Background: TRPC3 channels are inhibited by PKC and PKG, which also induce cerebellar LTD. We investigate if PKC- and PKG-mediated modulation of cerebellar TRPC3 channels contributes to cerebellar LTD.
Results: TRPC3 channel-dependent currents are not significantly modulated by PKC or PKG.
Conclusion: TRPC3 channel modulation is unlikely to be involved in cerebellar LTD.
Significance: TRPC3 channels can be regulated in a cell-specific manner.
Keywords: G protein-coupled receptors (GPCR), Ion Channels, Protein Phosphorylation, Signal Transduction, Synapses, Transient Receptor Potential Channels, Cellular Signaling, Metabotropic Glutamate Receptors, Synaptic Signaling
Abstract
Canonical transient receptor potential (TRPC) channels are widely expressed in the brain and play several roles in development and normal neuronal function. In the cerebellum, Purkinje cell TRPC3 channels underlie the slow excitatory postsynaptic potential observed after parallel fiber stimulation. In these cells TRPC3 channel opening requires stimulation of metabotropic glutamate receptor 1, activation of which can also lead to the induction of long term depression (LTD), which underlies cerebellar motor learning. LTD induction requires protein kinase C (PKC) and protein kinase G (PKG) activation, and although PKC phosphorylation targets are well established, virtually nothing is known about PKG targets in LTD. Because TRPC3 channels are inhibited after phosphorylation by PKC and PKG in expression systems, we examined whether native TRPC3 channels in Purkinje cells are a target for PKG or PKC, thereby contributing to cerebellar LTD. We find that in Purkinje cells, activation of TRPC3-dependent currents is not inhibited by conventional PKC or PKG to any significant extent and that inhibition of these kinases does not significantly impact on TRPC3-mediated currents either. Based on these and previous findings, we propose that TRPC3-dependent currents may differ significantly in their regulation from those overexpressed in expression systems.
Introduction
Canonical transient receptor potential (TRPC)2 channels belong to the superfamily of TRP channels that form cation channels that can be activated by a myriad of distinct mechanisms (for review, see Refs. 1 and 2). Seven distinct genes code for the seven subunits that make up the TRPC family, TRPC1–7. In humans, TRPC2 is a pseudogene, and in rodents it is mainly expressed in the vomeronasal organ where it is thought to play a key role in pheromone detection (for a recent review, see Ref. 3). The remaining TRPC subunits can be divided into three groups comprising TRPC1, TRPC3/6/7, and TRPC4/5 (TRPC1 is sometimes placed in the same group as TRPC4/5). TRPC channels are activated in response to stimulation of phospholipase C as well as phospholipase D (4–6), although other activation mechanisms have also been reported (7).
TRPC channels are widely expressed in the brain and have been shown to promote key aspects of neuronal development such as proliferation, differentiation, and morpho- and synaptogenesis (for recent reviews, see Refs. 8 and 9). It is becoming increasingly evident that TRPC channels also play important roles in the fully developed brain. TRPC channels regulate presynaptic neurotransmitter release (for reviews, see Refs. 9 and 10) and underlie the slow excitatory postsynaptic potentials (EPSPs) observed in a number of neurons after release of brain-derived neurotrophic factor or glutamate (10–13). Brain-derived neurotrophic factor-mediated activation of TRPC induced changes in dendritic spine density, which suggests that they can influence synaptic plasticity and may hence be crucially involved in processes such as memory formation (10, 14). However, there is very little information about a distinct physiological role of an identified brain TRPC channel in the behaving animal. To date, the only behavioral function of a neuronal TRPC channel is that of TRPC3 channels expressed in the cerebellum, a part of the brain involved in motor execution, coordination, and learning.
In the cerebellum TRPC3 is predominantly expressed in Purkinje cells (soma and dendrites), the principal and only output neuron of the cerebellar cortex. TRPC3 expression increases during postnatal cerebellar development, thereby mirroring Purkinje cell dendritic tree development (15). Importantly, TRPC3 expression remains high in the adult, suggesting that TRPC3 channels have important physiological functions in the fully developed cerebellum. Cerebellar Purkinje cells receive two main excitatory synaptic inputs: climbing fiber input and parallel fiber inputs. At the parallel fiber-Purkinje cell synapse glutamate release from presynaptic parallel fibers generates two distinct postsynaptic responses in Purkinje cells: fast EPSPs mediated by AMPA receptors and slow EPSPs mediated by TRPC3 channels after activation of mGluR1 (11–13).
Climbing fiber-Purkinje cell synapses and parallel fiber- Purkinje cell synapses are thought to play a crucial role in motor learning in the cerebellum. Simultaneous stimulation of climbing fiber and parallel fiber inputs leads to the selective suppression of parallel fiber inputs; this phenomenon is termed long term depression (LTD). Cerebellar LTD has been intensely studied, and certain key events have to take place to induce LTD at the parallel fiber-Purkinje cell synapse, including activation of postsynaptic mGluR1 receptors at the parallel fiber-Purkinje cell synapse. This ultimately leads to the internalization of AMPA receptors at this synapse, thereby causing LTD of the AMPA receptor-dependent fast EPSP (16, 17).
Induction of cerebellar LTD depends on the activity of a number of distinct protein kinases, notably protein kinase C (PKC; conventional PKCα and/or -β (18)) and protein kinase G (PKG). PKC has a number of distinct targets in cerebellar Purkinje cells (19–21), and phosphorylation of Ser-880 on the GluR2 subunit of AMPA receptors is necessary for internalization of these receptors and, hence, LTD induction (22). PKG has equally been shown to be involved in the induction of cerebellar LTD, although its protein target(s) remains unclear.
A lot of effort has gone into understanding the mGluR1-dependent regulation of AMPA receptors at the parallel fiber-Purkinje cell synapse, but comparatively little is known about the mGluR1-dependent TRPC3-mediated slow EPSC that is also activated after parallel fiber stimulation. Importantly, it is unknown whether or not the TRPC3-mediated slow EPSP is involved in cerebellar LTD. In expression systems, TRPC3 channel activity is inhibited after phosphorylation by both PKC (23, 24) and PKG (25), and the aim of this study was to investigate if native TRPC3 channels are subject to modulation by these protein kinases.
EXPERIMENTAL PROCEDURES
Tissue and Slices Preparation
Sprague-Dawley rats (postnatal days P10-P15) and CB57/BL6 mice (P11-P14) were killed by cervical dislocation, and sagittal cerebellar slices of 200 or 300 μm thickness (for electrophysiological recordings or immunohistochemical experiments, respectively) were obtained as described in Glitsch (5).
Cell Culture
HEK cells stably transfected with human TRPC3 (a kind gift from Prof. J. Putney, NIEHS, National Institutes of Health) were maintained at 37 °C, 5% CO2, in DMEM supplemented with 10% fetal bovine serum, 2 mm glutamine and 0.5 mg/ml G-418. They were plated in 6-cm culture dishes; adherent cells were subcultured at 70% confluency.
Solutions and Drugs for Electrophysiology
The standard external bicarbonate-buffered solution (BBS) contained 125 mm NaCl, 2.5 mm KCl, 26 mm NaHCO3, 1.25 mm NaH2PO4, 2 mm CaCl2, 1 mm MgCl2, and 10 mm glucose (pH 7.4 when oxygenated with carbogen). This was supplemented with 200 μm Cd2+ for slices obtained from animals older than P12 and in ramp experiments (see below). The standard intracellular solution contained 145 mm CsCl, 10 mm HEPES, 1 mm EGTA, 0.1 mm CaCl2, 4 mm Na-ATP, 0.4 mm Na-GTP, 4.6 mm MgCl2 (pH adjusted to 7.3 with CsOH); this was supplemented with 4 μg of TRPC3 antibody for experiments looking at the impact of TRPC3 antibody on (S)-3,5-dihydroxyphenylglycine (DHPG)-mediated inward current. TRPC3 antibody was generous present from Prof. William Schilling (Cleveland, Ohio).
