introduced into the germ line by microinjection. All PCR products were confirmed by DNA sequencing. Drosophila stocks were maintained in standard food conditions at 25 °C. The parkin null mutant line, park1, and PINK1B9 were gifts from J. Chung (Seoul National University) (18, 19). The TH-Gal4 line was a gift from S. Birman (CNRS-Université de la Méditerranée) (20). The Tub-Gal4 fly line and the 24B-Gal4 line were obtained from the Bloomington Stock Center. We obtained the ATP synthase β subunit RNAi lines, CG11154R-1 and R-3, from the NIG-FLY stock center (National Institute of Genetics, Mishima, Japan).
Exposure to Paraquat
One- to two-day-old male flies were starved for 5 h and then kept in vials with 3M filter paper soaked with 20 mm paraquat (Methyl Viologen, Sigma-Aldrich) in 5% sucrose. Flies were kept in the dark at all times.
Immunoblot Analysis
Protein extracts for immunoblot analysis were prepared by homogenizing 10 fly thoraces from 3-day-old male flies in lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 0.5% Nonidet P-40) containing a protease inhibitor mixture (1×; Calbiochem-Merck4Biosciences). Total protein (20 μg) was separated by 8 or 10% SDS-PAGE and transferred to PVDF membranes (Millipore). Membranes were blocked by Tris-buffered saline (TBS) with 4% nonfat dry milk or 4% BSA for 1 h. We used the following primary antibodies: rabbit anti-phospho-JNK (1:1,000; Promega), rabbit anti-JNK (1:1,000; Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)), rabbit anti-β-actin (1:5,000; Sigma-Aldrich), mouse anti-α-tubulin (1:4,000; Sigma-Aldrich), mouse anti-β-tubulin (1:3,000; Sigma-Aldrich), rabbit anti-phospho-eIF2α Ser-51 (1:1,000; Cell Signaling Technology), rabbit anti-eIF2α (1:200; Abcam), rabbit anti-HSP60 (1:1,000; Stressgen Bioreagents, Kampenhout, Belgium), mouse anti-HSP/HSC70 (1:1,000; Stressgen Bioreagents), mouse anti-ATP synthase α subunit (1:20,000; Mitosciences), rabbit anti-prohibitin (1:500; Abcam), and rabbit anti-Drosophila ATP synthase β subunit (1:20,000; a kind gift from Rafael Garesse, Universidad Autonoma de Madrid). Detection of the primary antibodies was carried out with HRP-conjugated secondary antibodies and an ECL-Plus detection kit (Amersham Biosciences). Polyclonal antibodies against Drosophila GSTO1A were produced by immunizing rabbits with a C-terminal synthetic peptide, 231EFQKSKTLGNPQY243, as the antigen (Abfrontier).
Muscle Histology
Muscle section analysis was carried out as described previously (21) with some modifications. For muscle tissue analyses, whole thoraces from 3-day-old male flies were fixed with 4% formaldehyde overnight at 4 °C. After fixation, the samples were oxidized with 1% OsO4 for 2 h at room temperature and then dehydrated in a series of acetone/water mixtures of increasing acetone concentration (50, 70, 80, 90, and 100% acetone). The samples were embedded in Spurr's resin. The thoraces were then trimmed and sectioned from the transverse side of the thorax. The sections were stained with a toluidine blue dye and observed by light microscopy (Carl Zeiss, Axio Imager A1).
Immunohistochemistry and TUNEL Assay
Adult brains and thoraces of 1-, 3-, 5-, and 20-day-old male flies were fixed with 4% formaldehyde in a fixative buffer (100 mm PIPES, 1 mm EGTA, 1% Triton X-100, and 2 mm MgSO4, pH 6.9) and blocked in a wash buffer (50 mm Tris, 150 mm NaCl, 0.1% Triton X-100, and 0.5 mg/ml BSA, pH 6.8) with 10 mg/ml BSA. The following antibodies were used: rabbit anti-TH (1:100; Pel-freeze), mouse anti-TH (1:100; Immunostar), rabbit anti-phospho-JNK (1:100; Promega), mouse anti-GFP (1:500; Roche Applied Science), and mouse anti-α-tubulin (1:500; Sigma-Aldrich). Alexa 488-conjugated streptavidin (1:100; Invitrogen) was used to identify mitochondria. Rhodamine phalloidin (Invitrogen) was used to visualize actin. All images were obtained on a Carl Zeiss confocal microscope (DE/LSM510 NLO). For the TUNEL assay, apoptosis in the indirect flight muscles (IFMs) of 3-day-old flies was detected using the In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science).
Quantitative RT-PCR and Real-time Quantitative RT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen), and cDNA was prepared from 2 μg of total RNA using Moloney murine leukemia virus reverse transcriptase (Promega). The PCRs were performed using Ex Taq (Takara Bio, Otsu, Japan) and TaqDNA polymerase (Bioneer, Daejeon, Korea) with a PTC-100 programmable thermal controller (MJ Research). We used the previously reported Drosophila GSTO primers for CG6776, CG6662, DmGSTO1A, and DmGSTO1B (8). The following dparkin and dPINK1 primers were used: dparkin-For (CAT ATG AGT TTT ATT TTT AAA TTT ATT GCC ACT TTT GTA C), dparkin-Rev (CTC GAG TTA GCC GAA CCA GTG GG), dPINK1-For (TTC TGC CAC CAC CGC CCC CAC ACT TC), and dPINK1-Rev (CCG CAG CAC ATT GGC AGC GGT GG).
The comparative cycle threshold (Ct) method was adapted to estimate transcript levels using an ABI7300 system (Applied Biosystems). The transcript levels were calculated as the relative -fold change over rp49 mRNA. We used the following primers for α-tubulin, and β-tubulin: α-tubulin-For (ACA ACG AGG CTA TCT ACG ACA TCT), α-tubulin-Rev (TTT TCA GTG TTG CAG TGA ATT TTT) (22), β-tubulin-For (CAA GGC TTC CAA CTC ACA CAC TC), and β-tubulin-Rev (AGG TGG CGG ACA TCT TCA GAC) (23).
Site-directed Mutagenesis and Expression of Mutant Proteins
The DmGSTO1AC31A mutant was generated by site-directed mutagenesis (Cosmo Genetech, Seoul, Korea). Cysteine 31 at the active site was mutated to alanine by changing the TGC codon encoding cysteine 31 to GCC. Mutated DNA was sequenced to confirm the single codon change. The DmGSTO1AC31A mutant was expressed in Escherichia coli strain BL21 after cloning the cDNA into a pET15b expression vector (Novagen-Merck4Biosciences).
In Vitro Glutathionylation Assay
Recombinant ATP synthase β subunit (5 μg) was incubated at 37 °C in 50 mm potassium phosphate (pH 7.6), and 10 mm GSH in the presence of either recombinant DmGSTO1A or DmGSTO1B protein. After 30 min, the samples were placed on ice, and 5× non-reducing SDS loading buffer was added to the mixtures. Samples were separated by 12% SDS-PAGE, transferred, and probed with mouse anti-GSH (1:1,000; ViroGen Corp.) and rabbit anti-Drosophila ATP synthase β subunit (1:4,000) antibodies.
Immunoprecipitation and Glutathionylation Assay
Thoraces from 3-day-old male flies were homogenized in lysis buffer containing 1× protease inhibitor mixture (Calbiochem-Merck4Biosciences). Thorax lysates were incubated with mouse anti-GSH antibodies (ViroGen) for 2 h at 4 °C and incubated overnight with 60 μl of a solution of 50% protein G-Sepharose beads (Amersham Biosciences) at 4 °C. The resins were collected by centrifugation at 1,000 × g for 20 s. Bound proteins, which were glutathionylated, were eluted by boiling in a 2× non-reducing SDS loading buffer for 5 min. Materials were subjected to SDS-PAGE and visualized with Coomassie Blue or silver staining.
After immunoprecipitation with mouse anti-GSH antibodies (ViroGen), proteins bound to resins were separated by 8% SDS-PAGE and transferred to PVDF membranes (Millipore) for the glutathionylation assay. Western blot analysis was carried out with rabbit anti-Drosophila ATP synthase β subunit antibodies (1:4,000). Signals were detected by HRP-conjugated secondary antibodies.
