Background: Cdk2 activity essential for chromosome replication is regulated by p27KIP1 the Cdk inhibitor.
Results: Cdc6 the AAA+ ATPase, known to assemble pre-replicative complexes and activate p21CIP1-bound Cdk2, also activates p27-bound Cdk2 but only after the p27 undergoes C-terminal phosphorylation.
Conclusion: An entirely new mechanism for regulating Cdk2 activity is discovered.
Significance: This makes key progress in understanding how the G1-S transition is controlled.
Keywords: CDK (Cyclin-dependent Kinase), Cell Adhesion, Cell Culture, Cell Cycle, Cyclins, Cdc6, Cdk2, Cdk6, Rock, p27KIP1
Abstract
In mammalian cells Cdk2 activity during the G1-S transition is mainly controlled by p27KIP1. Although the amount and subcellular localization of p27 influence Cdk2 activity, how Cdk2 activity is regulated during this phase transition still remains virtually unknown. Here we report an entirely new mechanism for this regulation. Cdc6 the AAA+ ATPase, known to assemble prereplicative complexes on chromosomal replication origins and activate p21CIP1-bound Cdk2, also activated p27-bound Cdk2 in its ATPase and cyclin binding motif-dependent manner but only after the p27 bound to the Cdk2 was phosphorylated at the C terminus. ROCK, which mediates a signal for cell anchorage to the extracellular matrix and activates the mTORC1 cascade as well as controls cytoskeleton assembly, was partly responsible for C-terminal phosphorylation of the p27. In vitro reconstitution demonstrated ROCK (Rho-associated kinase)-mediated phosphorylation of Cdk2-bound p27 at the C terminus and subsequent activation of the Cdk2 by Cdc6.
Introduction
The onset of S phase is prepared in advance by the assembly of prereplication complexes that takes place in late M and G1 phases (1, 2). Cdc6 the AAA+ ATPase, aided by Cdt1, assembles prereplicative complexes by loading the minichromosome maintenance helicase complex on the origin recognition complex (ORC)2-bound origins of replication. After prereplicative complexes are assembled, several factors, some of which require activation by Cdk2, which is negatively regulated by association with Cdk inhibitors such as p27 and p21, are further loaded on the origins (3). Minichromosome maintenance helicase is then activated by Cdc7, and finally, DNA polymerases are recruited to those origins to initiate DNA replication.
Cdc6 is known to possess two biological functions for the onset and progression of S phase. Besides assembling prereplicative complexes, it activates p21-bound Cdk2 and thereby governs utilization of p21-dependent DNA damage checkpoint (4, 5). Cdc6 contains a cyclin binding motif and an ATPase domain, and these two functional domains are essential to activate p21-bound Cdk2. During the G1-S transition, Cdk2 activity is regulated mainly by p27, which undergoes modification by phosphorylation at Ser10, Thr187, and Thr197 in rodent fibroblasts (6, 7). Phosphorylation of this inhibitor at Ser10 and Thr197 occurs early in growth-stimulated cells and facilitates its own stabilization and nuclear exclusion, whereas Cdk2-mediated Thr187 phosphorylation promotes its own degradation by proteasomes (8).
The cells constituting solid organs of animals require an anchorage to the extracellular matrix for their proliferation, and without such an anchorage they arrest in G1 and eventually die of apoptosis known as anoikis (9, 10). The G1 arrest is caused at least in part by inactivation of Cdk4/Cdk6 and Cdk2 with repression of cyclins A, D1, D3, and induction of p27 (11, 12). Inactivation of Cdk4/Cdk6 results in activation of retinoblastoma protein (Rb) and its cognates, which in turn inactivates the E2F transcriptional factors to shut down a subset of genes essential or important for S phase onset, such as Cdc6, cyclin A, and E2F1 (13). Furthermore, Cdc6 protein is rapidly eliminated by proteasomal degradation executed mainly by the APC/CCDH1 ubiquitin ligase (14).
The evolutionarily conserved Tsc1/Tsc2-Rheb-mTORC1 pathway mediates growth and metabolic signals to control cell proliferation (15, 16). Growth factor-activated AKT/PKB stimulates the Rheb small G protein by inactivating the Tsc1/Tsc2 GTPase activating protein complex. Stimulated Rheb activates mTORC1 to phosphorylate S6K1 and 4EBP to enhance translation. In addition, this cascade conveys a cellular anchorage signal to control the G1-S transition. ROCK (Rho-associated kinase) activated by an anchorage signal originated from RhoA and integrins up-regulates the mTORC1 pathway by directly phosphorylating Tsc2 at Thr1203 (17). Consequently, when cells are deprived of anchorage, not only Cdk4/Cdk6 and Cdk2 but also mTORC1 undergo inactivation. Forced activation of mTORC1, however, restores only Cdk4/Cdk6 activity despite marked up-regulation of both cyclin A and D-type cyclins and additional enforced Cdc6 expression. By contrast, expression of both a constitutively active ROCK and Cdc6 stimulates not only Cdk4/Cdk6 but also Cdk2 (17). Although ROCK may participate therein, how anchorage signals regulate Cdk2 activity is unknown.
During a search for a minimum combination of G1 cell cycle factors the manipulation of which invokes anchorage-independent proliferation of rodent fibroblasts, we came across finding an entirely new mechanism for regulating Cdk2 activity that involves ROCK-mediated C-terminal phosphorylation of Cdk2-bound p27 and subsequent activation of the Cdk2 by Cdc6.
EXPERIMENTAL PROCEDURES
Antibodies and Chemicals
Anti-S6K1, anti-phospho-S6K1 (Thr389), anti-phospho-Rb (Ser807/811), anti-Pim1, anti-Pim3, anti-RSK1, anti-phospho-RSK (Thr573), anti-AKT, anti-phospho-AKT (Ser473), anti-ROCK1, anti-ROCK2, anti-LIMK1, and anti-phospho-LIMK1 (Thr508) antibodies were purchased from Cell Signaling; anti-phospho-p27KIP1 (Ser10) was from Epitomics; anti-phospho-p27KIP1 (Thr198) was from R&D Systems, anti-Rb was from BD Biosciences; anti-Cdk4, anti-Cdk6, and anti-β-actin were from Sigma; anti-phospho-Rb (Ser780) and anti-cyclin D3 from MBL; anti-Cdc6 was from NeoMarker. Agarose-conjugated anti-Cdk2 antibody and the rest of the antibodies used were obtained from Santa Cruz.