DHPG, tetrodotoxin, 2,3-dioxo-6-nitro-1,2,3,4,-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX; disodium salt), and (−)-bicuculline methochloride (Bicuculline) were obtained from Tocris Cookson (Bristol) and dissolved in water. 8-Bromo-cyclic GMP was purchased from Sigma. Fura 2-AM was obtained from Molecular Probes, and phorbol 12-myristate 13-acetate (PMA), 4α-phorbol-12,13-didecanoate (referred to in the text as inactive PMA control), and 1-oleoyl-2-acetyl-sn-glycerol (OAG) were bought from Calbiochem whereas okadaic acid, Go6983, KT5823, N-[4-[3,5-bis(trifluoromethyl)-1H-p yrazol-1-yl]phenyl]-4-methyl-1,2,3-thiadiazole-5-carboxamide (BTP2) and 2-aminoethoxydiphenylborane (2-APB) were obtained from Tocris Cookson; all these drugs were dissolved in DMSO. Pyr3 was kindly given by Prof. Yasuo Mori (Kyoto, Japan) and dissolved in DMSO. Drug stocks were separated into aliquots and frozen at −20 °C.
All slice experiments were carried out in the presence of 0.2 μm tetrodotoxin, 20 μm NBQX, and 10 μm bicuculline to block synaptic currents; for PMA experiments investigating the impact of PMA on frequency of miniature inhibitory synaptic currents (mIPSCs), bicuculline was omitted from the extracellular BBS. Drugs were directly dissolved in BBS; final drug concentrations and preincubation times are indicated in results for each drug. The final DMSO concentration never exceeded 0.2% (v/v). Pyr3 had to be sonicated after the addition of stock aliquot to BBS.
Electrophysiology
Cerebellar Slices
Experiments were carried out as described in Glitsch (5). Membrane currents were recorded for 3 min, starting 2 min after establishing the whole-cell configuration (except for experiments with TRPC3 antibody in which case whole cell recordings were resumed 13 min after break-in to allow sufficient time for the antibody to diffuse into the cell). DHPG was applied after the first minute of whole cell recording for 1 min and then washed out for the remaining minute of the 3-min recording; perfusion rates exceeded 7 ml/min. When testing the effect of drugs on the DHPG-mediated inward current, the drug of interest was present throughout the experiment to avoid wash-out. Control and test experiments were all carried out on different slices from the same animal; exceptions are experiments in which the effect of PMA on mIPSC frequency was tested. Here, control mIPSC frequency and test mIPSC frequency were obtained from the same cell; control mIPSC frequency was recorded for 3 min (after 2 min in whole cell configuration), then PMA was added, and mIPSC frequency was recorded after 11 min in the presence of PMA; experiments were repeated with slices that had been preincubated in Go6983 for 50 min to test the effect of this compound on PMA-mediated increase on mIPSC frequency. For current-voltage relationships (IVs) of the TRPC3-mediated inward current, ramps from −100 to +100 mV were given from the holding potential of −60 over 100 ms. Ramps were given under control conditions and then after application of 50 μm DHPG at peak inward current; ramp used in the figure is leak-subtracted (test ramp − control ramp). Experiments were carried out in rat cerebellar slices unless otherwise stated.
Human TRPC3 Stably Transfected HEK293 Cells
Cells (either untreated or after preincubation with 1 μm PMA for 10–15 min) were clamped at −40 mV, and membrane currents were recorded over a 3-min period during which ATP (100 μm) was applied (for PMA experiments in the continued presence of PMA). Peak ATP-currents were normalized to cell size by dividing peak current by cell capacitance and normalized to average control peak ATP response. Intracellular solutions were the same as for cerebellar slice recordings, and extracellular solutions were the same as used for Ca2+ imaging experiments (see below). Pipettes had resistance of 3–4 megaohms, and experiments were discarded if series resistance exceeded 12 megaohms at the end of the experiment.
Fluorescence Ca2+ Imaging Experiments
Experiments were carried out as described in Glitsch (5). In experiments in which cells were preincubated with Go6983 and 8-bromo-cGMP, these drugs were added to the Fura-2-AM-containing incubation solution and also to the recovering medium to ensure continued presence and minimize potential washout or loss. For PMA incubations (including the inactive control), the drug was only added 5–10 min after the actual experiment to prevent down-regulation of PKC. Application of 50 μm OAG was performed at 100 s of recording, and cells remained in new solution until the end of the recording. Fluorescence changes were analyzed as the ratio of fluorescence excited at 356 nm over fluorescence excited at 380 nm (F356/F380). All experimental conditions for a given series of experiments were carried out on the same day using the same passage of cultured cells to avoid differences in results due to differences between cell passages.
Solutions for Ca2+ Imaging Experiments
Standard external solution contained 145 mm NaCl, 10 mm HEPES, 2.8 mm KCl, 2 mm CaCl2, mm 2 MgCl2, and 10 mm glucose (pH 7.35 with NaOH).
Immunohistochemical Staining and Confocal Microscopy
For PKC translocation experiments, slices were left in BBS with 0.2 μm tetrodotoxin, 20 μm NBQX, and 10 μm bicuculline in either the presence (test) of absence (control) of PMA for 5–10 min. Both control and test slices were fixed immediately after treatment in 4% paraformaldehyde for 1 h. After washing with 10 mm PBS, slices were transferred to a cryoprotectant (30% sucrose in PBS), resectioned using a cryostat (10-μm sections), then mounted on slides and rehydrated with PBS. Sections were blocked with 2% bovine serum albumin (BSA) and 1% normal goat serum for 2 h at room temperature. Sections were washed and treated with primary PKCα polyclonal rabbit antibody (Santa Cruz Biotechnology) at a dilution of 1:500 in carrier (0.2% BSA, 0.1% normal goat serum) for 3 h at room temperature. The sections were washed 3 × 15 min, then incubated in secondary antibody at 1:1000 dilution in PBS (Alexa Fluor 568 coupled goat anti-rabbit IgG antibody) for 1.5 h at room temperature. Sections were washed, mounted in Vectashield fluorescent mount (Vector Laboratories), and viewed with a Leica confocal microscope.
Quantification of Staining
ImageJ was used for quantification of fluorescence of membrane as opposed to cytosolic area of Purkinje cells after staining with PKC antibody. A small region of interest was placed on the periphery of the cell, and maximal fluorescence intensity was measured and then compared with maximal fluorescence intensity of the same region of interest placed in the cytosol of the cell. An increase in the ratio of membrane fluorescence/cytosolic fluorescence was then used as an indicator for movement of PKC to the membrane.
Statistical Analysis
ANOVA or Student's t tests were used for statistical analysis (Instat 2.03 software and SPSS 12.0 for Windows), and differences were considered significant for p < 0.05. Results are shown as average ± S.E.