Mitochondrial ATP Synthase Activity Assay
To isolate mitochondria from fly thoraces, a mitchondria isolation kit (Pierce) was used according to the manufacturer's protocol. Freshly prepared total mitochondrial protein was used for the ATP synthase activity assay.
ATP synthase activity was measured by ATP hydrolysis using a spectrophotometric method described previously (24). ATP synthase was assayed in 20 mm Hepes (pH 7.5), 5 mm KCl, 5 mm MgCl2, 5 mm KCN, 2.5 mm phosphoenolpyruvate (Sigma-Aldrich), 15 units of pyruvate kinase (Sigma-Aldrich), 15 units of lactate dehydrogenase (Sigma-Aldrich), 300 μm NADH (Sigma-Aldrich), and 20 μg of total mitochondrial proteins. After 2 min of preincubation at 37 °C, the reaction was initiated by the addition of 2 mm ATP. The initial velocity of the reaction was followed for 2 min at 340 nm at 37 °C. The molar extinction coefficient of NADH is 6220 m−1.
ATP Assay
Five thoraces from 3-day-old male flies were homogenized in 100 μl of 1× reporter lysis buffer (Promega) on ice. The homogenized samples were quickly frozen in liquid nitrogen to inhibit ATP synthase activity. The frozen samples were boiled for 8 min to destroy endogenous ATP synthase and then centrifuged at 20,000 × g for 15 min. ATP was quantified in the supernatant using the ATP bioluminescent assay kit (Sigma-Aldrich) according to the manufacturer's protocol.
Blue Native Electrophoresis
Mitochondria were isolated from 3-day-old male fly thoraces using the mitochondrial isolation kit (Pierce). Mitochondrial proteins (10 μg) were dissolved in NativePAGETM sample buffer (Invitrogen), 1% digitonin (Invitrogen), and 2% dodecylmaltoside (Invitrogen). Samples were incubated for 15 min on ice and centrifuged at 12,000 × g for 25 min. Blue native electrophoresis was performed on NativePAGETM Novex 3–12% BisTris gels (Invitrogen).
RESULTS
DmGSTO1 Mutants Are Sensitive to Oxidative Stress
We previously reported that there are four different GSTO genes in D. melanogaster: CG6781 (sepia), CG6776, CG6673, and CG6662. CG6781 is the structural gene for sepia, which encodes pyrimidodiazepine synthase and is expressed exclusively in Drosophila heads (8). CG6673 is also called D. melanogaster GSTO1 (DmGSTO1) and has the highest thiol transferase and DHA reductase activity among the GSTO genes, reminiscent of thioredoxin and glutaredoxin (8). CG6776 responds to heat stress in Drosophila (25). Although the function of CG6662 is not known, CG6662 transcripts are primarily expressed in the ovary and testes (26). Thus, we focused our study on CG6673 (DmGSTO1) and excluded other Drosophila GSTO genes.
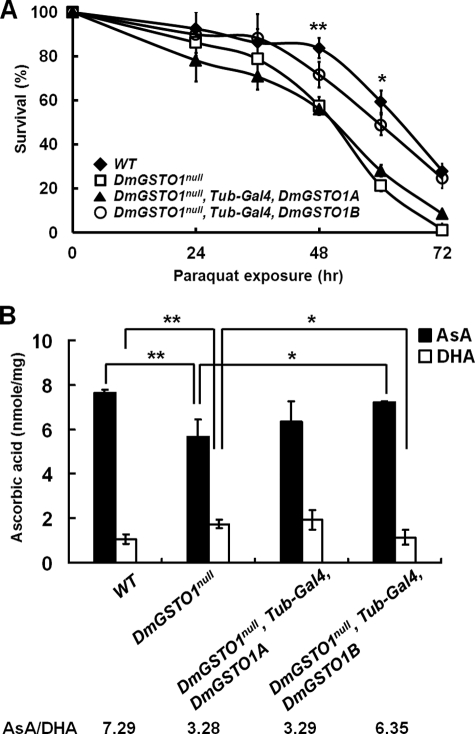
We generated both loss-of-function and gain-of-function DmGSTO1 mutant flies. Because DmGSTO1 encodes two alternatively spliced transcripts, A and B, we created both DmGSTO1A- and DmGSTO1B-overexpressing fly lines. Loss-of-function mutant DmGSTO1null was generated by imprecise P-element excision, which resulted in partial deletion of the DmGSTO1 gene (supplemental Fig. S1A). The DmGSTO1null mutant was viable and fertile, and it exhibited no obvious defects in adult morphology. In the DmGSTO1 null mutant fly, there were no detectable levels of either DmGSTO1A or DmGSTO1B transcripts (supplemental Fig. S1B). GSTO enzymes exhibit higher glutathione-dependent thiol transferase and dehydroascorbate reductase (DHAR) activity than any other class of GSTs (27). We have shown that DmGSTO1B has much higher DHAR activity than DmGSTO1A in vitro (8). The DHAR activity in DmGSTO1null flies was dramatically decreased to ∼5% of the DHAR activity found in wild type flies (supplemental Fig. S1C). Furthermore, DmGSTO1null mutants were sensitive to paraquat, an oxidative stress inducer (Fig. 1A). Using a ubiquitous driver, Tub-Gal4, we directed expression of DmGSTO1A or DmGSTO1B in the DmGSTO1null mutant background. The paraquat sensitivity was rescued by expression of DmGSTO1B, which exhibited more oxidative stress protection than DmGSTO1A (Fig. 1A). These results suggest that the paraquat-sensitive phenotype in the DmGSTO1null mutant is primarily due to the loss of DmGSTO1B function. Because DHAR catalyzes the conversion of dehydroascorbate (DHA) to ascorbate (AsA) using glutathione as a reducing agent, we investigated the level of DHA and AsA in the mutant flies. As shown in Fig. 1B, only the overexpression of DmGSTO1B rescued the AsA/DHA ratio in DmGSTO1null mutants. These results indicate that DmGSTO1B has a protective role in response to paraquat-induced stress and plays an important role in the in vivo conversion of DHA to AsA.
FIGURE 1.
Sensitivity of DmGSTO1 mutants to oxidative stress. A, survival rates of paraquat-treated (20 mm) flies overexpressing DmGSTO1A, and DmGSTO1B under the control of Tubulin-Gal4, a ubiquitously expressed driver (n ≥ 80). DmGSTO1null mutants were sensitive to oxidative stress. Overexpression of DmGSTO1B in a DmGSTO1null mutant background reduced sensitivity to treatment with paraquat. Error bars, S.D. The significance was determined by one-way ANOVA (**, p < 0.05; *, p < 0.01). B, ascorbic acid, and dehydroascorbic acid content in fly extracts. The level of AsA was lower in DmGSTO1null flies than in WT flies, whereas DHA was higher in DmGSTO1null flies than in WT flies. The AsA/DHA ratio decreased from 7.29 in WT to 3.28 in DmGSTO1null. Overexpression of DmGSTO1B in a DmGSTO1null mutant background restored the ratio of AsA/DHA to 6.35. Error bars, S.D. Experimental significance was determined by one-way ANOVA (**, p < 0.01; *, p < 0.05). Experiments were performed in triplicate.