Cell Construction
Rat embryonic fibroblasts (REFs) constitutively expressing human CDK6, mouse cyclin D3, rat Cdc6, C-terminal-truncated constitutively active human ROCK1 and/or constitutively active human Rheb from the cytomegalovirus promoter were constructed as described (14, 17). The drugs used for selection were G418, hygromycin, puromycin, blasticidin, and zeocin. All the cells were maintained in DMEM containing 10% FCS and selection drugs. REF-K6D3 cells inducible for rat p27, p27T197A, Cdc6, Cdc6WB, or Cdc6Cy were constructed and maintained as described (4).
Cdc6 siRNA
Cells were transfected with the rat Cdc6-specific 23/27mer RNA duplex (Integrated DNA Technologies) or a universal negative control duplex at 10 nm according to vender instructions. The rat Cdc6-specific RNA duplex used was 5′-rGrGrUrUrUrArGrArArArGrArUrGrArArArCrGrGrArArUGA-3′ and 3′-rCrUrCrCrArArArUrCrUrUrUrCrUrArCrUrUrUrGrCrCrUrUrArCrU-5′ (r indicates ribonucleotides).
In Vitro Cdk2 Reactivation Assay
Proliferating REF cells were cultured in methylcellulose medium (MC) for 12 h, lysed with lysis buffer (14), and immunoprecipitated for Cdk2 with the agarose beads-conjugated anti-Cdk2 antibody. The Cdk2-bound agarose beads were then incubated at 30 ºC in 20 μl of 50 mm Tris-HCl (pH 7.5) buffer containing 30 mm MgCl2 and 10 mm ATP for 30 min with the addition of 1 μl of either Escherichia coli-expressed active ROCK1 (17) or its control preparation and then for another 30 min with the addition of 1 μl of either recombinant Cdc6/Cdc6WB/Cdc6Cy or its control preparation. The bead-bound Cdk2 was determined for its amount and activity as well as coprecipitated p27, its Thr197 phosphorylated form, and Cdc6 as described (4). Recombinant Cdc6, Cdc6WB, and Cdc6Cy were synthesized in reticulocyte lysates and affinity-purified as described (4).
The cDNA encoding rat Cdc6 tagged with 3 × FLAG and 6 × His at its C terminus was inserted into the pFASTBAC plasmid (Invitrogen) and then converted to a recombinant baculovirus (constructed by Q. Kan). The recombinant Cdc6 protein expressed in SF9 cells was double affinity-purified with nickel-nitrilotriacetic acid beads and anti-FLAG M2 gels. A phosphomimetic mutant p27S10D N-terminal-tagged with His6 and an empty vector control was expressed in E. coli, purified with nickel-nitrilotriacetic acid beads, and used for inactivation of baculovirus-expressed affinity-purified Cdk2-cyclin A complexes (46% purity) (Upstate Biotechnology) in a buffer containing 50 mm Tris-HCl (pH 7.5) and 10 mm MgCl2. The inactivated Cdk2 was immunoprecipitated with anti-Cdk2 antibody-conjugated beads and used for subsequent reactivation assay as above.
RESULTS
Cdk2 Remains Activated in REF-overexpressing Cdk6 and Cyclin D3 Despite Anchorage Deprivation
To achieve the initial goal, we began to examine the potentially anchorage-independent cell cycle-promoting effects of Cdk6-cyclin D3 complexes (K6D3) that are refractory to CDK inhibitors (18, 19). REFs overexpressing both Cdk6 and cyclin D3 (REF-K6D3) were constructed with a retroviral vector. Overexpression of K6D3 did not influence the cell cycle distribution of the original REF cells during logarithmic proliferation (supplemental Fig. S1).
Both original REF and REF-K6D3 cells were deprived of anchorage by culturing in MC for 48 h with every 12-h sampling and analyzed for major G1 cell cycle factors by immunoblotting (Fig. 1A). Confirming our previous results, in REF, expression of Cdc6, G1 cyclins, and E2F-1 diminished or disappeared with mTORC1 inactivation as indicated by a loss of both S6K1 protein and its Thr389 phosphorylation. In addition, Rb quickly lost Cdk4/Cdk6-specific Ser780 and Cdk2-specific Ser807/811 phosphorylation, consistent with inactivation of these kinases as shown by the parallel in vitro kinase assays (Fig. 1B).
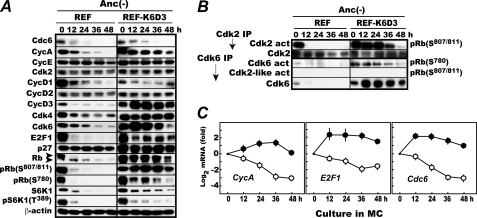
FIGURE 1.
Enforced expression of Cdk6 and cyclin D3 activates Cdk2 in the absence of anchorage. A, Rb remains phosphorylated at Ser807/811, a Cdk2-specific phosphorylation site in anchorage-deprived REF-K6D3 cells. Rapidly proliferating REF and REF-K6D3 were cultured in MC for 48 h and analyzed for the indicated factors by immunoblotting as described (14). B, Cdk2 activation is prolonged in anchorage-deprived REF-K6D3. Cdk2 and Cdk6 in A were assayed for their amounts and activities as described (14). C, E2F-dependent genes driving S phase onset are up-regulated in anchorage-deprived REF-K6D3. RNA was prepared from the cells harvested in A, and cyclin A, E2F1, and Cdc6 mRNAs were quantified by reverse transcription-coupled real time PCR as described (14). The data shown are averaged values with S.D obtained from three independently isolated samples.