RESULTS
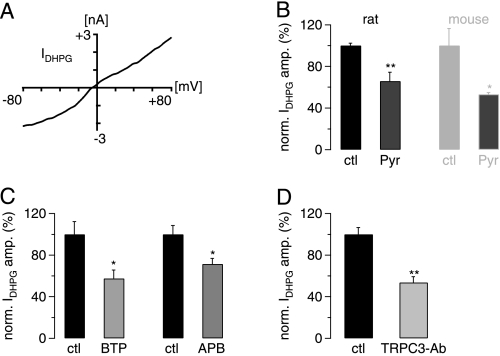
It is well established that in mouse cerebellum the channels underlying the mGluR1-mediated slow EPSC are dependent on TRPC3 (11, 12). To confirm that this is also the case in rat cerebellum, we investigated the current-voltage relationship of the mGluR1-mediated current in rat Purkinje cells by applying the mGluR1 agonist DHPG (50 μm) to rat cerebellar slices and giving voltage ramps at peak inward current (see “Experimental Procedures”). We found that the DHPG-dependent current had a near-linear current-voltage profile that is typical for TRPC3 currents reported in the literature (26–28) (Fig. 1A). We next investigated if the specific TRPC3 channel blocker Pyr3 (29) was able to interfere with the DHPG-mediated inward current. In rat Purkinje cells, the DHPG-mediated current was significantly suppressed after preincubation of cerebellar slices in 10 μm Pyr3 compared with DHPG-mediated currents in slices that were not pretreated with Pyr3 (∼35% inhibition; p = 0.0066, unpaired Student's t test). Repetition of these experiments but this time in mouse cerebellar Purkinje cells yielded a similar suppression of the DHPG-mediated inward current (∼47%; Fig. 1B) that was not significantly different from the inhibition observed in rat Purkinje cells (p = 0.3484; unpaired Student's t test). We also tested the ability of two further TPRC3 channel blockers, BTP2 and 2-APB. Both led to a significant reduction in peak DHPG-mediated current (Fig. 1C).
FIGURE 1.
The mGluR1-mediated slow EPSC in juvenile rat cerebellar slices is TRPC3-dependent. A, shown is the current-voltage relationship of the DHPG-induced inward current (IDHPG) (20 μm DHPG in P15 rat). B, DHPG-mediated inward current (IDHPG) (50 μm DHPG) was significantly blocked in the presence of 10 μm Pyr3 (Pyr) both in juvenile rat (P12–14; left pair of black bars; n = 5 for control (ctl) and 6 for Pyr3) and in juvenile mice (P12–14; right pair of gray bars; n = 3 for both control and Pyr3). IDHPG amplitudes in the presence of Pyr3 were normalized (norm.) to average control amplitude in absence of Pyr3. There was no significant difference in the extent of Pyr3-mediated block between rat and mouse (p = 0.3484; unpaired Student's t test). Preincubation in Pyr3 was 40 min. C, DHPG-mediated inward current (IDHPG) (50 μm DHPG) was significantly blocked by 15 μm BTP2 (left two columns; n = 4 for control (ctl) and 6 for BTP2 (BTP)) and by 10 μm 2-APB (APB; right two columns; n = 3 for each control and test; p = 0.0481, unpaired Student's t test). D, DHPG-mediated inward current (IDHPG) (50 μm DHPG) was significantly blocked by intracellular TRPC3-antibody (TRPC3-Ab); n = 5 for control (ctl) and 4 antibody experiments. IDHPG amplitudes in the presence of TRPC3 antibody were normalized (norm.) to average control amplitude in the absence of the antibody. Intracellular solution was supplemented with 4 μg/ml TRPC3 antibody. Reduction in current amplitude is significant (p = 0.0013; unpaired Student's t test).
It was recently shown that TRPC3 antibodies can inhibit TRPC3-mediated currents when applied to the intracellular side (30); this finding was confirmed by Dr. Diana Kunze and Prof. William Schilling.3 We, therefore, included the inhibitory TRPC3 antibody in our intracellular solution and repeated DHPG applications under these conditions. There was a significant reduction of the DHPG-mediated inward current when the intracellular solution contained the TRPC3 antibody compared with control experiments in which the intracellular solution did not contain the antibody (Fig. 1D). The incomplete block of the slow EPSC by inclusion of TRPC3 antibody likely reflects incomplete perfusion of the elaborate dendritic tree with the antibody. Taken together these findings demonstrate that the DHPG-mediated inward current in rat cerebellar Purkinje cells depends on TRPC3-containing channels, like its murine counterpart.
Human TRPC3 channels contain three identified phosphorylation sites that are targeted by PKC and PKG. Trebak et al. (23) demonstrated that phosphorylation of TRPC3 by PKC blocked channel activation and that Ser-712 was the phosphorylation site for PKC; mutation of this residue (S712A) prevented PKC from inhibiting TRPC3 activation, suggesting that S712 was the only target for PKC-dependent inhibition of human TRPC3 channels. Moreover, PKC activity may promote PKG-mediated inhibition of TRPC3 channels (31), and hence, PKC activity may inhibit TRPC3 activity through two independent mechanisms.
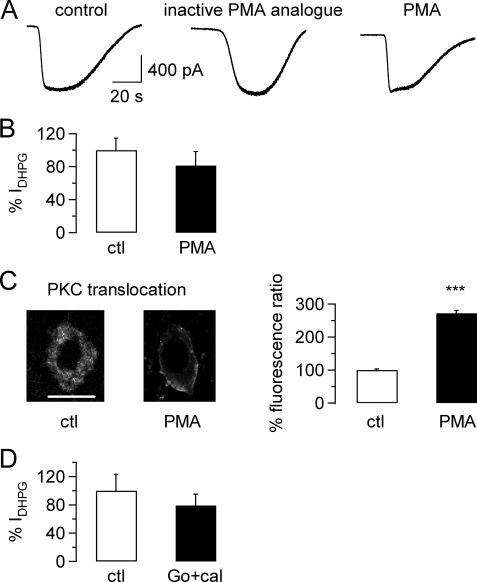
We, therefore, wanted to test the effect of activation of conventional PKC on DHPG-activated TRPC3-mediated inward currents by applying DHPG (50 μm) to rat cerebellar slices under control conditions (Fig. 2A, left panel), in the presence of an inactive PKC activator analog (Fig. 2A, middle panel; 1 μm) and in the presence of the conventional PKC activator PMA (Fig. 2A, right panel; 1 μm). The amplitude of the DHPG-mediated inward current was very similar for the different conditions (Fig. 2A). Because there was no significant difference between DHPG-mediated TRPC3-dependent inward currents under control conditions or in the presence of the inactive PKC activator analog (−916.3 ± 233 pA for control conditions, n = 8; −729.6 ± 115 pA for inactive PMA analog, n = 7; p = 0.5049, unpaired Student's t test), results for these two conditions were pooled. There was no significant difference in the amplitude of the DHPG-mediated inward current under control conditions and after exposure to 1 μm PMA (Fig. 2B; p = 0.3622, unpaired Student's t test), suggesting that PKC activation did not interfere with TRPC3-mediated inward currents in native Purkinje cells. To confirm that the PMA we used could trigger PKC translocation to the plasma membrane and hence activation of PKC under our experimental conditions, we exposed cerebellar slices for 5–10 min to 1 μm PMA and compared PKC localization in Purkinje cells treated this way with PKC localization in Purkinje cells that had not been exposed to PMA. There was a clear translocation of PKC from the cytoplasm to the plasma membrane in PMA-treated Purkinje cells (Fig. 2C, left two pictures), and this translocation of PKC to the plasma membrane was highly significant (Fig. 2C, right panels; p < 0.0001).
FIGURE 2.