DmGSTO1 Partially Rescues park1 Mutant Phenotypes
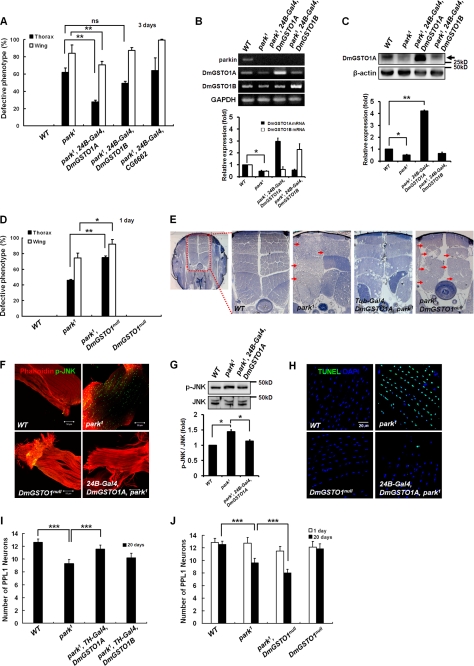
Although it has been reported that GSTs are involved in neurodegenerative disease (13, 28, 29), the molecular function of GSTOs remains unknown. To investigate the biological function of DmGSTO1 in PD, we conducted genetic studies with park1 mutants. As reported previously (18, 19), park1 mutants showed collapsed thorax and downturned wing phenotypes. Surprisingly, increasing DmGSTO1A expression using the muscle-specific 24B-Gal4 driver significantly suppressed both thorax and wing phenotypes in park1 mutants, whereas increased DmGSTO1B expression showed no effects (Fig. 2A and supplemental Fig. S2, A and B). Of the GSTO genes, only the transcriptional expression of DmGSTO1, (CG6673) was decreased in park1 mutants (Fig. 2B and supplemental Fig. S1D). Because the level of DmGSTO1 mRNA decreased in park1 mutants (Fig. 2B), we investigated whether the protein levels were also decreased in park1 mutants. We generated a specific antibody against DmGSTO1A. As shown in Fig. 2C, the DmGSTO1A protein level was dramatically decreased in park1 mutants compared with wild type flies. These results indicate that parkin regulates transcriptional expression of the DmGSTO1 gene. We further examined the genetic interactions between parkin and DmGSTO1 by introducing a DmGSTO1 null mutation in the park1 mutants. We found that the loss of function of DmGSTO1 further enhanced the downturned wing and collapsed thorax phenotypes in the one-day-old park1 mutants (Fig. 2D). In the DmGSTO1null mutant, neither DmGSTO1 nor CG6662 transcripts were detected (supplemental Fig. S1B). We could eliminate the possibility that CG6662 played a role in suppressing the park1 mutant phenotype by demonstrating that CG6662 overexpression in park1 mutants had no effect on the park1 mutant phenotype (Fig. 2A). These results indicate that CG6662 is not involved in the suppression of parkin mutant phenotypes.
FIGURE 2.
Up-regulation of DmGSTO1 suppresses phenotypes caused by parkin loss of function. A, statistical analysis of the percentage of collapsed thorax (n > 120) and downturned wing (n > 90) phenotypes in 3-day-old flies. DmGSTO1A overexpression by the 24B-Gal4 muscle-specific driver suppresses the thorax and wing defects of parkin mutant flies. Experimental significance was determined by one-way ANOVA (**, p < 0.05; ns, not significant). B, DmGSTO1 mRNA levels determined by RT-PCR were also reduced in park1 mutants. Error bars, S.D. Significance was determined by one-way ANOVA (*, p < 0.01). Experiments were performed in triplicate. C, endogenous DmGSTO1A levels (black arrow) were dramatically reduced in park1 mutants. Error bars, S.D. The significance was determined by one-way ANOVA (**, p < 0.0001; *, p < 0.01). D, percentage of collapsed thorax (n > 300) and downturned wing (n > 110) phenotypes in 1-day-old park1/DmGSTO1null double mutants. The DmGSTO1 mutation enhanced the thorax and wing defects exhibited by parkin mutants. Error bars, S.D. Statistical analysis was carried out with one-way ANOVA (**, p < 0.001; *, p < 0.05). E, light microscopy was used to examine IFM morphology (red arrow, muscle degeneration). Shown are magnified views of the dorsal longitudinal muscle (×200). Tubulin-Gal4-driven DmGSTO1A expression rescues muscle degeneration of park1 mutant flies. The park1/DmGSTO1null double mutants showed enhanced degeneration of muscles compared with park1 single mutants. F, muscle-specific expression of DmGSTO1A in park1 mutants reduced phosphorylated JNK. Activated JNK (phospho-JNK; p-JNK) is visualized in green, and phalloidin-labeled muscle tissues are shown in red. G, Western blot analysis of phospho-JNK. Error bars, S.D. Experimental significance was determined by one-way ANOVA (*, p < 0.05). Experiments were performed in triplicate. H, merged images of apoptotic cells (TUNEL, green) and nuclei (DAPI, blue). Up-regulation of DmGSTO1A resulted in fewer apoptotic cells in the muscle. I and J, quantification of TH-positive neurons in PPL1 clusters in 1- and 20-day-old flies (n > 10). Up-regulation of DmGSTO1A by the TH-Gal4 DA neuron-specific driver can rescue DA neuron loss in parkin mutants. Error bars, S.D. Experimental significance was determined by one-way ANOVA (***, p < 0.0001).
The downturned wing and thorax disruption phenotypes of parkin mutant flies are caused by the degeneration of the IFMs (21, 30). Therefore, we investigated whether DmGSTO1 prevented muscle degeneration in park1 mutants. As determined by histological analysis of thoracic IFMs, the integrity of IFMs in dorsal longitudinal muscles, which regulate adult wing posture, was clearly disrupted in park1 mutants (Fig. 2E). Although DmGSTO1null mutants showed a normal muscle phenotype, park1/DmGSTO1null double mutants showed dramatically enhanced degeneration of IFMs compared with park1 single mutants (Fig. 2E). Furthermore, overexpression of DmGSTO1A using a ubiquitous driver, Tub-Gal4, suppressed the degeneration of IFMs in park1 mutants. Overexpression of DmGSTO1A in park1 mutants resulted in regular and compact muscle tissues in the dorsal longitudinal IFMs, which were similar to those of wild type flies except for occasional vacuoles (Fig. 2E). These data suggest that DmGSTO1 expression partially rescues the morphological defects and muscle degeneration in park1 mutants.
Phospho-JNK Signal and Apoptosis Are Suppressed by DmGSTO1 in park1 Mutants
Many studies have suggested that neuronal cell death in various neurodegenerative diseases is closely related to JNK activation. Cha et al. (18) reported that parkin inhibits the JNK pathway. As shown in Fig. 2F, phospho-JNK was dramatically increased in IFMs of the park1 mutant, whereas there was no change in phospho-JNK in the DmGSTO1null mutant compared with wild type. The expression of DmGSTO1A suppressed activation of phospho-JNK in park1 mutants (Fig. 2F), and the degree of suppression was confirmed by Western blot analysis (Fig. 2G). Degeneration of IFMs in parkin mutants occurs through an apoptotic mechanism (30). The IFMs in park1 mutants were examined by a terminal deoxynucleotidyltransferase-mediated dUTP end labeling (TUNEL) assay. As shown in Fig. 2H, the increased TUNEL signal in park1 mutants was suppressed by DmGSTO1A expression, similar to the suppression in the phospho-JNK signal. These data suggest that DmGSTO1 prevents degeneration of IFMs by blocking the activation of JNK and apoptosis in park1 mutants.
DmGSTO1 Suppresses Dopaminergic Neuronal Degeneration in park1 Mutants
park1 mutants show an age-dependent degeneration of DA neurons, especially in the protocerebral posterior lateral 1 (PPL1) cluster (18, 31). To further clarify the effect of DmGSTO1 on park1 mutants, we examined the degeneration of DA neurons that is characteristic of PD. As shown in Fig. 2I, DmGSTO1A expression under the control of a DA neuron-specific tyrosine hydroxylase (TH)-Gal4 driver resulted in significant restoration of the lost DA neurons in 20-day-old park1 mutant flies. In addition, 20-day-old park1/DmGSTO1null double mutants showed significantly increased DA neuron degeneration in the PPL1 cluster compared with the park1 single mutants of the same age, whereas there was no difference in neuronal degeneration between park1/DmGSTO1null double mutants and park1 single mutants in 1-day-old flies (Fig. 2J). These results indicate that DmGSTO1 protects the DA neurons from age-dependent degeneration in park1 mutants.