On the other hand, in anchorage-deprived REF-K6D3, G1 cyclins and E2F1 remained expressed at least for 48 h, but Cdc6 disappeared gradually. Due to the overexpressed K6D3, Rb phosphorylation at Ser780 persisted for 48 h, albeit significantly reduced perhaps, partly caused by destabilization of Cdk6 protein. Both S6K1 and its Thr389 phosphorylation markedly diminished but, unlike in REF, persisted at a low level, indicating that mTORC1 was not completely inactivated in this cell despite anchorage loss. Highly interestingly, Rb continued to be phosphorylated at Ser807/811 for 48 h. We speculated that this phosphorylation might be attributable to the overexpressed K6D3 because there was a report showing that Cdk6 bound to a viral cyclin is refractory to p27KIP1 like K6D3 and has a Cdk2-like activity (20). But this speculation was wrong. In in vitro kinase assays, Cdk6 showed no ability to phosphorylate Rb Ser807/811 despite its robust activity toward Rb Ser780 (Fig. 1B). Instead, Cdk2 was found highly active, accounting for the in vivo Rb Ser807/811 phosphorylation.
Consistent with the continued Cdk6 activation, mRNAs for E2F-regulated genes, such as Cdc6, cyclin A, and E2F1, were up-regulated in REF-K6D3 (Fig. 1C). Thus, in this cell overexpressed Cdk6 was active without anchorage as initially intended. But unexpectedly, Cdk2 was also active albeit transiently, as opposed to its rapid inactivation in anchorage-deprived REF.
Close Association of p27 Thr197 Phosphorylation with Cdk2 Activation
The factors that would affect Cdk2 activity in G1-S are the partner cyclins, p27 and p21, and its own modification by activating Thr160 phosphorylation and checkpoint-associated inhibitory Tyr15 phosphorylation (21). As already noted Cdk2 was quickly inactivated upon anchorage deprivation in REF despite continued expression of cyclin E (Fig. 1A) and even in mTORC1-active REFs that express both cyclins E and A (14). These observations indicate that the availability of the partner cyclins is not the determinant of Cdk2 activity during anchorage deprivation.
Consequently we next focused on Cdk2 phosphorylation at Thr160 and Tyr15 and examined its causal relation to Cdk2 activity. Rapidly proliferating REF and REF-K6D3 were cultured in MC as in Fig. 1A and analyzed for the levels of Cdk2, its phosphorylation at Thr160 and Tyr15, and others as well as Cdk2 activity (Fig. 2A). In REF, Cdk2 remained Thr160-phosphorylated at least for 12 h during MC culture, whereas Cdk2 activity vanished within 12 h. In REF-K6D3, this phosphorylation persisted for 48 h, whereas Cdk2 activity almost disappeared at 36 h. In addition, throughout this experiment Tyr15 phosphorylation of Cdk2 could not be detected. Thus there was no direct correlation between the loss of Cdk2 activity and phosphorylation at these sites, although Thr160 phosphorylation is absolutely essential for Cdk2 activity. The level of p21 was similar between the two during anchorage deprivation (Fig. 2A).
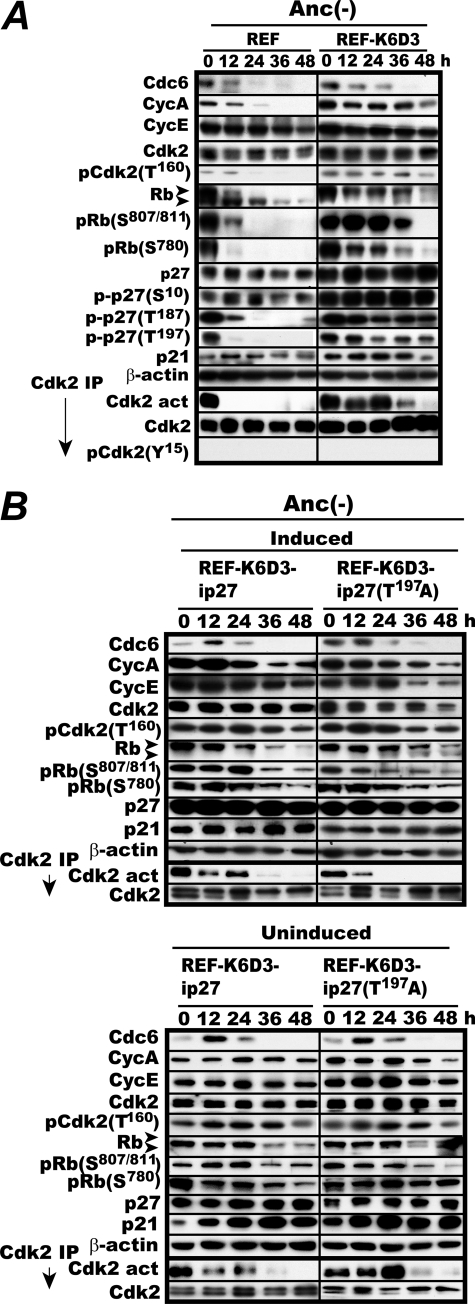
FIGURE 2.
C-terminal phosphorylation of p27 is essential for the continued activation of Cdk2 in anchorage-deprived REF-K6D3. A, REF and REF-K6D3 were incubated in MC for 48 h and analyzed for the levels of Cdk2 phosphorylation at Thr160 and p27 phosphorylation at Ser10, Thr187, and Thr197 and the indicated factors by immunoblotting. In parallel, Cdk2 was immunoprecipitated and determined for its activity and the level of Tyr15 phosphorylation. B, overexpression of T197A mutant p27 shortens Cdk2 activation in anchorage-deprived REF-K6D3. REF-K6D3-ip27 and REF-K6D3-ip27T197A cells were induced or uninduced by withdrawal of doxycycline or not and 4 days later, incubated in MC as in A and determined for Cdk2 activity and the levels of the indicated factors.
On the other hand, despite the continued Cdk2 activity, p27 expression was constitutively elevated 2–3-fold in REF-K6D3 with no significant fluctuations during MC culture. Because modification of p27 by phosphorylation controls its subcellular localization and ultimate fate, we next examined the levels of p27 phosphorylated at the three sites, Ser10, Thr187, and Thr197, in REF and REF-K6D3 during MC culture. Ser10 phosphorylation was elevated in REF-K6D3 proportional to the p27 amount. Interestingly, there was a great difference in the levels of both Thr187 and Thr197 phosphorylation between the two. In REF, in which Cdk2 was quickly inactivated, phosphorylation at both sites fell sharply to an undetectable level, whereas in REF-K6D3, where Cdk2 was active for 36 h, phosphorylation at these sites persisted for 48 h with a gradual reduction. Because Thr187 is the site phosphorylated by Cdk2, we attributed the Thr187 phosphorylation to the activated Cdk2. Consequently, we speculated that the C-terminal phosphorylation of p27 might be causally related to the Cdk2 activation. This speculation is consistent with the previous report showing that overexpression of a C-terminally unphosphorylatable p27 mutant retards both activation of Cdk2 and S phase entry (22).