Lack of inhibition on mGluR1-mediated TRPC3 currents in rat Purkinje cells after activation of protein kinase C. A, raw traces show TRPC3 current in response to application of 50 μm DHPG under control conditions (left graph) after preincubation in an inactive PMA-analog (1 μm; preincubation time 13 min; middle graph) and after preincubation in the PKC activator PMA (1 μm; preincubation time 14 min; right graph). B, aggregate data compare the size of the DHPG-mediated TRPC3 current (IDHPG) under control (ctl) conditions (including inactive PMA analog; n = 16; white column) and after exposure to PMA (n = 15; black column). Current amplitude was normalized to average control current amplitude. There was no significant difference between current amplitudes under control and test conditions (p = 0.3622, unpaired Student's t test). Average preincubation time in 1 μm PMA was 15.3 ± 1.3 min (n = 15 cells) and in the inactive PMA analog, 14.1 ± 1.2 min (n = 7 cells). C, right, representative images show distribution of PKC under control conditions and after incubation with PMA (5–10 min) in 2 Purkinje cells; scale bar = 50 μm and applies to both images; left, shown is a significant increase in membrane fluorescence ratio in Purkinje cells exposed to PMA compared with control Purkinje cells, demonstrating movement and, hence, activation of PKC upon exposure to PMA (n = 9 for PMA exposed cells and n = 6 for control cells; p < 0.0001, unpaired Student's t test). D, aggregate data compare the size of the DHPG-mediated TRPC3 current under control conditions and after preincubation with 0.5 μm calphostin (cal) and 1 μm Go6976 (Go), or just 1 μm Go6976 for 45.2 ± 2.9 min (n = 8 for control and 9 for PKC inhibitor-exposed cells; p = 0.4626, unpaired Student's t test). All drugs under investigation were present during the OAG applications to avoid wash-out of the drugs.
It was possible that activation of mGluR1 with DHPG led to maximal stimulation of PKC or that PKC was constitutively active, which could explain why we failed to see an effect of PMA on the amplitude of the TRPC3 current. We hence investigated the effect of PKC inhibitors, which should prevent PKC activation and should, therefore, give rise to a larger inward current after DHPG application than under control conditions (i.e. in the absence of the PKC inhibitor). We preincubated cerebellar slices in the PKC inhibitors calphostin (0.5 μm) and/or Go6983 (1 μm) and then repeated DHPG applications. There was no difference in the amplitude of the DHPG-mediated inward current between control conditions and the presence of calphostin and Go6983 or Go6983 alone (Fig. 2D; p = 0.4626, unpaired Student's t test).
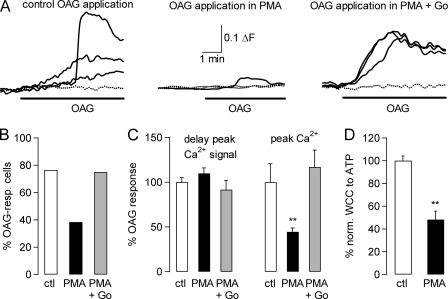
We next considered that translocation of PKC may not necessarily translate into activation of the enzyme, and it was possible that the PKC inhibitors used were not active in our hands. We, therefore, tested the drugs (PMA as PKC activator and Go6983 as PKC inhibitor) in HEK cells stably expressing TRPC3 (23). In this system we activated TRPC3 channels by application of 50 μm OAG and studied OAG-mediated Ca2+ influx into TRPC3-expressing HEK cells; an increase in intracellular Ca2+ concentration reflects the activation of TRPC3 channels. We carried out OAG applications under control conditions (Fig. 3A, left panel) after preincubation with PMA for 5–10 min (Fig. 3A, middle panel) and after treatment of cells with 1 μm Go6983 after PMA incubation and OAG application (Fig. 3A, right panel). There was a clear decrease in OAG-responsiveness of PMA-treated TRPC3-expressing HEK cells. Importantly, exposure of TRPC3-expressing HEK cells to the PKC inhibitor Go6983 after PMA incubation restored the original OAG responsiveness (Fig. 3B). There was no significant difference in the activation kinetics of the intracellular Ca2+ signal (p = 0.2621, ANOVA test), but the peak Ca2+ signal was significantly reduced after PMA treatment compared with control conditions or after preincubation in the Go6983 after PMA application (Fig. 3C; p = 0.0034, ANOVA test). To confirm that this PMA-dependent decrease in TRPC3-mediated current was also seen after receptor stimulation (which is how TRPC3 currents were evoked in the slice preparation), we carried out whole cell patch clamp experiments in the stably transfected HEK293 cells in which we evoked TRPC3-dependent currents in response to ATP application, taking advantage of endogenous P2Y receptors coupling to phospholipase C. The whole cell current was significantly reduced in PMA-treated compared with control cells (Fig. 3D; p = 0.0007, unpaired Student's t test).
FIGURE 3.
Inhibition of TRPC3 currents by protein kinase C in expression system. A, representative fluorescence Ca2+ imaging traces recorded from HEK298 cells stably transfected with human TRPC3 show increases in intracellular Ca2+ concentration after application of 50 μm OAG under control conditions (ctl, left graph), after preincubation in 1 μm PMA (7 min), and after exposure of cells to PMA (n = 6 min) that had also been exposed to the PKC inhibitor Go6983 (Go) for 70 min; increases in intracellular Ca2+ concentration are monitored as changes in fluorescence ratio (ΔF) as described under “Experimental Procedures.” Dotted lines show fluorescence signals in cells that were considered to be non-responders. B, aggregate data show that there is a clear decrease in OAG-responsive (resp.) cells after incubation of TRPC3-transfected cells with 1 μm PMA (5–10 min; black bars indicate PMA-incubated cells, and white bars indicated control cells); this decrease can be reversed by preincubating cells with 1 μm Go6983 (65–75 min in Go6983; 5–10 min in PMA; gray bar). Numbers of OAG-responsive cells (cells that responded to OAG application with increase in intracellular Ca2+ concentration) are: for control conditions, 13/17 (76.4%); for PMA, 13/33 (39.4%); for PMA+Go6983, 17/24 (70.8%). C, aggregate data show that PMA treatment of TRPC3-expressing cells did not significantly interfere with timing of peak Ca2+ signal (measured as time lapsed between application of OAG and peak fluorescence signal; left three columns of the bar chart; p = 0.2621, ANOVA test). Peak Ca2+ signal (measured as peak fluorescence signal compared with base line; right three columns of the bar chart) was significantly decreased after preincubation with PMA alone (p = 0.0034; ANOVA test). White columns, control (ctl) conditions (n = 13); black columns, after exposure to PMA only (n = 13); gray columns, exposure to PMA after pretreatment with Go6983 (n = 17). All drugs under investigation were present during the OAG applications to avoid wash-out of the drugs. D, aggregate data from four control (ctl) and 5 test (PMA) HEK293 cells stably transfected with human TRPC show that TRPC3 whole cell current (WCC) responses to ATP application (100 μm) are significantly inhibited after preincubation in PMA (10–15 min); p = 0.0007, unpaired Student's t test. Two cells did not respond to ATP with an inward current after PMA preincubation.
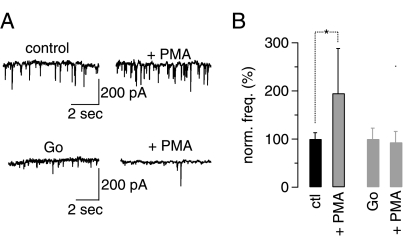
We then wanted to ensure that the drugs were effective not only in cell cultures but also in the rat cerebellar slice preparation that we had used in the previous experiments. Activation of PKC leads to increased spontaneous inhibitory neurotransmitter release (measured in the form of increased mIPSC frequency) at the interneuron-Purkinje cell synapse in mouse cerebellum (32). We, therefore, investigated if the concentration of PMA used in our previous slice experiments was sufficiently high to trigger an increase in mIPSC frequency and whether the concentration of the PKC inhibitor Go6983 used in our slice experiments could counter these effects. Application of 1 μm PMA for 11 min resulted in a robust and significant increase in mIPSC frequency in rat cerebellar slices (Fig. 4A, the top panels show the results of one representative Purkinje cell; Fig. 4B, the black bars show aggregate data; p = 0.016, paired Student's t test). This increase could be prevented when slices were preincubated (40 min) in 1 μm Go6983 after PMA addition (recordings were resumed 11 min after PMA application in the continued presence of both Go6983 and PMA) (Fig. 4A, the bottom panels show the results of one representative Purkinje cell; Fig. 4B, the gray bars show aggregate data; p = 0.868; paired Student's t test). Intriguingly, we also found that there was a significant reduction in mIPSC frequency between slices preincubated in 1 μm Go6983 and slices under control conditions. In the Go6983-treated slices, mIPSC frequency was only 38.9 ± 8.8% that of control mIPSC frequency (n = 4 for control and Go6983-pretreated cells; p = 0.0086; unpaired Student's t test).