DmGSTO1 Restores Accumulation of Tubulin in IFMs in park1 Mutants
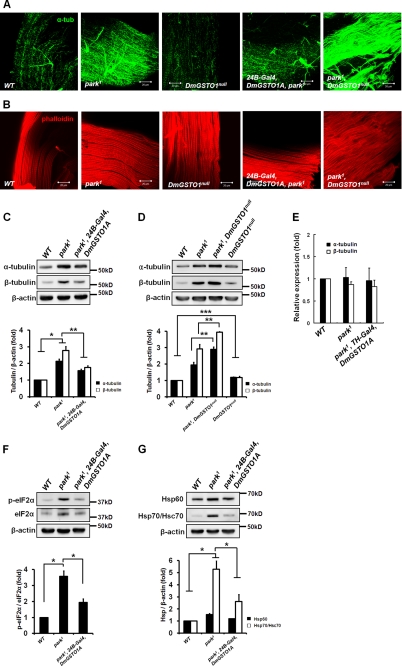
Parkin functions as an E3 ubiquitin ligase and has important roles in the degradation of many substrates (32, 33). Recent in vitro studies demonstrated that parkin binds to microtubule and tubulin proteins with high affinity and that parkin ubiquitinates and promotes the degradation of α/β-tubulin (34). It remains controversial whether putative in vitro substrates are relevant in vivo. Therefore, we investigated the α/β-tubulin protein levels in park1 mutants in vivo. Levels of α-tubulin were increased in park1 mutant muscles (Fig. 3A). Interestingly, the accumulation of α-tubulin in park1 mutant muscles was dramatically reduced by DmGSTO1A expression, and it was enhanced in park1/DmGSTO1null double mutant muscles (Fig. 3A). The levels of actin filaments in IFMs were unchanged in all mutants (Fig. 3B). These changes were confirmed by Western blot analysis (Fig. 3, C and D). We found that tubulin was slightly increased in DmGSTO1null mutants by Western blot analysis (Fig. 3D). We also detected tubulin accumulation in DA neurons in park1 mutants and park1/DmGSTO1null double mutants (supplemental Fig. S3). These data indicate that parkin does not directly regulate the level of tubulin in Drosophila in vivo. In contrast to the change in total protein levels (Fig. 3C), there were no detectable changes in the α/β-tubulin transcript levels in park1 mutants regardless of DmGSTO1A expression level (Fig. 3E). Thus, the increased α/β-tubulin levels were not caused by increased transcription but by protein accumulation. These results indicate that parkin is required for the regulation of tubulin levels and that DmGSTO1 suppresses the accumulation of tubulin in park1 mutants.
FIGURE 3.
Up-regulation of DmGSTO1 in muscle decreases tubulin accumulation and UPR activation in park1 mutants. Shown are representative images of flight muscle stained with anti-α-tubulin antibody and phalloidin. A, α-tubulin accumulates in park1 mutant muscle. The accumulation of tubulin in the park1 mutant was suppressed by the muscle-specific up-regulation of DmGSTO1A. B, the levels of actin filaments in muscle did not change in any mutants as visualized with phalloidin. C, the increased levels of α/β-tubulin in park1 mutants were rescued by DmGSTO1A expression. Error bars, S.D. The experimental significance was determined by one-way ANOVA (**, p < 0.05; *, p < 0.01). D, tubulin levels were higher in the double mutants than in the park1 single mutants. Error bars, S.D. The significance was determined by one-way ANOVA (***, p < 0.01; **, p < 0.05). E, mRNA levels of male thoraces were determined by real-time PCR analysis. Relative amounts of tubulin mRNA were unchanged in all mutants. F, Western blot analysis of phosphorylated and total eIF2α. G, increased HSPs in park1 mutants were decreased with the up-regulation of DmGSTO1A. F and G, error bars, S.D. The significance was determined by one-way ANOVA (*, p < 0.05). β-Actin was used as a loading control. Experiments were performed in triplicate.
DmGSTO1 Suppresses Activation of Unfolded Protein Response (UPR) in park1 Mutant Muscles
Many studies have claimed that ER stress is involved in the progression of neurodegenerative diseases, such as PD. The UPR is a signaling pathway that is activated in response to ER stress. UPR activation has been observed in DA neurons of PD patients and is exemplified by an increase in phospho-PERK and eIF2α (35). To determine whether the parkin mutation induces UPR activation, we examined eIF2α, which is one of the components of the UPR signaling pathway and is activated by the upstream kinase PERK (36). The level of active phospho-eIF2α was highly increased in park1 mutants and dramatically restored by DmGSTO1A expression (Fig. 3F). The ER and mitochondrial UPR share similar pathways that increase chaperone levels to promote protein homeostasis in the cytoplasm and mitochondria (37). We also examined heat shock proteins (HSPs), including Hsp60, and Hsp/Hsc70. As shown in Fig. 3G, the levels of HSPs were increased in park1 mutants and reduced by DmGSTO1A expression. These data indicate that DmGSTO1 suppresses UPR activation in park1 mutants.
DmGSTO1 Restores Mitochondrial ATP Synthase Activity in park1 Mutants
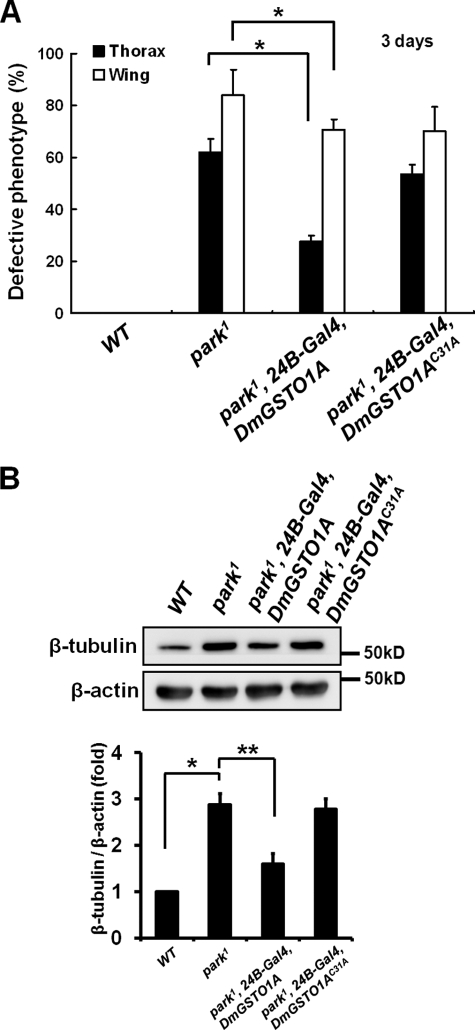
We next examined whether the catalytic activity of DmGSTO1A was critical for the rescue the park1 mutant phenotypes. GSTOs have a unique cysteine at their active site that binds to GSH (27). We constructed a catalytically inactive form of DmGSTO1A, DmGSTO1AC31A, in which cysteine 31 was mutated to alanine. In contrast to wild type DmGSTO1A, expression of DmGSTO1AC31A in muscle did not rescue the collapsed thorax and downturned wing phenotypes in park1 mutants (Fig. 4A). Moreover, tubulin accumulation was not suppressed by DmGSTO1AC31A expression in park1 mutants (Fig. 4B). Our data demonstrate that DmGSTO1 catalytic activity is required to rescue the defective phenotypes of park1 mutants.
FIGURE 4.
The catalytic activity of DmGSTO1 is critical for rescue of the defective phenotypes of the park1 mutant. A, expression of DmGSTO1AC31A in the park1 mutant background did not suppress the collapsed thorax (n > 200) and downturned wing (n > 160) phenotypes of parkin mutants. Error bars, S.D. The experimental significance was determined by one-way ANOVA (*, p < 0.05). B, Western blot analysis of adult thorax extracts using anti-β-tubulin antibodies. DmGSTO1AC31A expressed in the park1 mutants did not rescue the tubulin accumulation phenotypes. Error bars, S.D. The significance was determined by one-way ANOVA (**, p < 0.05; *, p < 0.01). β-Actin was used as a loading control. Experiments were performed in triplicate.