Enforced Expression of T197A Substitution Mutant of p27 Reduces Cdk2 Activity in REF-K6D3
To prove or disprove the speculation, we examined the effect of overexpression of an unphosphorylatable T197A mutant of p27 on Cdk2 activity in REF-K6D3 cells during MC culture. REF-K6D3 inducible for wild-type and T197A mutant p27 (REF-K6D3-ip27 and REF-K6D3-ip27T197A) were constructed. Both cells were first induced or not, then cultured in MC and analyzed for Cdk2 activity and relevant factors (Fig. 2B). When p27T197A was induced, Cdk2 activity disappeared within 24 h. Consistently, the lower band of Rb became visible with a significantly reduced Ser807/811 phosphorylation at 24 h. By contrast, induction of wild-type p27 had little effect on Cdk2 activity despite a marked elevation in its level. Without induction, the levels of Cdk2 activity, Rb phosphorylation, and all other G1 factors examined were virtually identical at each time point between the two. These results indicate that Thr197 phosphorylation of p27 is required for the prolonged Cdk2 activation observed in anchorage-deprived REF-K6D3.
ROCK and Pim Are Major Kinases Responsible for p27 Thr197 Phosphorylation in Anchorage-deprived REF-K6D3
Given the results, we began to search for the kinase(s) responsible for p27 Thr197 phosphorylation in anchorage-deprived REF-K6D3. Three kinases have been reported to phosphorylate p27 Thr197, AKT (23), Pim1, Pim2, and Pim3 (24), and Rsk1 (25). In addition to these kinases, we included ROCK in the candidates for in-depth examination not only because this kinase minimum target sequence (RRX(S/T) or RX(S/T)) perfectly matches with the evolutionarily conserved C-terminal sequence of p27 (RRQT) but also because unlike forced mTORC1 activation, expression of a constitutively active ROCK1 activated Cdk2 in the absence of anchorage albeit weakly (17).
To narrow down candidates, we first examined the expression levels of these four kinases and the presence or absence of the physical or biological representations of their activation state in anchorage-deprived REF and REF-K6D3. Both cells were incubated in MC and analyzed for the levels of the four kinases and additionally, Ser473-phosphorylated AKT and Thr573-phosphorylated Rsk1 because these phosphorylated forms are absolutely essential for their activity (26), whereas ROCK activity was monitored by detecting Thr508 phosphorylation of LIMK1, a specific ROCK substrate (27). AKT remained expressed but was inactive in REF-K6D3 because its Ser473 phosphorylation disappeared quickly in this cell, like in REF (Fig. 3A). On the other hand, Pim-1 protein was up-regulated, but Pim-3 was unaltered, whereas Pim-2 was not expressed. Rsk1 was expressed and active in both cells as indicated by continued phosphorylation at the Thr573. Finally, ROCK1 and ROCK2 were expressed in both cells, but at least either one was active in REF-K6D3 as Thr508 phosphorylation of LIMK1 persisted in these cells, albeit as a gradual loss. Thus, both Pim and ROCK appeared to nicely fit the aimed kinase.
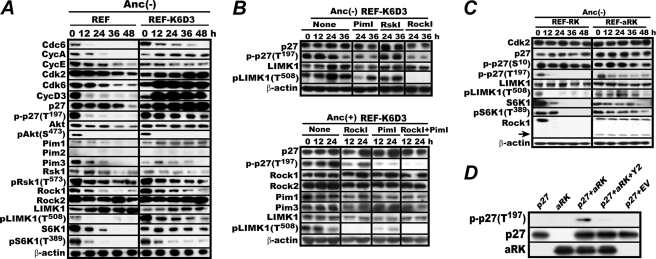
FIGURE 3.
ROCK1 phosphorylates C-terminal Thr197 of p27. A, ROCK is active in anchorage-deprived REF-K6D3. The same experiment as in Fig. 1 was carried out with immunoblot analysis of the indicated factors. B, treatment with Y29762, a specific ROCK inhibitor, diminishes p27 Thr197 phosphorylation. REF-K6D3 was incubated in MC for 12 h and then with no inhibitor, 20 μm GW5074, 20 μm BI-D1870, or 30 μm Y27632 for an additional 36 h. The cells were lysed and analyzed for p27 Thr197 phosphorylation. C, in REF expressing an active ROCK1, Thr197 phosphorylation of p27 persists during anchorage deprivation. REF-RK and REF-aRK were cultured in MC and analyzed for the indicated factors as in Fig. 2A. The arrow indicates the constitutively active truncated ROCK1. D, E. coli-expressed active ROCK1 phosphorylates E. coli-expressed p27 Thr197 in vitro. N-terminal histidine oligomer-tagged, C-terminal-truncated aRK was incubated with similarly tagged p27 or its E. coli empty vector control lysate (EV) in the reaction mixture for 30 min with or without the addition of 50 μm Y29762 (Y2) as described (17) and analyzed for p27 Thr197 phosphorylation by immune-blotting.
We, therefore, examined the effects of specific inhibitors to these two kinases. An Rsk1 inhibitor was also included in the experiment as a provisional negative control. The inhibitors used were GW 5074 for Pim-1 and Pim-3, Y27632 for ROCK1 and ROCK2, and BI-D1870 for Rsk1 (28). Proliferating REF-K6D3 cells were cultured in MC for 12 h first, then for 24 h with or without the addition of the inhibitors and analyzed for the levels of p27 and its Thr197 phosphorylation (Fig. 3B, upper panel). Without these additions, the levels of p27 Thr197 phosphorylation were similar for 24 h. Of the three, the ROCK and Pim inhibitors significantly lowered p27 Thr197 phosphorylation at 24 h. In this experiment, LIMK1 Thr508 phosphorylation completely vanished on treatment with the ROCK inhibitor as expected but was also markedly reduced by treatment with the Pim inhibitor. On the other hand, the Rsk1 inhibitor had no inhibitory effect.