FIGURE 4.
PMA and Go6983 are active in the juvenile rat slice preparation at the concentrations used in previous slice experiments. A, representative raw data show that application of 1 μm PMA to rat cerebella slices leads to an increase in mIPSC amplitude (top left, control mIPSC frequency; top right, mIPSC frequency after exposure to PMA for 11 min; data were obtained from one cell). This increase in mIPSC frequency can be inhibited by pretreatment of slices with 1 μm Go6983 (Go) (bottom left) mIPSC frequency after exposure of slices to 1 μm Go6983 for 40 min; bottom right, mIPSC frequency after additional exposure to 1 μm PMA for 11 min; data were obtained from one cell). Top and bottom traces were recorded from two different Purkinje cells. Experiments were carried out in the absence of bicuculline. B, aggregate data show that under control (ctl) conditions application of 1 μm PMA leads to a significant increase in mIPSC frequency (n = 4; p = 0.016, paired Student's t test), whereas application of 1 μm PMA after preincubation to 1 μm Go6983 (Go) prevents an increase in mIPSC frequency (p = 0.868 for mIPSC frequency in Go6983 compared with mIPSC frequency in Go693 and PMA, paired Student's t test).
Taken together, these findings show that the lack of effect of activators and inhibitors of PKC (PMA and Go6983, respectively) was not due to a lack of effect of the drugs in the slice preparation. Hence, native TRPC3-containing channels in cerebellar Purkinje cells are not subject to inhibition by PKC phosphorylation.
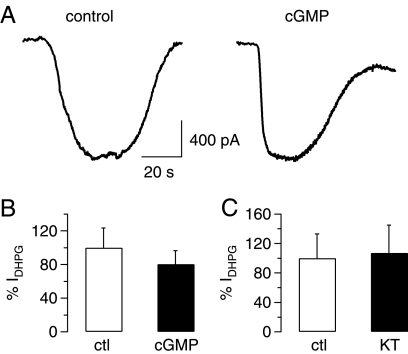
We then considered the possibility that Purkinje cell TRPC3 channels can be modulated in their activity by PKG (25) in a manner that is independent of PKC. Kwan et al. (25) showed that TRPC3 was phosphorylated by PKG at two distinct residues, Thr-11 and Ser-263. PKG-dependent phosphorylation of TRPC3 was reduced in TRPC3 channels in which either Thr-11 or Ser-263 had been mutated (T11A and S263Q, respectively), demonstrating that both phosphorylation sites were targeted independently by PKG (25). We carried out experiments like those described for Fig. 2, but this time we exposed cerebellar slices to 1 mm 8-bromo-cGMP, a membrane-permeable cGMP analog, to activate PKG after application of DHPG. There was no effect of activation of PKG on the amplitude of DHPG-induced TRPC3-mediated inward currents (Fig. 5); Fig. 5A shows representative current traces under control (left panel) and test (right panel) conditions; Fig. 5B shows aggregate data of 6 control experiments and 6 experiments after preincubation in 8-bromo-cGMP (average incubation time 41.4 ± 3.8 min; p = 0.5073, unpaired Student's t test). We also found no effect of the PKG inhibitor KT5823 (2 μm) on the DHPG-induced TRPC3-mediated inward current (Fig. 5C; preincubation with KT5823 for 62 ± 5.8 min; p = 0.895, unpaired Student's t test), suggesting that TRPC3 channels in Purkinje cells are not subject to inhibition after PKG activation.
FIGURE 5.
Lack of inhibition on mGluR1-mediated TRPC3 currents in rat Purkinje cells after activation of protein kinase G. A, raw traces show TRPC3 current in response to application of 50 μm DHPG under control conditions (left graph) or after preincubation in 1 mm 8-bromo-cGMP (cGMP; 43 min; right graph). B, aggregate data compare the size of DHPG-mediated TRPC3 current (IDHPG) under control conditions (ctl; white column; n = 6) and after preincubation with 1 mm 8-bromo-cGMP (cGMP; preincubation for 41.4 ± 3.8 min; n = 5). Current amplitude was normalized to average control current amplitude. There was no significant difference between current amplitudes under control and test conditions (p = 0.5073, unpaired Student's t test). C, aggregate data compare the size of DHPG-mediated TRPC3 current (IDHPG) under control conditions (white column; n = 6) and after preincubation in the protein kinase G inhibitor KT5823 (KT, 1 μm, preincubation for 62 ± 5.8 min; n = 4). Current amplitude was normalized to average control current amplitude. There was no significant difference between current amplitudes under control and test conditions (p = 0.8950, unpaired Student's t test). All drugs under investigation were present during the OAG applications to avoid wash-out of the drugs.
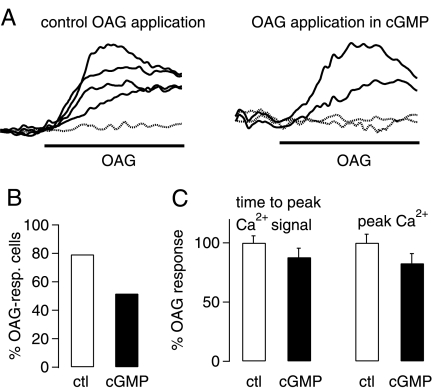
To verify that the drugs were active under our experimental conditions, we tested their efficacy in TRPC3-expressing HEK298 cells. There was a clear reduction in the number of OAG-responsive TRPC3-expressing HEK cells after preincubation of cells with 1 mm 8-bromo-cGMP (Fig. 6A for individual fluorescence signals recorded under control conditions (left panel) and test conditions (right panel); see Fig. 6B for aggregate data). There was no significant difference in the development of the intracellular Ca2+ signal or the peak Ca2+ signal under the different conditions tested (Fig. 6C). We tried to confirm that KT5823 could prevent the 8-bromo-cGMP-induced decrease in OAG-responsive TRPC3-transfected HEK cells but found that KT5823 either interfered with the fluorescent dye or gave rise to elevated Ca2+ levels on its own (data not shown).
FIGURE 6.
Inhibition of TRPC3 currents by protein kinase G in expression system. A, representative fluorescence Ca2+ imaging traces show increases in intracellular Ca2+ concentration in HEK298 cells stably transfected with human TRPC3 after application of 50 μm OAG under control conditions (left graph) and after preincubation in 1 mm 8-bromo-cGMP (65 min); increases in intracellular Ca2+ concentration are monitored as changes in fluorescence ratio (ΔF), as described under “Experimental Procedures.” Dotted lines show fluorescence signals in cells that were considered to be non-responders. B, aggregate data show that there is a clear decrease in OAG-responsive cells after incubation of TRPC3-transfected cells with 1 mm 8-bromo-cGMP (60–75 min). C, aggregate data show that 8-bromo-cGMP treatment of TRPC3-expressing cells does not significantly interfere with timing of peak Ca2+ signal (measured as time lapsed between application of OAG and peak fluorescence signal; left part of the bar chart; p = 0.8694) or peak Ca2+ signal (measured as peak fluorescence signal compared with base line; right part of the bar chart; p = 0.2838, unpaired Student's t test). White columns, control (ctl; n = 38) conditions; black columns, after exposure to 8-bromo-cGMP (n = 31). All drugs under investigation were present during the OAG applications to avoid wash-out of the drugs.