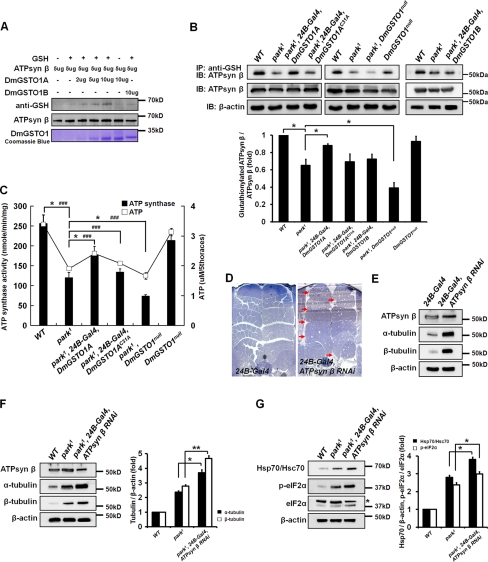
Although the ability of DmGSTO1B to respond to oxidative stress was higher than that of DmGSTO1A, only up-regulation of DmGSTO1A suppressed the defective phenotypes in parkin mutants (Figs. 1A and 2A). Therefore, DmGSTO1A may suppress parkin mutant phenotypes by other mechanisms. The catalytic detoxification functions of the GST family have been studied by several research groups. However, some members of the GST family have physiological functions unrelated to detoxification (38). Previous reports revealed that the rate of protein glutathionylation, a post-translational modification that regulates the function of proteins, is enhanced by the presence of active GSTP (GST Pi family) (39). Based on the result that DmGSTO1 enzyme activity is required to suppress the parkin mutant phenotypes, we screened in vivo targets of DmGSTO1 by immnunoprecipitation with an anti-GSH antibody in adult thorax extracts. Only one glutathionylated protein showed altered abundance in park1 mutants. DmGSTO1A expression increased the level of glutathionylation of this protein, whereas DmGSTO1AC31A did not. The protein was identified by MALDI-TOF mass spectrometric analysis as the mitochondrial F1F0-ATP synthase β subunit. We also confirmed that DmGSTO1A can directly glutathionylate the ATP synthase β subunit in a dose-dependent manner in the presence of GSH (Fig. 5A). Furthermore, the endogenous levels of the glutathionylated form of the ATP synthase β subunit in thorax extracts were decreased in park1 mutants and decreased even more in park1/DmGSTO1null double mutants, whereas the total expression levels of the ATP synthase β subunit were unchanged in all fly lines. DmGSTO1A expression in park1 mutants restored the levels of glutathionylated ATP synthase β subunit to wild type levels, whereas the expression of DmGSTO1AC31A or DmGSTO1B in park1 mutants had no effect on the level of ATP synthase β subunit glutathionylation (Fig. 5B). However, in the DmGSTO1null mutant, the level of glutathionylation of the ATP synthase β subunit was only slightly decreased compared with control. This finding suggests that a compensatory mechanism related to glutathionylation of the ATP synthase β subunit exists. Another DmGSTO, CG6662, did not affect glutathionylation of the ATP synthase β subunit (supplemental Fig. S4). These data indicate that the ATP synthase β subunit is a novel and specific target of DmGSTO1A in Drosophila and that only DmGSTO1A expression in park1 mutants is sufficient to partially restore glutathionylation of the ATP synthase β subunit.
FIGURE 5.
DmGSTO1 partially restored mitochondrial F1F0-ATP synthase activity in park1 mutants. A, in the presence of GSH, recombinant ATP synthase β subunit was glutathionylated by DmGSTO1A in a dose-dependent manner. B, glutathionylated proteins were immunoprecipitated from thorax extracts with an anti-GSH antibody and were immunoblotted with an anti-ATP synthase β antibody. Glutathionylation of endogenous ATP synthase β subunit in park1 mutants was regulated by the GSH-conjugating catalytic activity of DmGSTO1A but not by DmGSTO1B. The endogenous levels of the glutathionylated form of the ATP synthase β subunit were decreased even more in park1/DmGSTO1null double mutants. Error bars, S.D. The experimental significance was determined by one-way ANOVA (*, p < 0.05). C, mitochondrial F1F0-ATP synthase activity and ATP levels in Drosophila thoraces. DmGSTO1A expression enhances ATP synthase activity and ATP level of park1 mutants. Error bars, S.D. One-way ANOVA was used for statistical analysis (ATP synthase activity: *, p < 0.05; ATP level: ###, p < 0.001). D, muscle morphology of wild type and ATP synthase β subunit RNAi mutant flies (arrow, muscle degeneration). Muscle-specific ATP synthase β subunit RNAi resulted in degeneration of the flight muscles. E, Western blot analysis of adult thorax extracts from the ATP synthase β subunit RNAi mutant flies. α- and β-tubulin showed significant accumulation in the RNAi mutant muscle. F, Western blot analysis of adult thorax extracts from the ATP synthase β subunit RNAi in a park1 mutant background. Accumulation of tubulin was increased more in the RNAi mutants than in the park1 single mutants. Error bars, S.D. The significance was determined by one-way ANOVA (**, p < 0.01; *, p < 0.05). G, Western blot analysis of Hsp70/Hsc70, phosphorylated eIF2α, and total eIF2α (*, nonspecific band). Error bars, S.D. The significance was determined by one-way ANOVA (*, p < 0.01). Experiments were performed in triplicate.
Glutathionylation is a reversible post-translational modification that can lead to alteration of protein or enzyme function, such as the Ca2+ uptake activity of the sarco-/endoplasmic reticulum calcium ATPase (40). To test the hypothesis that glutathionylation of the ATP synthase β subunit by DmGSTO1A in park1 mutants regulates ATP synthase activity, we isolated mitochondria from the thorax and measured F1F0-ATP synthase activity. As shown in Fig. 5C, the level of mitochondrial F1F0-ATP synthase activity was partially rescued by DmGSTO1A expression in park1 mutants and was decreased in park1/DmGSTO1null double mutants. Indeed, the change in mitochondrial F1F0-ATP synthase activity was correlated to the change in glutathionylation of the ATP synthase β subunit. Consistent with the ATP synthase activity results, we observed a change in the total ATP levels in all of the mutant lines (Fig. 5C). Impaired mitochondrial respiration caused by decreased ATP production has been reported in PD, and agents that improve mitochondrial respiration can exert beneficial effects in animal models of PD (2). These reports are consistent with our findings; ATP synthase activity and ATP levels are significantly decreased in park1 mutants and can be modulated by DmGSTO1 expression.
Decreased ATP Synthase β Subunit in Drosophila Muscle Leads to park1 Mutant-like Phenotype
Mutations of ATP synthase subunits are known to exhibit mitochondrial dysfunction and neuromuscular impairment (41, 42). To determine whether decreased ATP synthase activity was directly linked to the phenotypes found in parkin mutants, we down-regulated the ATP synthase β subunit in Drosophila thorax muscle using a UAS-RNAi line (obtained from the NIG-FLY stock center) together with the muscle-specific 24B-Gal4 driver. Because the ATP synthase β subunit knockdown flies reared at 25 °C displayed pupal lethality, we performed all knockdown experiments at 18 °C to increase adult viability. When ATP synthase β subunit knockdown was induced at 18 °C in muscle using 24B-Gal4, fewer than 20% of the flies were able to eclose. We observed abnormal muscle structure in the knockdown mutant flies, similar to the phenotypes found in parkin mutants (Fig. 5D). Moreover, the ATP synthase β subunit RNAi flies showed accumulation of total α/β-tubulin (Fig. 5E). These results demonstrate that loss of the ATP synthase β subunit induces some of the parkin mutant phenotypes, including muscle degeneration and accumulation of tubulin.
Next, to examine whether the ATP synthase β subunit RNAi enhances the parkin mutant phenotype, the ATP synthase β subunit was knocked down in park1 mutant muscles. As shown in Fig. 5F, we observed an increase in α/β-tubulin accumulation. Moreover, the levels of Hsp70/Hsc70 and phosphorylated eIF2α in the park1 mutant were further increased by RNAi knockdown of the ATP synthase β subunit (Fig. 5G). These data indicate that down-regulation of ATP synthase activity in the park1 mutant results in a more severe phenotype compared with that of park1 single mutants.