To confirm the inhibitory effect and examine a potential synergism between the ROCK and Pim inhibitors, a similar inhibition experiment was carried out for REF-K6D3 proliferating in anchorage-furnished dishes. In this experiment inhibitors were added at 0 h, and cells were incubated for 24 h with every 12 h harvests (Fig. 3B, lower panel). Both inhibitors markedly reduced Thr197 phosphorylation at 12 and 24 h when used separately. But the simultaneous use of both inhibitors reduced Thr197 phosphorylation further. These results indicate that both Rock and Pim are mainly responsible for C-terminal phosphorylation of p27 in REF-K6D3 regardless of the presence or absence of anchorage.
Expression of Active ROCK1 Leads to Continued p27 Thr197 Phosphorylation during Anchorage Deprivation
Because Pim was already known to phosphorylate p27 at Thr197, we decided to focus on ROCK and sought to investigate a possible physical interaction between this kinase and p27. REF cells expressing wild-type or a constitutively active ROCK1 (REF-RK and REF-aRK) were constructed as previously (17) and examined for phosphorylation of both p27 Thr197 and LIMK1 Thr508 and others (Fig. 3C). Unlike in REF-RK, in which ROCK1 was quickly inactivated, p27 Thr197 continued to be phosphorylated in REF-aRK, albeit at a lowered level, consistent with the inhibitor data.
ROCK1 Phosphorylates p27 at Thr197 in Vitro
Consequently we examined whether or not active ROCK1 can directly phosphorylate p27 Thr197 in vitro. Both E. coli-expressed affinity-purified p27, and similarly, E. coli-expressed affinity-purified active ROCK1 (aRK) were incubated in an ATP-containing reaction mixture in the presence or absence of Y27632 and analyzed for p27 Thr197 phosphorylation by immunoblotting (Fig. 3D). Active ROCK1 but not the empty vector lysate phosphorylated p27 Thr197 when the inhibitor was absent. Thus ROCK1 could physically interact with p27 and phosphorylate its C terminus.
Cdc6 Facilitates Cdk2 Activation in Anchorage-deprived REF-K6D3 Cells
Although essential, p27 Thr197 phosphorylation was not sufficient for Cdk2 activation in REF-K6D3 because Cdk2 lost activity at 48 h despite the presence of both p27 Thr197 and Cdk2 Thr160 phosphorylation (Fig. 2). This indicates that an additional factor(s) is likely to be needed for the Cdk2 activation. Cdc6 is known to activate p21-bound Cdk2 (4, 5). Moreover, the loss of Cdk2 activity roughly coincided with the disappearance of Cdc6 protein (Figs. 1 and 2). We, therefore, speculated that Cdc6 might be the additional factor and examined whether or not siRNA-mediated knockdown or antipodal overexpression of Cdc6 would influence the prolonged Cdk2 activation. A duplex siRNA designed for Cdc6 knockdown was prepared and transfected into REF-K6D3. Meanwhile, REF-K6D3 cells 2–3-fold-overexpressing Cdc6 (REF-K6D3-Cdc6) were constructed. Two days before, REF-K6D3 was transfected with the Cdc6 siRNA or a control RNA. These transfected REF-K6D3 and logarithmically proliferating REF-K6D3-Cdc6 were then cultured in MC and similarly analyzed for Cdk2 activity and others (Fig. 4A). In the siRNA-transfected REF-K6D3, Cdk2 activity markedly diminished with reduced Cdc6 levels. By contrast, in the Cdc6 overexpressor, Cdk2 stayed active with continued Cdc6 expression. These results indicate that Cdc6 is indeed the additional factor required for the Cdk2 activation.
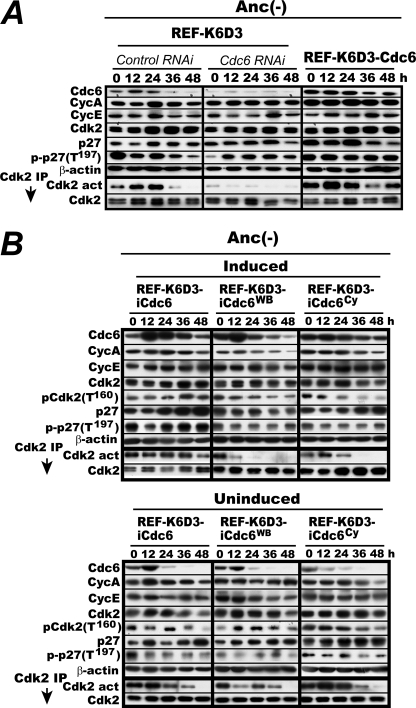
FIGURE 4.
Cdc6 is required for the Cdk2 activation in anchorage-deprived REF-K6D3 cells. A, proliferating REF-K6D3 was transfected with the Cdc6-specific siRNA or a control RNA. Two days later the transfected cells and rapidly proliferating REF-K6D3-Cdc6 cells were cultured in MC and determined for Cdk2 activity and the indicated factors as in Fig. 2A. IP, immunoprecipitate. B, both the ATPase domain and the Cy motif are essential for Cdc6 to activate Cdk2. Rapidly proliferating REF-K6D3-iCdc6, REF-K6D3-iCdc6Cy, and REF-K6D3-iCdc6WB cells were induced for the corresponding Cdc6 proteins by withdrawal of doxycycline, then cultured in MC and determined for Cdk2 activity and the levels of the indicated factors as in Fig. 2B.
Cdc6 Defective in ATPase Domain or Cy Motif Cannot Activate Cdk2 in Vivo
We next examined a mechanistic similarity in Cdc6-mediated activation between p27-bound Cdk2 and p21-bound Cdk2 in vivo. Activation of the latter requires both ATPase domain and cyclin binding (Cy) motif of Cdc6 (4). REF-K6D3 cells inducible for wild type, an ATPase-defective Walker B (WB) mutant, or a Cy motif-deficient mutant of Cdc6 (REF-K6D3-iCdc6, REF-K6D3-iCdc6WB, and REF-K6D3-iCdc6Cy) were constructed with the same doxycycline-repressible system as in Fig. 2 and analyzed similarly (Fig. 4B). When wild-type Cdc6 was induced, Cdk2 remained active throughout the MC culture, confirming the Fig. 4A results. But when the WB or Cy mutant was induced, Cdk2 lost activity within 36 h. Loss of Cdk2 activity was more evident with the WB mutant. By contrast, without induction, Cdk2 activity was indistinguishable among them and similar to that in original REF-K6D3 cells. Thus both the ATPase domain and the cyclin binding motif were required for Cdc6 to induce the prolonged activation of Cdk2.