Finally, we carried out experiments in which we exposed cerebellar Purkinje cells to the phosphatase inhibitor okadaic acid to test for a role of phosphatases in regulating TRPC3 channel activity. It has been shown that in the cerebellum protein phosphatases are crucial for the induction of cerebellar LTP and that phosphatase activity is involved in motor learning (33, 34). We found that there was no significant difference between the DHPG-mediated TRPC3-dependent inward current elicited under control conditions or after preincuabtion in 1 μm okadaic acid (supplemental Fig. 1; p = 0.3336, unpaired Student's t test), suggesting that phosphatase inhibition did not impact on TRPC3 channel activity.
DISCUSSION
They key finding of this study is that native TRPC3-dependent currents elicited in cerebellar Purkinje cells are unlikely to be targets of conventional PKC or PKG, which is in contrast to PKC- and PKG-dependent inhibition of recombinant TRPC3 channels expressed in HEK293 cells (23–25). The discrepancy between these and our findings can be explained in four ways.
First, it may be that the channel(s) underlying the mGluR1-mediated inward current in rat Purkinje cells are not mediated by TRPC3. Although an essential role for TRPC3 has been clearly demonstrated for the murine slow EPSC, there is no genetic evidence for involvement of TRPC3 in the rat slow EPSC. We addressed this issue in Fig. 1 and provide evidence for an important role of TRPC3 in the mGluR1-mediated slow EPSC in rat Purkinje cells that complements previously published findings. We have demonstrated previously that the slow DHPG-dependent EPSC does not require Ca2+ release from intracellular Ca2+ stores but is activated in response to stimulation of Gq-coupled mGluR1, suggesting that it is mediated by TRPC channels rather than by Ca2+-activated or store-operated channels (15). We have also shown that TRPC3 is strongly expressed in rat cerebellar Purkinje cells and that its expression increases in parallel with Purkinje cell dendritic tree development (15). Crucially, TRPC3 is the only TRPC subunit in rat cerebellum that increases during development, and its levels remain high in the adult (15), suggesting that TRPC3 is involved in adult cerebellar function.
This study shows that the rat mGluR1-mediated inward current is reduced by Pyr3, a highly selective blocker of TRPC3 channels (Fig. 1B), and by BTP2 and 2-APB, two pharmacologically distinct blockers that are equally used to interfere with TRPC3 channel activation (Fig. 1C). Furthermore, the rat mGluR1-mediated slow EPSC was significantly inhibited by inclusion of an inhibitory TRPC3 antibody (Fig. 1D) that was shown to inhibit native TRPC3 channels in rabbit artery myocytes (30). All these lines of evidence strongly suggest crucial involvement of TRPC3 in rat cerebellar slow EPSC.
The second possibility to explain the lack of effect of phosphorylation on rat TRPC3 is that PKC- and PKG-dependent inhibition of TRPC3 channels may only apply to human TRPC3 channels. An alignment of the rat and human sequence (supplemental Fig. 2) shows that the first PKG phosphorylation site of the human TRPC3 sequence (human T11) is absent from the rat TRPC3 sequence, which might account for the lack of effect of PKG in rat cerebellar Purkinje cells. However, the second PKG phosphorylation site (human Ser-263) is present in both human and rat TRPC3 sequences, and importantly, the area around it is highly conserved. The same is true for the PKC phosphorylation site (human Thr-712). It would, therefore, seem unlikely that the lack of inhibition of native rat TRPC3 channels after PKC and PKG stimulation was due to differential folding of the TRPC3 protein, which might restrict access of kinases to their phosphorylation sites. However, we cannot rule out that TRPC3 channels were phosphorylated in our experiments and that phosphorylation of TRPC3 in the rat does not lead to inhibition of the channels. Again, similarities in the sequences would suggest that this is unlikely to be the case; the TRPC3 subunits have an almost identical amino acid sequences, and it seems implausible that phosphorylation of the rat and human channel should affect the human but not the rat channel.
Third, it is possible that PKC and PKG are restricted from phosphorylating TRPC3 channels in rat cerebellar Purkinje cells because they are not physically near the channel protein. TRPC channels have been shown to be integrated in caveolar lipid rafts that create a unique protein microenvironment, which is thought to facilitate interactions between TRPC channels and proteins involved in their regulation (35–37). It could be that the TRPC3 protein microenvironment in cerebellar Purkinje cells lacks PKC and PKG. Hence, the lack of inhibition of TRPC3 channels after activation of PKC and PKG would be the result of a lack of vicinity of TRPC3 channels to these two kinases, i.e. lack of opportunity rather than lack of ability to phosphorylate the channels. Overexpression of TRPC3 channels in an expression system, however, may result in less specific/controlled insertion of channels in the plasma membrane so that their phosphorylation by kinases is more easily accomplished.
Fourth, it is possible that PKC and PKG only phosphorylate and inhibit a subset of TRPC3 channels in rat cerebellar Purkinje cells. Given the relatively large variability of current amplitudes in response to DHPG between Purkinje cells, we cannot rule out that a comparatively small proportion of TRPC3 channels (around 20% at most) was subject to inhibition after phosphorylation by PKC/PKG and that this inhibitory effect on TRPC3 channels was obscured by the variance in the response. Regardless, the majority of the DHPG-induced current was unaffected.
The apparent lack of effect of phosphorylation on native TRPC3-containing channels has interesting implications. It has been shown that loss of the so-called moonwalker phosphorylation site in cerebellar Purkinje cells led to up-regulation of TRPC3 channel function in moonwalker mice (11). We demonstrate that in rat cerebellar Purkinje cells, neither enhancing nor inhibiting conventional PKC activity interferes with TRPC3-mediated currents. This suggests that in rat the moonwalker phosphorylation site does not mediate acute regulation of TRPC3-mediated currents. It is, however, possible that the site influences TRPC3 channel activity in the long term. Some voltage-gated K+ channels are translocated to the plasma membrane in a phosphorylation-dependent manner (38–40), as are aquaporins (41) and AMPA receptors (for review, see Refs. 42 and 43). It is hence feasible that in moonwalker mice lack of phosphorylation of the channel protein leads to aberrant translocation of more channel protein to the plasma membrane, resulting in larger whole cell currents in moonwalker than wild type mice. Alternatively, it could be that the moonwalker phosphorylation site controls subunit composition of the DHPG-activated TRPC channel, as was demonstrated for synaptic NMDA receptors (44). In this model lack of phosphorylation of the moonwalker DHPG-activated channel would result in insertion of TRPC channels with a distinct subunit composition, resulting in altered channel properties. The larger whole cell current observed in the moonwalker mouse in this scenario would be the result of a longer mean open time and/or larger conductance of the ion channel pore.