DmGSTO1 Rescues Mitochondrial ATP Synthase Assembly in park1 Mutants
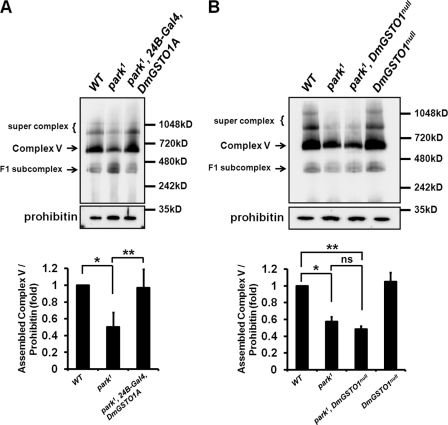
To investigate how expression of DmGSTO1A affects ATP synthase activity, we examined the assembly level of the F1F0-ATP synthase complex (Complex V) by blue native PAGE and Western blot analysis with an anti-ATP synthase α subunit antibody. Three bands (supercomplex, >800 kDa; assembled ATP synthase, Complex V, >600 kDa; F1 subcomplex, >400 kDa) were detected (Fig. 6). All three bands associated with the mitochondrial F1F0-ATP synthase complex were dramatically decreased by RNAi knockdown of the ATP synthase β subunit (supplemental Fig. S5). The amount of assembled ATP synthase was normalized using mitochondrial prohibitin. In comparison with the wild type, the amount of assembled ATP synthase complex was decreased in park1 mutants. Expression of DmGSTO1A in park1 mutants increased the amount of assembled ATP synthase complex (Fig. 6A, Complex V). Interestingly, park1/DmGSTO1null double mutants tend to exhibit lower amounts of assembled ATP synthase than those in park1 mutants, although the effect was not statistically significant (Fig. 6B). Blue native electrophoresis analysis is not sensitive to small changes; nevertheless, the change in the amount of assembled ATP synthase complex correlated to the change in ATP synthase activity. These results indicate that DmGSTO1A affects mitochondrial ATP synthase activity by regulating the assembly efficiency of ATP synthase in park1 mutants.
FIGURE 6.
Mitochondrial F1F0-ATP synthase (Complex V) assembly is affected by DmGSTO1 expression in park1 mutants. A and B, mitochondrial protein extracts from the thorax of mutant fly lines were subjected to blue native PAGE, followed by Western blot analysis with anti-ATP synthase α subunit antibody. Three bands were detected: supercomplex (>800 kDa), assembled ATP synthase (Complex V, >600 kDa), and F1 subcomplex (>400 kDa). Prohibitin was used as a mitochondrial loading control. A, the assembly of ATP synthase (Complex V) was significantly decreased in park1 mutants. The amount of assembled ATP synthase was restored by DmGSTO1A up-regulation in park1 mutants. Error bars, S.D. The experimental significance was determined by one-way ANOVA (**, p < 0.01; *, p < 0.0001). Experiments were performed in quintuplicate. B, The amount of assembled ATP synthase tended to decrease more in park1/DmGSTO1null double mutants than park1 mutants; however, the effect was not statistically significant. Error bars, S.D. The experimental significance was determined by one-way ANOVA (**, p < 0.0001; *, p < 0.001; ns, not significant). Experiments were performed in quadruplicate.
DISCUSSION
In this study, we suggest that DmGSTO1 is a novel genetic suppressor of parkin dysfunction and has a protective role in a model of PD. Moreover, we showed that the ATP synthase β subunit is a novel target of DmGSTO1 in Drosophila. The ATP synthase β subunit is an essential catalytic core component of the F1F0-ATP synthase complex in mitochondria. We found that levels of glutathionylation of the ATP synthase β subunit were significantly decreased in park1 mutants, whereas its total protein level remained unchanged. Glutathionylation is a mechanism of post-translational regulation of several proteins, including protein-tyrosine phosphatase 1B (PTP1B), and MEKK1. The glutathionylation of a calcium channel, ryanodine receptor 1 (RyR1), activates it and enhances calcium release (43). Our results suggest that expression of DmGSTO1A in park1 mutants increases the level of glutathionylated ATP synthase β subunit and restores mitochondrial F1F0-ATP synthase activity, thereby partially rescuing park1 mutant phenotypes, including the degeneration of DA neurons.
The increase in F1F0-ATP synthase activity and assembly by DmGSTO1A in the park1 mutant led to a recovery of ATP depletion. Interestingly, because mitochondrial F1F0-ATP synthase has a role in maintaining inner membrane morphology, and mitochondrial membrane potential (41, 44) and mutation of F1F0-ATP synthase ϵ subunit can cause mitochondrial dysfunction (42), the restoration of F1F0-ATP synthase activity in the park1 mutant is important for rescuing the parkin mutant phenotypes. Moreover, RNAi mutants of the ATP synthase β subunit exhibit phenotypes similar to park1 mutants, including α/β-tubulin accumulation, locomotor dysfunction, UPR activation, and muscle degeneration.
Although the change in mitochondrial F1F0-ATP synthase activity and ATP levels correlated with the degree of glutathionylation of the ATP synthase β subunit, the exact regulatory mechanism between glutathionylation of the ATP synthase β subunit and ATP synthase activity is not known. It is technically difficult to show a direct relationship between glutathionylation of the ATP synthase β subunit and ATP synthase activity because in Drosophila, mitochondrial ATP synthase is a large multiprotein complex composed of eight different subunits. Further studies will be required to determine how glutathionylation of the ATP synthase β subunit regulates mitochondrial ATP synthase activity and assembly at the molecular level.
However, the loss-of-function mutant DmGSTO1null exhibited no obvious morphological defects and slightly reduced glutathionylation of the ATP synthase β subunit and ATP synthase activity. It is not clear why DmGSTO1null single mutants show a weak effect on the glutathionylation and activity of ATP synthase. One possible explanation is that compensatory mechanisms related to glutathionylation of the ATP synthase β subunit exist in vivo.
Although our current study focused on the specific target of GSTO related to neurodegeneration, previous studies have shown that GSTs have protective functions against oxidative stress in neurodegenerative diseases. Loss of function of the yeast GSTS1 homolog, gtt-1, enhances α-synuclein toxicity (45), and mouse GST Pi contributes to the sensitivity to xenobiotics in idiopathic PD (29). Additionally, increased GST Pi expression protected cells from rotenone-induced neurotoxicity (46). However, the various roles of GSTs remain controversial because of the diversity and complexity of these proteins. Because the expression levels of several GSTs increase due to oxidative stress, we measured endogenous DmGSTO1A expression in park1 mutants and found that the protein and mRNA levels of DmGSTO1A were significantly lowered in park1 mutants. Transcriptional profiling of parkin mutants showed that the oxidative stress response genes are up-regulated, and overexpression of GSTS1 in DA neurons suppressed neurodegeneration in parkin mutants (11, 12). DmGSTS1 was also up-regulated by the 4E-BP1-mediated stress response (47). Therefore, DmGSTS1 in park1 mutants may be increased in an effort to decrease the stress induced by the parkin mutation, but its increased level is not sufficient to rescue the park1 phenotype. Both DmGSTO1 mRNA and protein levels were decreased in park1 mutants, suggesting that the normal function of parkin is critical for the regulation of DmGSTO1 expression. Little is known about the factors regulating DmGSTO1 gene expression, but we can make the following speculation. Because the DmGSTO1 gene contains a potential NF-κB-like transcription factor binding motif (48), and parkin stimulates NF-κB-dependent transcription (49), DmGSTO1 may be down-regulated in the park1 mutant. Thus, the mechanism underscoring the protective role of DmGSTO1 in park1 mutants is distinct from the mechanisms used by general antioxidants and the detoxifying enzyme DmGSTS1. Furthermore, GSTO1 has an active site cysteine residue, which is distinct from the tyrosine residue found in GSTS1. This cysteine residue could enable GSTO1 to modulate the disulfide status of cysteine residues on substrate proteins (27). Although the specific substrates of individual GSTs remain unknown, the functional diversity of GSTs might explain why they have different substrates against various stresses in vivo.
DmGSTO1B more strongly protects against oxidative stress than DmGSTO1A, but only the up-regulation of DmGSTO1A improved the defective phenotypes in parkin mutants. Although DHAR activity and the reduced form of ascorbate have protective roles under oxidative stress conditions (50), they are not sufficient to rescue parkin mutant phenotypes. As shown in Fig. 5, A and B, DmGSTO1B was not able to glutathionylate the ATP synthase β subunit. Thus, we propose that these two isoforms, DmGSTO1A and DmGSTO1B, have different substrates and act differently on the stress response pathway in vivo.