ROCK-dependent Activation of p27-Bound Cdk2 by Cdc6
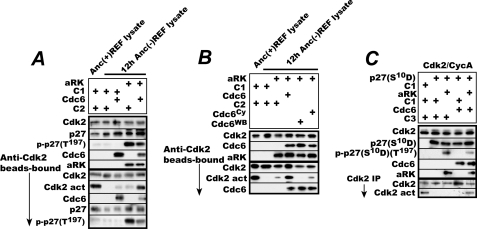
Finally, to establish that Cdc6 activates p27-bound Cdk2 in its ATPase- and Cy-dependent manner only after the bound p27 is C-terminal-phosphorylated, we performed in vitro reactivation assays with E. coli-expressed affinity-purified aRK and in vitro reticulocyte lysate-synthesized affinity-purified Cdc6. First, inactive Cdk2 was immunoprecipitated from 12-h MC-cultured REF with anti-Cdk2 antibody-conjugated agarose beads. In this cell, Cdk2 was inactivated completely, and Thr197 phosphorylation of p27 disappeared also nearly completely, yet Thr160 phosphorylation of Cdk2 essential for its activity was retained (Fig. 2A). The immunoprecipitated Cdk2 was then incubated in an ATP-containing reaction mixture added with either aRK or Cdc6 or with aRK first and Cdc6 next and split into halves. One-half was used to determine the amounts of Cdk2, p27, aRK, and Cdc6 in the reaction mixture. From the other half, the bead-bound Cdk2 was recovered by a brief centrifugation, incubated in the Cdk2 assay mixture, and determined for Cdk2 activity and the amounts of Cdk2, coprecipitated 27, its Thr197 phosphorylation, and Cdc6 (Fig. 5A). In parallel, Cdk2 immunoprecipitated from logarithmically proliferating REF cells was similarly incubated with E. coli and reticulocyte control preparations (C1 and C2) and assayed for its activity as a positive control. When the inactive Cdk2 was incubated with active ROCK1, p27 bound to the Cdk2 was phosphorylated at Thr197, but the Cdk2 was only marginally activated. When the Cdk2 was incubated with Cdc6, it was activated marginally too. By contrast, when incubated with ROCK1 first and Cdc6 next, the Cdk2 was activated nearly as high as the fully active positive control Cdk2. The activated Cdk2 was associated with Cdc6, a slightly less amount of p27, and a much less amount of its C-terminal-phosphorylated form. On the other hand, the active Cdk2 recovered from proliferating cells did not contain any significant amounts of Cdc6 or C-terminal-phosphorylated p27, perhaps because of their rapid removal from the Cdk2 in proliferating cells. Instead it was associated with C-terminal-nonphosphorylated p27. Consistent with the in vivo results, the WB or Cy-motif mutant Cdc6 failed to reactivate the Cdk2 in the same in vitro assay although they bound to the Cdk2 similarly (Fig. 5B). Furthermore, consistently the WB mutant even suppressed the slight reactivation induced upon incubation with active ROCK1 perhaps by a residual amount of endogenous Cdc6 associated with the inactive Cdk2.
FIGURE 5.
Active ROCK1 phosphorylates the C terminus of Cdk2-bound p27 and Cdc6 activates the Cdk2 in its ATPase- and Cy-dependent manner only after bound p27 is C-terminal-phosphorylated. A, agarose bead-bound inactive Cdk2 immunoprecipitated (IP) from 12-h anchorage-deprived REF cells was incubated first with either E. coli-expressed aRK or its negative control preparation (C1) and then with either reticulocyte-synthesized Cdc6 or its negative control preparation (C2). After incubation, a half of the reaction mixture was denatured and analyzed for the amounts of Cdk2, Cdc6, p27, and its Thr197 phosphorylated form. From the other half, bead-bound Cdk2 was collected and analyzed for its activity and coprecipitated p27-, Cdc6-, and Thr197-phosphorylated p27. B, the immunoprecipitated Cdk2 as above was incubated first with aRK and then with in vitro synthesized recombinant Cdc6, Cdc6WB, or Cdc6Cy and analyzed as in A. C, baculovirus-produced and highly purified Cdk2-cyclin A complexes were incubated with E. coli-expressed p27S10D or an empty vector counterpart (C1) and immunoprecipitated with anti-Cdk2 antibody-conjugated beads. The bead-bound Cdk2 was then incubated with aRK or C1 and finally with baculovirus-produced double affinity-purified Cdc6 or its negative control preparation (C3) and analyzed as in A.
As for activation of p21-bound Cdk2, no other cellular protein may be required for this activation because the same reactivation occurred with baculovirus-expressed highly purified recombinant Cdk2-cyclin A complexes inactivated by bacterially expressed p27S10D, bacterially expressed active ROCK1, and baculovirus-expressed double affinity-purified Cdc6 (Fig. 5C). In this experiment, a phospho-mimetic S10D substitution mutant p27S10D was used to reconstitute inactive Cdk2-p27 complexes because p27 in REF was phosphorylated at Ser10 regardless of the presence or absence of anchorage (Fig. 2). These results demonstrate that ROCK1 phosphorylates the C terminus of Cdk2-bound p27 and that Cdc6 can activate the Cdk2 in its ATPase- and Cy-dependent manner only after the bound p27 is C-terminal-phosphorylated.