Another implication of this study is that channels may display distinct properties in native cells compared with expression systems and that there may even be differential regulation of the same channel by different enzymes in different native cells. TRPC3 channels are known to be activated in response to phospholipase C stimulation; however, neither mouse nor rat slow EPSC is inhibited after pharmacological block of phospholipase C in cerebellar Purkinje cells (5, 45, 46). Instead, another class of phospholipases, phospholipase D, was found to regulate activation of the (rat) slow EPSC (5). This suggests that cerebellar TRPC3 channels differ in their activation mechanism from TRPC3 channels in other systems. A recent review highlights the importance of the source and species of diacylglycerol in phospholipase-dependent regulation of TRPC3 channels (47), pointing out that different types of diacylglycerol may have varying affinities for TRPC3. Interestingly, we found that 100 μm OAG failed to activate any significant inward current in both mouse and rat Purkinje cells (data not shown). OAG activation is thought to be a hallmark of TRPC3 channels and a lack of activation of TRPC3 channels in native systems after OAG application is usually explained in terms of OAG-mediated PKC activation and subsequent inhibition of TRPC3 channels (47). This study, however, finds that conventional PKC does not necessarily inhibit TRPC3-mediated currents, and hence, a lack of effect of OAG in the rat cerebellar slice preparation is unlikely to be due to OAG-mediated PKC activation and subsequent TRPC3 inhibition. Importantly, similar results were found for cerebellar granule cells in which OAG failed to trigger TRPC3-dependent growth cone-turning (48). Hence, OAG may not open TRPC3 channels in all native systems.
All these findings together suggest that TRPC3-containing channels in (rat) cerebellar Purkinje cells may not be regulated by the same enzymes as TRPC3 channels in expression systems or other native systems. This discrepancy could reflect heteromeric TRPC channel expression rather than TRPC3 homomers in Purkinje cells. Neither rat nor mouse slow EPSCs were completely inhibited by 10 μm Pyr3, a concentration routinely used to block TRPC3 channels, but the extent of the block was the same for both preparations. Furthermore, both BTP2 and 2-APB only partially inhibited the slow EPSC. The incomplete block of the current may indicate that the channels underlying the slow EPSC in (juvenile) rats (and mice) may not be homomeric TRPC3 channels but heteromeric TRPC channels containing TRPC3 (as suggested in Ref. 13). In the TRPC3 knock-out mouse the slow EPSC was completely suppressed (12), which can be interpreted as TRPC3 being the only subunit contributing to the channel underlying the slow EPSC. Although this result clearly demonstrates that TRPC3 subunits are essential for the channel protein (e.g. form channel pore), it does not rule out a role for other subunits in modulating the properties of the channel protein. Likewise, the fact that the moonwalker mouse showed increased responses to DHPG does not necessitate that the channel is a homomer, but the same result might also be observed with a heteromeric channel protein. Hence, it is possible that the DHPG-dependent slow EPSC in rodent Purkinje involves heteromeric rather than homomeric TRPC3 channels. Experiments showing TRPC3 phosphorylation and inhibition by PKC and/or PKG were carried out in HEK293 cells overexpressing TRPC3 homomers. It is feasible that TRPC3 heteromers, unlike TRPC3 homomers, are not subject to phosphorylation after activation of PKC and/or PKG or that phosphorylation of heteromeric TRPC channels containing TRPC3 does not result in channel block.
As discussed above it is also possible that Purkinje cells create a specific microenvironment for TRPC3 channels that prevents inhibition of these channels by PKC or PKG. Future experiments will need to address whether the slow EPSC in Purkinje cells is mediated by TRPC3 homo- or heteromers and whether there are regional differences in TRPC3-mediated currents in Purkinje cells (e.g. dendrites versus soma or proximal dendrites versus distal dendrites). If the channel is a TRPC3 homomer, our results demonstrate that the same channel protein can be differentially regulated depending on the cell type in which it is expressed.
Supplementary Material

This article contains supplemental Figs. 1 and 2.
D. Kunze and W. Schilling, unpublished observation.
- TRPC
- canonical transient receptor potential
- EPSP
- slow excitatory postsynaptic potentials
- mGluR1
- metabotropic glutamate receptor 1
- LTD
- long term depression
- BBS
- bicarbonate-buffered solution
- DHPG
- (S)-3,5-dihydroxyphenylglycine
- NBQX
- 2,3-dioxo-6-nitro-1,2,3,4,-tetrahydrobenzo[f]quinoxaline-7-sulfonamide
- BTP2
- N-[4-[3,5-bis(trifluoromethyl)-1H-p yrazol-1-yl]phenyl]-4-methyl-1,2,3-thiadiazole-5-carboxamide
- 2-APB
- 2-aminoethoxydiphenylborane
- mIPSC
- miniature inhibitory synaptic current
- PKG
- protein kinase G
- PMA
- phorbol 12-myristate 13-acetate
- OAG
- 1-oleoyl-2-acetyl-sn-glycerol
- ANOVA
- analysis of variance.
REFERENCES
- 1. Yuan J. X. J., Ward J. P. T., Song M. Y., Yuan J. X. J. (2010) Membrane Receptors, Channels and Transporters in Pulmonary Circulation (Yuan J. X. J., Ward J. P. T., eds) pp. 99–109, Humana Press Inc., Totowa, NJ [Google Scholar]
- 2. Venkatachalam K., Montell C. (2007) TRP channels. Annu. Rev. Biochem. 76, 387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiselyov K., van Rossum D. B., Patterson R. L., Gerald L. (2010) in Vitamins and Hormones (Litwack G., ed) pp. 197–213, Academic Press, New York: [DOI] [PubMed] [Google Scholar]
- 4. Albert A. P., Piper A. S., Large W. A. (2005) Role of phospholipase D and diacylglycerol in activating constitutive TRPC-like cation channels in rabbit ear artery myocytes. J. Physiol. 566, 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glitsch M. D. (2010) Activation of native TRPC3 cation channels by phospholipase D. FASEB J. 24, 318–325 [DOI] [PubMed] [Google Scholar]
- 6. Kwan H. Y., Wong C. O., Chen Z. Y., Dominic Chan T. W., Huang Y., Yao X. (2009) Stimulation of histamine H2 receptors activates TRPC3 channels through both phospholipase C and phospholipase D. Eur. J. Pharmacol. 602, 181–187 [DOI] [PubMed] [Google Scholar]
- 7. Trebak M., Lemonnier L., Smyth J. T., Vazquez G., Putney J. W., Jr. (2007) Phospholipase C-coupled receptors and activation of TRPC channels. Handb. Exp. Pharmacol. 179, 593–614 [DOI] [PubMed] [Google Scholar]
- 8. Tai Y., Feng S., Du W., Wang Y. (2009) Functional roles of TRPC channels in the developing brain. Pflügers Arch. 458, 283–289 [DOI] [PubMed] [Google Scholar]
- 9. Selvaraj S., Sun Y., Singh B. B. (2010) TRPC channels and their implication in neurological diseases. CNS Neurol. Disord. Drug Targets 9, 94–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amaral M. D., Chapleau C. A., Pozzo-Miller L. (2007) Transient receptor potential channels as novel effectors of brain-derived neurotrophic factor signaling. Potential implications for Rett syndrome. Pharmacol Ther. 113, 394–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Becker E. B., Oliver P. L., Glitsch M. D., Banks G. T., Achilli F., Hardy A., Nolan P. M., Fisher E. M., Davies K. E. (2009) A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc. Natl. Acad. Sci. U. S. A. 106, 6706–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartmann J., Dragicevic E., Adelsberger H., Henning H. A., Sumser M., Abramowitz J., Blum R., Dietrich A., Freichel M., Flockerzi V., Birnbaumer L., Konnerth A. (2008) TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59, 392–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartmann J., Konnerth A. (2009) Acta Physiol. 195, 79 [Google Scholar]