Mitochondrial defects have been detected in PD cases with parkin mutations. Recent studies suggest that parkin promotes mitochondrial fission and/or inhibits mitochondrial fusion in muscle tissues and DA neurons (51–53). Staining mitochondria with streptavidin revealed severe defects in mitochondrial integrity in the IFMs of park1 mutants. Interestingly, these defects were not restored by DmGSTO1A expression, and park1/DmGSTO1null double mutants were not distinguishable from park1 single mutants (supplemental Fig. S6). These findings were further confirmed by expressing mito-GFP in thorax muscles (supplemental Fig. S6). In contrast to the park1 mutants, the ATP synthase β subunit RNAi mutants did not show disrupted mitochondrial morphology (supplemental Fig. S6). These results suggest that DmGSTO1 is not important for restoring mitochondrial morphology in park1 mutants and might act downstream or in parallel to the mitochondrial dynamics pathway.
PTEN-induced putative kinase 1 (PINK1) is a Ser/Thr kinase containing a mitochondrial targeting motif (54). Previous studies reported that mutations of PINK1 lead to muscle degeneration, DA neuron loss, and mitochondrial dysfunction. PINK1 and parkin are linked in the same pathway, and PINK1 acts upstream of parkin (19, 51–53, 55). We therefore hypothesized that DmGSTO1A would genetically interact with the PINK1 null mutant PINK1B9. Interestingly, consistent with park1 mutants, we found that DmGSTO1 and parkin mRNA were decreased in PINK1B9 mutants (supplemental Fig. S7A). Glutathionylation of the mitochondrial ATP synthase β subunit was significantly decreased in PINK1B9 mutants (supplemental Fig. S7B). Therefore, it seems possible that DmGSTO1A up-regulation may also contribute to the prevention of degeneration in PINK1B9 mutants. A detailed study of PINK1B9 mutants is currently under way in our group.
Our results support the hypothesis that DmGSTO1 is linked to the pathogenic phenotypes displayed in parkin mutants. We found that DmGSTO1 is a novel genetic suppressor of parkin dysfunction. The two isoforms of DmGSTO1 have different functions. DmGSTO1B in Drosophila is required to protect the flies against oxidative stress. Although the exact molecular mechanism is not clear, glutathionylation of the ATP synthase β subunit by DmGSTO1A regulates mitochondrial F1F0-ATP synthase activity, and the restoration of ATP synthase activity by DmGSTO1A expression is critically important for partial rescue of the mitochondrial function in park1 mutants. These findings present a novel mechanism of regulation of ATP synthase by DmGSTO1 in parkin mutants. Our results strongly suggest that promoting DmGSTO1 activity could alleviate neurodegeneration in parkin mutants. These findings will lead us to a better understanding of the molecular mechanism of neuroprotection due to GSTO in PD and could help in developing new therapeutic approaches for PD.
Supplementary Material
Acknowledgments
We thank Drs. J. Chung, S. Birman, H. J. Bellen, the Bloomington Stock Center, the KAIST GenExel Drosophila library, and the NIG-FLY Stock Center for Drosophila stocks. We also thank Dr. Rafael Garesse for providing the rabbit anti-Drosophila ATP synthase β subunit antibody.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by Ministry of Education, Science, and Technology (MEST) Grants KRF-2008-313-E00068 and 2011-0004860 and by Brain Korea 21 Research Fellowships from the Ministry of Education, Science, and Technology of Korea.

This article contains supplemental Figs. S1–S7.
- PD
- Parkinson disease
- UAS
- upstream activation sequence
- IFM
- indirect flight muscle
- DA
- dopaminergic
- UPR
- unfolded protein response
- ANOVA
- analysis of variance
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Dawson T. M., Dawson V. L. (2003) Molecular pathways of neurodegeneration in Parkinson's disease. Science 302, 819–822 [DOI] [PubMed] [Google Scholar]
- 2. Abou-Sleiman P. M., Muqit M. M., Wood N. W. (2006) Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat. Rev. Neurosci. 7, 207–219 [DOI] [PubMed] [Google Scholar]
- 3. Hayes J. D., Flanagan J. U., Jowsey I. R. (2005) Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 45, 51–88 [DOI] [PubMed] [Google Scholar]
- 4. Sheehan D., Meade G., Foley V. M., Dowd C. A. (2001) Structure, function, and evolution of glutathione transferases. Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 360, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dulhunty A., Gage P., Curtis S., Chelvanayagam G., Board P. (2001) The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J. Biol. Chem. 276, 3319–3323 [DOI] [PubMed] [Google Scholar]
- 6. Laliberte R. E., Perregaux D. G., Hoth L. R., Rosner P. J., Jordan C. K., Peese K. M., Eggler J. F., Dombroski M. A., Geoghegan K. F., Gabel C. A. (2003) Glutathione S-transferase Omega 1-1 is a target of cytokine release-inhibitory drugs and may be responsible for their effect on interleukin-1β posttranslational processing. J. Biol. Chem. 278, 16567–16578 [DOI] [PubMed] [Google Scholar]
- 7. Yin S., Li X., Meng Y., Finley R. L., Jr., Sakr W., Yang H., Reddy N., Sheng S. (2005) Tumor-suppressive maspin regulates cell response to oxidative stress by direct interaction with glutathione S-transferase. J. Biol. Chem. 280, 34985–34996 [DOI] [PubMed] [Google Scholar]
- 8. Kim J., Suh H., Kim S., Kim K., Ahn C., Yim J. (2006) Identification and characteristics of the structural gene for the Drosophila eye color mutant sepia, encoding PDA synthase, a member of the Omega class glutathione S-transferases. Biochem. J. 398, 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y. J., Oliveira S. A., Xu P., Martin E. R., Stenger J. E., Scherzer C. R., Hauser M. A., Scott W. K., Small G. W., Nance M. A., Watts R. L., Hubble J. P., Koller W. C., Pahwa R., Stern M. B., Hiner B. C., Jankovic J., Goetz C. G., Mastaglia F., Middleton L. T., Roses A. D., Saunders A. M., Schmechel D. E., Gullans S. R., Haines J. L., Gilbert J. R., Vance J. M., Pericak-Vance M. A., Hulette C., Welsh-Bohmer K. A. (2003) Glutathione S-transferase Omega-1 modifies age-at-onset of Alzheimer disease and Parkinson disease. Hum. Mol. Genet. 12, 3259–3267 [DOI] [PubMed] [Google Scholar]
- 10. Li Y. J., Scott W. K., Zhang L., Lin P. I., Oliveira S. A., Skelly T., Doraiswamy M. P., Welsh-Bohmer K. A., Martin E. R., Haines J. L., Pericak-Vance M. A., Vance J. M. (2006) Revealing the role of glutathione S-transferase Omega in age-at-onset of Alzheimer and Parkinson diseases. Neurobiol. Aging 27, 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greene J. C., Whitworth A. J., Andrews L. A., Parker T. J., Pallanck L. J. (2005) Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Hum. Mol. Genet. 14, 799–811 [DOI] [PubMed] [Google Scholar]
- 12. Whitworth A. J., Theodore D. A., Greene J. C., Benes H., Wes P. D., Pallanck L. J. (2005) Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 102, 8024–8029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trinh K., Moore K., Wes P. D., Muchowski P. J., Dey J., Andrews L., Pallanck L. J. (2008) Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson's disease. J. Neurosci. 28, 465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peña P., Garesse R. (1993) The β subunit of the Drosophila melanogaster ATP synthase. cDNA cloning, amino acid analysis, and identification of the protein in adult flies. Biochem. Biophys. Res. Commun. 195, 785–791 [DOI] [PubMed] [Google Scholar]
- 15. Capaldi R. A., Aggeler R. (2002) Mechanism of the F1F0-type ATP synthase, a biological rotary motor. Trends Biochem. Sci. 27, 154–160 [DOI] [PubMed] [Google Scholar]
- 16. Cross R. L., Müller V. (2004) The evolution of A-, F-, and V-type ATP synthases and ATPases. Reversals in function and changes in the H+/ATP coupling ratio. FEBS Lett. 576, 1–4 [DOI] [PubMed] [Google Scholar]
- 17. Verstreken P., Ly C. V., Venken K. J., Koh T. W., Zhou Y., Bellen H. J. (2005) Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47, 365–378 [DOI] [PubMed] [Google Scholar]
- 18. Cha G. H., Kim S., Park J., Lee E., Kim M., Lee S. B., Kim J. M., Chung J., Cho K. S. (2005) Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc. Natl. Acad. Sci. U.S.A. 102, 10345–10350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park J., Lee S. B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J. M., Chung J. (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 [DOI] [PubMed] [Google Scholar]