Combined Overexpression of Cdk6, Cyclin D3, Cdc6, and Active Rheb Induces Anchorage-independent Proliferation of Rodent Embryonic Fibroblasts
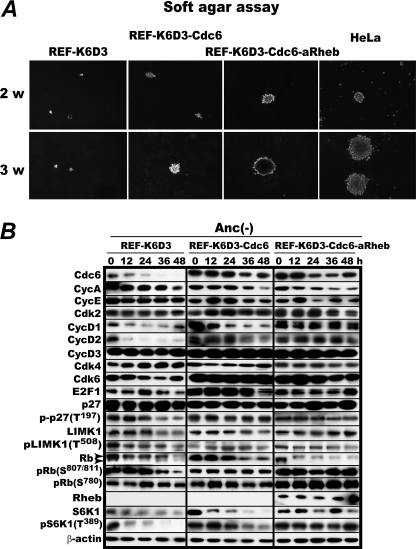
As expected from the anchorage-independent Cdk2 activation by combined overexpression of Cdk6, cyclin D3, and Cdc6 as described above, REF-K6D3-Cdc6 and the same cell but with additional overexpression of active Rheb to enhance mRNA translation (REF-K6D3-Cdc6-aRheb) proliferated in soft agar and formed colonies. Remarkably, the active Rheb overexpressor proliferated in soft agar as rapidly as HeLa, a fully developed human cancer cell line (Fig. 6A). In this cell line all the G1 cell cycle factors examined continued to be expressed with activated mTORC1 despite the absence of anchorage at least for 48 h (Fig. 6B). Mouse embryonic fibroblasts overexpressing the same combination of the factors formed smaller colonies infrequently mingled with large ones (supplemental Fig. S2). Thus, overexpression of four G1 cell cycle or related factors can induce sustainable anchorage-independent proliferation of otherwise absolutely anchorage-dependent rodent embryonic fibroblasts.
FIGURE 6.
Anchorage-independent proliferation of rat embryonic fibroblasts is induced by overexpression of Cdk6, cyclin D3, Cdc6, and a constitutively active Rheb. A, logarithmically proliferating REF-K6D3, REF-K6D3-Cdc6, and REF-K6D3-Cdc6-aRheb cells were cultured in DMEM growth medium containing 0.33% Noble agar layered on 0.5% bottom agar for 2 and 3 weeks with HeLa cells as a reference. B, the same set of the cells was cultured in methylcellulose medium and analyzed for the levels of the specified factors.
DISCUSSION
Cdc6 is the bifunctional AAA+ ATPase initially discovered to assemble prereplicative complexes on ORC-bound replication origins and later to activate p21-bound Cdk2. The most striking finding in this study is that Cdc6 can activate also p27-bound Cdk2 but only after the bound p27 acquires C-terminal phosphorylation. Despite this difference, both the ATPase domain and the Cy motif are required for this ATPase to activate p27-bound Cdk2, just like p21-bound Cdk2. This implies that the basic mechanism for the activation is perhaps similar between the two, but understanding the reason for the requirement of C-terminal phosphorylation of the bound p27 awaits three-dimensional structural analysis of the tetrameric complex. From the regulatory point of view, this Cdc6-driven mechanism provides cells with a highly effective tool to overcome inhibition of Cdk2 by high levels of p27 that have accumulated during G0 or G1 arrest invoked by growth factor withdrawal or anchorage deprivation.
It is to our surprise that p27 Thr197 phosphorylation was exerted not only by Pim but also ROCK, which mediates anchorage signals to control cytoskeleton as well as activate mTORC1, because this kinase has never been implicated to phosphorylate p27. As already noted, we previously observed that unlike mTORC1 activation, expression of an active ROCK1 together with Cdc6 restored both Cdk4 and Cdk2 activities and induced anchorage-independent proliferation albeit weakly (17). In light of the current finding, the mechanistic basis for the Cdk2 activation by expression of active ROCK1 and Cdc6 is now understood.
Although ROCK1 can phosphorylate the C terminus of free p27 as demonstrated in Fig. 3D, it appears that this kinase more efficiently phosphorylates the p27 molecule that properly binds and thereby inactivates Cdk2. Notably, upon Cdk2 activation by Cdc6, the amount of the Thr197-phosphorylated form of bound p27 markedly diminished, whereas the total amount of bound p27 decreased only marginally at most in the in vitro reactivation assay (Fig. 5A). This observation implies the following scenario. As well documented, multiple molecules of p27 or p21 bind one molecule of Cdk2 (29), but only one molecule of these inhibitors properly binds Cdk2 and causes its inactivation. The properly bound p27 molecule is preferentially phosphorylated by ROCK and removed by Cdc6 with full activation of the Cdk2. This scenario also well explains the strange association of C-terminal-nonphosphorylated p27 with the active Cdk2 in proliferating cells (Fig. 5A) as well as only the marginal reduction in the amount of Cdk2-associated p21 despite full reactivation of the Cdk2 by Cdc6 in vitro (4).
Finally, we would like to briefly comment on the induction of anchorage-independent proliferation by manipulation of G1 cell cycle factors or their related. As shown in Fig. 6, combined overexpression of Cdk6, cyclin D3, Cdc6, and active Rheb was sufficient to induce proliferation of rat embryonic fibroblasts in soft agar as rapidly as HeLa, the fully developed human cancer cells. There are numerous reports documenting that many of these factors are highly expressed in various cancer cells (30–34). In light of our finding, overexpression of these factors might in part account for their anchorage-independent proliferation capability.
Supplementary Material
This work was supported by grants-in-aid for Scientific Research (S) and the Global Center of Excellence from the Ministry of Education, Science, and Culture of Japan.

This article contains supplemental Figs. S1 and S2.
- ORC
- origin recognition complex
- p27
- p27KIP1
- p21
- p21CIP1
- REF
- rat embryonic fibroblast
- MC
- methylcellulose medium
- Rb
- retinoblastoma protein
- ROCK
- Rho-associated kinase
- aRK
- active ROCK1
- Cy
- cyclin-binding
- WB
- Walker B
- Anc
- anchorage.