- 14. Bollimuntha S., Selvaraj S., Singh B. B. (2011) Emerging roles of canonical TRP channels in neuronal function. Adv. Exp. Med. Biol. 704, 573–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang W. C., Young J. S., Glitsch M. D. (2007) Changes in TRPC channel expression during postnatal development of cerebellar neurons. Cell Calcium 42, 1–10 [DOI] [PubMed] [Google Scholar]
- 16. Jörntell H., Hansel C. (2006) Synaptic memories upside down. Bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 52, 227–238 [DOI] [PubMed] [Google Scholar]
- 17. Kano M., Hashimoto K., Tabata T. (2008) Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells. A key molecule responsible for long term depression, endocannabinoid signaling, and synapse elimination. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 363, 2173–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirono M., Sugiyama T., Kishimoto Y., Sakai I., Miyazawa T., Kishio M., Inoue H., Nakao K., Ikeda M., Kawahara S., Kirino Y., Katsuki M., Horie H., Ishikawa Y., Yoshioka T. (2001) Phospholipase Cβ4 and protein kinase Cα and/or protein kinase CβI are involved in the induction of long term depression in cerebellar Purkinje cells. J. Biol. Chem. 276, 45236–45242 [DOI] [PubMed] [Google Scholar]
- 19. Xia J., Chung H. J., Wihler C., Huganir R. L., Linden D. J. (2000) Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron 28, 499–510 [DOI] [PubMed] [Google Scholar]
- 20. Eto M., Bock R., Brautigan D. L., Linden D. J. (2002) Cerebellar long term synaptic depression requires PKC-mediated activation of CPI-17, a myosin/moesin phosphatase inhibitor. Neuron 36, 1145–1158 [DOI] [PubMed] [Google Scholar]
- 21. Kondo T., Kakegawa W., Yuzaki M. (2005) Induction of long term depression and phosphorylation of the δ2 glutamate receptor by protein kinase C in cerebellar slices. Eur. J. Neurosci. 22, 1817–1820 [DOI] [PubMed] [Google Scholar]
- 22. Chung H. J., Steinberg J. P., Huganir R. L., Linden D. J. (2003) Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long term depression. Science 300, 1751–1755 [DOI] [PubMed] [Google Scholar]
- 23. Trebak M., Hempel N., Wedel B. J., Smyth J. T., Bird G. S., Putney J. W., Jr. (2005) Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol. Pharmacol. 67, 558–563 [DOI] [PubMed] [Google Scholar]
- 24. Venkatachalam K., Zheng F., Gill D. L. (2003) Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J. Biol. Chem. 278, 29031–29040 [DOI] [PubMed] [Google Scholar]
- 25. Kwan H. Y., Huang Y., Yao X. (2004) Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc. Natl. Acad. Sci. U. S. A. 101, 2625–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hurst R. S., Zhu X., Boulay G., Birnbaumer L., Stefani E. (1998) Ionic currents underlying HTRP3-mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Lett. 422, 333–338 [DOI] [PubMed] [Google Scholar]
- 27. Kamouchi M., Philipp S., Flockerzi V., Wissenbach U., Mamin A., Raeymaekers L., Eggermont J., Droogmans G., Nilius B. (1999) Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J. Physiol. 518, 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zitt C., Obukhov A. G., Strübing C., Zobel A., Kalkbrenner F., Lückhoff A., Schultz G. (1997) Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J. Cell Biol. 138, 1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiyonaka S., Kato K., Nishida M., Mio K., Numaga T., Sawaguchi Y., Yoshida T., Wakamori M., Mori E., Numata T., Ishii M., Takemoto H., Ojida A., Watanabe K., Uemura A., Kurose H., Morii T., Kobayashi T., Sato Y., Sato C., Hamachi I., Mori Y. (2009) Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc. Natl. Acad. Sci. U. S. A. 106, 5400–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albert A. P., Pucovsky V., Prestwich S. A., Large W. A. (2006) TRPC3 properties of a native constitutively active Ca2+-permeable cation channel in rabbit ear artery myocytes. J. Physiol. 571, 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwan H. Y., Huang Y., Yao X. (2006) Protein kinase C can inhibit TRPC3 channels indirectly via stimulating protein kinase G. J. Cell. Physiol. 207, 315–321 [DOI] [PubMed] [Google Scholar]
- 32. Harvey V. L., Stephens G. J. (2004) Mechanism of GABA receptor-mediated inhibition of spontaneous GABA release onto cerebellar Purkinje cells. Eur. J. Neurosci. 20, 684–700 [DOI] [PubMed] [Google Scholar]
- 33. Belmeguenai A., Hansel C. (2005) A role for protein phosphatases 1, 2A, and 2B in cerebellar long term potentiation. J. Neurosci. 25, 10768–10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schonewille M., Belmeguenai A., Koekkoek S. K., Houtman S. H., Boele H. J., van Beugen B. J., Gao Z., Badura A., Ohtsuki G., Amerika W. E., Hosy E., Hoebeek F. E., Elgersma Y., Hansel C., De Zeeuw C. I. (2010) Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron 67, 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pani B., Singh B. B. (2009) Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 45, 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ambudkar I. S., Brazer S. C., Liu X., Lockwich T., Singh B. (2004) Plasma membrane localization of TRPC channels. Role of caveolar lipid rafts. Novartis Found. Symp. 258, 63–70; discussion 70–4, 98–102, 263–6 [PubMed] [Google Scholar]
- 37. Remillard C. V., Yuan J. X. (2006) Transient receptor potential channels and caveolin-1. Good friends in tight spaces. Mol. Pharmacol. 70, 1151–1154 [DOI] [PubMed] [Google Scholar]
- 38. Winklhofer M., Matthias K., Seifert G., Stocker M., Sewing S., Herget T., Steinhäuser C., Saaler-Reinhardt S. (2003) Analysis of phosphorylation-dependent modulation of Kv1.1 potassium channels. Neuropharmacology 44, 829–842 [DOI] [PubMed] [Google Scholar]
- 39. Yoo D., Fang L., Mason A., Kim B. Y., Welling P. A. (2005) A phosphorylation-dependent export structure in ROMK (Kir 1.1) channel overrides an endoplasmic reticulum localization signal. J. Biol. Chem. 280, 35281–35289 [DOI] [PubMed] [Google Scholar]
- 40. Yang J. W., Vacher H., Park K. S., Clark E., Trimmer J. S. (2007) Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc. Natl. Acad. Sci. U. S. A. 104, 20055–20060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Conner M. T., Conner A. C., Brown J. E., Bill R. M. (2010) Membrane trafficking of aquaporin 1 is mediated by protein kinase C via microtubules and regulated by tonicity. Biochemistry 49, 821–823 [DOI] [PubMed] [Google Scholar]
- 42. Boehm J., Malinow R. (2005) AMPA receptor phosphorylation during synaptic plasticity. Biochem. Soc. Trans. 33, 1354–1356 [DOI] [PubMed] [Google Scholar]
- 43. Lu W., Roche K. W. (2011) Posttranslational regulation of AMPA receptor trafficking and function. Curr. Opin. Neurobiol. 22, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanz-Clemente A., Matta J. A., Isaac J. T., Roche K. W. (2010) Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 67, 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirono M., Konishi S., Yoshioka T. (1998) Phospholipase C-independent group I metabotropic glutamate receptor-mediated inward current in mouse purkinje cells. Biochem. Biophys. Res. Commun 251, 753–758 [DOI] [PubMed] [Google Scholar]
- 46. Canepari M., Ogden D. (2006) Kinetic, pharmacological, and activity-dependent separation of two Ca2+ signaling pathways mediated by type 1 metabotropic glutamate receptors in rat Purkinje neurones. J. Physiol. 573, 65–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vazquez G., Tano J. Y., Smedlund K. (2010) On the potential role of source and species of diacylglycerol in phospholipase-dependent regulation of TRPC3 channels. Channels 4, 232–240 [DOI] [PubMed] [Google Scholar]
- 48. Li Y., Jia Y. C., Cui K., Li N., Zheng Z. Y., Wang Y. Z., Yuan X. B. (2005) Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434, 894–898 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.