- 20. Friggi-Grelin F., Coulom H., Meller M., Gomez D., Hirsh J., Birman S. (2003) Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54, 618–627 [DOI] [PubMed] [Google Scholar]
- 21. Pesah Y., Pham T., Burgess H., Middlebrooks B., Verstreken P., Zhou Y., Harding M., Bellen H., Mardon G. (2004) Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development 131, 2183–2194 [DOI] [PubMed] [Google Scholar]
- 22. Hoopfer E. D., Penton A., Watts R. J., Luo L. (2008) Genomic analysis of Drosophila neuronal remodeling. A role for the RNA-binding protein Boule as a negative regulator of axon pruning. J. Neurosci. 28, 6092–6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matyunina L. V., Bowen N. J., McDonald J. F. (2008) LTR retrotransposons and the evolution of dosage compensation in Drosophila. BMC Mol. Biol. 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosing J., Harris D. A., Kemp A., Jr., Slater E. C. (1975) Nucleotide-binding properties of native and cold-treated mitochondrial ATPase. Biochim. Biophys. Acta 376, 13–26 [DOI] [PubMed] [Google Scholar]
- 25. Sørensen J. G., Nielsen M. M., Kruhøffer M., Justesen J., Loeschcke V. (2005) Full genome gene expression analysis of the heat stress response in Drosophila melanogaster. Cell Stress Chaperones 10, 312–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walters K. B., Grant P., Johnson D. L. (2009) Evolution of the GST Omega gene family in 12 Drosophila species. J. Hered. 100, 742–753 [DOI] [PubMed] [Google Scholar]
- 27. Board P. G., Coggan M., Chelvanayagam G., Easteal S., Jermiin L. S., Schulte G. K., Danley D. E., Hoth L. R., Griffor M. C., Kamath A. V., Rosner M. H., Chrunyk B. A., Perregaux D. E., Gabel C. A., Geoghegan K. F., Pandit J. (2000) Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 275, 24798–24806 [DOI] [PubMed] [Google Scholar]
- 28. Stroombergen M. C., Waring R. H. (1999) Determination of glutathione S-transferase Mu and Theta polymorphisms in neurological disease. Hum. Exp. Toxicol. 18, 141–145 [DOI] [PubMed] [Google Scholar]
- 29. Smeyne M., Boyd J., Raviie Shepherd K., Jiao Y., Pond B. B., Hatler M., Wolf R., Henderson C., Smeyne R. J. (2007) GST Pi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc. Natl. Acad. Sci. U.S.A. 104, 1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greene J. C., Whitworth A. J., Kuo I., Andrews L. A., Feany M. B., Pallanck L. J. (2003) Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. U.S.A. 100, 4078–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang C., Lu R., Ouyang X., Ho M. W., Chia W., Yu F., Lim K. L. (2007) Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities. J. Neurosci. 27, 8563–8570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y., Gao J., Chung K. K., Huang H., Dawson V. L., Dawson T. M. (2000) Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc. Natl. Acad. Sci. U.S.A. 97, 13354–13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., Suzuki T. (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 25, 302–305 [DOI] [PubMed] [Google Scholar]
- 34. Ren Y., Zhao J., Feng J. (2003) Parkin binds to α/β tubulin and increases their ubiquitination and degradation. J. Neurosci. 23, 3316–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoozemans J. J., van Haastert E. S., Eikelenboom P., de Vos R. A., Rozemuller J. M., Scheper W. (2007) Activation of the unfolded protein response in Parkinson's disease. Biochem. Biophys. Res. Commun. 354, 707–711 [DOI] [PubMed] [Google Scholar]
- 36. Smith W. W., Jiang H., Pei Z., Tanaka Y., Morita H., Sawa A., Dawson V. L., Dawson T. M., Ross C. A. (2005) Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant α-synuclein-induced toxicity. Hum. Mol. Genet. 14, 3801–3811 [DOI] [PubMed] [Google Scholar]
- 37. Haynes C. M., Ron D. (2010) The mitochondrial UPR. Protecting organelle protein homeostasis. J. Cell Sci. 123, 3849–3855 [DOI] [PubMed] [Google Scholar]
- 38. Tew K. D., Townsend D. M. (2011) Regulatory functions of glutathione S-transferase P1-1 unrelated to detoxification. Drug Metab. Rev. 43, 179–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Townsend D. M., Manevich Y., He L., Hutchens S., Pazoles C. J., Tew K. D. (2009) Novel role for glutathione S-transferase Pi. Regulator of protein S-glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 284, 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adachi T., Weisbrod R. M., Pimentel D. R., Ying J., Sharov V. S., Schöneich C., Cohen R. A. (2004) S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 10, 1200–1207 [DOI] [PubMed] [Google Scholar]
- 41. Celotto A. M., Frank A. C., McGrath S. W., Fergestad T., Van Voorhies W. A., Buttle K. F., Mannella C. A., Palladino M. J. (2006) Mitochondrial encephalomyopathy in Drosophila. J. Neurosci. 26, 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mayr J. A., Havlícková V., Zimmermann F., Magler I., Kaplanová V., Jesina P., Pecinová A., Nusková H., Koch J., Sperl W., Houstek J. (2010) Mitochondrial ATP synthase deficiency due to a mutation in the ATP5E gene for the F1 ϵ subunit. Hum. Mol. Genet. 19, 3430–3439 [DOI] [PubMed] [Google Scholar]
- 43. Hidalgo C., Sánchez G., Barrientos G., Aracena-Parks P. (2006) A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J. Biol. Chem. 281, 26473–26482 [DOI] [PubMed] [Google Scholar]
- 44. Brown S. V., Hosking P., Li J., Williams N. (2006) ATP synthase is responsible for maintaining mitochondrial membrane potential in bloodstream form Trypanosoma brucei. Eukaryot. Cell 5, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willingham S., Outeiro T. F., DeVit M. J., Lindquist S. L., Muchowski P. J. (2003) Yeast genes that enhance the toxicity of a mutant huntingtin fragment or α-synuclein. Science 302, 1769–1772 [DOI] [PubMed] [Google Scholar]
- 46. Shi M., Bradner J., Bammler T. K., Eaton D. L., Zhang J., Ye Z., Wilson A. M., Montine T. J., Pan C. (2009) Identification of glutathione S-transferase Pi as a protein involved in Parkinson disease progression. Am. J. Pathol. 175, 54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tain L. S., Mortiboys H., Tao R. N., Ziviani E., Bandmann O., Whitworth A. J. (2009) Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat. Neurosci. 12, 1129–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li H. M., Buczkowski G., Mittapalli O., Xie J., Wu J., Westerman R., Schemerhorn B. J., Murdock L. L., Pittendrigh B. R. (2008) Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Insect Mol. Biol. 17, 325–339 [DOI] [PubMed] [Google Scholar]
- 49. Henn I. H., Bouman L., Schlehe J. S., Schlierf A., Schramm J. E., Wegener E., Nakaso K., Culmsee C., Berninger B., Krappmann D., Tatzelt J., Winklhofer K. F. (2007) Parkin mediates neuroprotection through activation of IκB kinase/nuclear factor-κB signaling. J. Neurosci. 27, 1868–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen Z., Young T. E., Ling J., Chang S. C., Gallie D. R. (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. U.S.A. 100, 3525–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deng H., Dodson M. W., Huang H., Guo M. (2008) The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 105, 14503–14508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Poole A. C., Thomas R. E., Andrews L. A., McBride H. M., Whitworth A. J., Pallanck L. J. (2008) The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl. Acad. Sci. U.S.A. 105, 1638–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang Y., Ouyang Y., Yang L., Beal M. F., McQuibban A., Vogel H., Lu B. (2008) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc. Natl. Acad. Sci. U.S.A. 105, 7070–7075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A. R., Healy D. G., Albanese A., Nussbaum R., González-Maldonado R., Deller T., Salvi S., Cortelli P., Gilks W. P., Latchman D. S., Harvey R. J., Dallapiccola B., Auburger G., Wood N. W. (2004) Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 [DOI] [PubMed] [Google Scholar]
- 55. Clark I. E., Dodson M. W., Jiang C., Cao J. H., Huh J. R., Seol J. H., Yoo S. J., Hay B. A., Guo M. (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162–1166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.