REFERENCES
- 1. Méndez J., Stillman B. (2000) Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle. Assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20, 8602–8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelly T. J., Brown G. W. (2000) Regulation of chromosome replication. Annu. Rev. Biochem. 69, 829–880 [DOI] [PubMed] [Google Scholar]
- 3. Araki H. (2010) Cyclin-dependent kinase-dependent initiation of chromosomal DNA replication. Curr. Opin. Cell Biol. 22, 766–771 [DOI] [PubMed] [Google Scholar]
- 4. Kan Q., Jinno S., Yamamoto H., Kobayashi K., Okayama H. (2008) ATP-dependent activation of p21WAF1/CIP1-associated Cdk2 by Cdc6. Proc. Natl. Acad. Sci. U.S.A. 105, 4757–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kan Q., Jinno S., Kobayashi K., Yamamoto H., Okayama H. (2008) Cdc6 determines utilization of p21(WAF1/CIP1)-dependent damage checkpoint in S phase cells. J. Biol. Chem. 283, 17864–17872 [DOI] [PubMed] [Google Scholar]
- 6. Coats S., Whyte P., Fero M. L., Lacy S., Chung G., Randel E., Firpo E., Roberts J. M. (1999) A new pathway for mitogen-dependent cdk2 regulation uncovered in p27(Kip1)-deficient cells. Curr. Biol. 9, 163–173 [DOI] [PubMed] [Google Scholar]
- 7. Vervoorts J., Lüscher B. (2008) Post-translational regulation of the tumor suppressor p27(KIP1). Cell. Mol. Life Sci. 65, 3255–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carrano A. C., Eytan E., Hershko A., Pagano M. (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1, 193–199 [DOI] [PubMed] [Google Scholar]
- 9. Otsuka H., Moskowitz M. (1975) Arrest of 3T3 cells in G1 phase in suspension culture. J. Cell Physiol. 87, 213–219 [DOI] [PubMed] [Google Scholar]
- 10. Reddig P. J., Juliano R. L. (2005) Clinging to life. Cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 24, 425–439 [DOI] [PubMed] [Google Scholar]
- 11. Schulze A., Zerfass-Thome K., Bergès J., Middendorp S., Jansen-Dürr P., Henglein B. (1996) Anchorage-dependent transcription of the cyclin A gene. Mol. Cell. Biol. 16, 4632–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu X., Ohtsubo M., Böhmer R. M., Roberts J. M., Assoian R. K. (1996) Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 133, 391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blais A., Dynlacht B. D. (2004) Hitting their targets. An emerging picture of E2F and cell cycle control. Curr. Opin. Genet. Dev. 14, 527–532 [DOI] [PubMed] [Google Scholar]
- 14. Arakawa-Takeuchi S., Kobayashi K., Park J. H., Uranbileg B., Yamamoto H., Jinno S., Okayama H. (2010) Mammalian target of rapamycin complex 1 signaling opposes the effects of anchorage loss, leading to activation of Cdk4 and Cdc6 stabilization. FEBS Lett. 584, 2779–2785 [DOI] [PubMed] [Google Scholar]
- 15. Manning B. D., Cantley L. C. (2003) United at last. The tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signaling. Biochem. Soc. Trans. 31, 573–578 [DOI] [PubMed] [Google Scholar]
- 16. Sarbassov D. D., Ali S. M., Sabatini D. M. (2005) Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17, 596–603 [DOI] [PubMed] [Google Scholar]
- 17. Park J. H., Arakawa-Takeuchi S., Jinno S., Okayama H. (2011) Rho-associated kinase connects a cell cycle-controlling anchorage signal to the mammalian target of rapamycin pathway. J. Biol. Chem. 286, 23132–23141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin J., Jinno S., Okayama H. (2001) Cdk6-cyclin D3 complex evades inhibition by inhibitor proteins and uniquely controls cell proliferation competence. Oncogene 20, 2000–2009 [DOI] [PubMed] [Google Scholar]
- 19. Faast R., White J., Cartwright P., Crocker L., Sarcevic B., Dalton S. (2004) Cdk6-cyclin D3 activity in murine ES cells is resistant to inhibition by p16(INK4a). Oncogene 23, 491–502 [DOI] [PubMed] [Google Scholar]
- 20. Swanton C., Mann D. J., Fleckenstein B., Neipel F., Peters G., Jones N. (1997) Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 390, 184–187 [DOI] [PubMed] [Google Scholar]
- 21. Gu Y., Rosenblatt J., Morgan D. O. (1992) Cell cycle regulation of CDK2 activity by phosphorylation of Thr-160 and Tyr-15. EMBO J. 11, 3995–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kossatz U., Vervoorts J., Nickeleit I., Sundberg H. A., Arthur J. S., Manns M. P., Malek N. P. (2006) C-terminal phosphorylation controls the stability and function of p27KIP1. EMBO J. 25, 5159–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujita N., Sato S., Katayama K., Tsuruo T. (2002) Akt-dependent phosphorylation of p27KIP1 promotes binding to 14-3-3and cytoplasmic localization. J. Biol. Chem. 277, 28706–28713 [DOI] [PubMed] [Google Scholar]
- 24. Morishita D., Katayama R., Sekimizu K., Tsuruo T., Fujita N. (2008) Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27KIP1 at the transcriptional and posttranscriptional levels. Cancer Res. 68, 5076–5085 [DOI] [PubMed] [Google Scholar]
- 25. Fujita N., Sato S., Tsuruo T. (2003) Phosphorylation of p27KIP1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 278, 49254–49260 [DOI] [PubMed] [Google Scholar]
- 26. Pearce L. R., Komander D., Alessi D. R. (2010) The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22 [DOI] [PubMed] [Google Scholar]
- 27. Pandey D., Goyal P., Bamburg J. R., Siess W. (2006) Regulation of LIM-kinase 1 and cofilin in thrombin-stimulated platelets. Blood 107, 575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. (2007) The selectivity of protein kinase inhibitors. a further update. Biochem. J. 408, 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang H., Hannon G. J., Beach D. (1994) p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 8, 1750–1758 [DOI] [PubMed] [Google Scholar]
- 30. Semple J. W., Duncker B. P. (2004) ORC-associated replication factors as biomarkers for cancer. Biotechnol. Adv. 22, 621–631 [DOI] [PubMed] [Google Scholar]
- 31. Chen D., Law M. E., Theis J. D., Gamez J. D., Caron L. B., Vrana J. A., Dogan A. (2009) Clinicopathologic features of CDK6 translocation-associated B-cell lymphoproliferative disorders. Am. J. Surg. Pathol. 33, 720–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendrzyk F., Radlwimmer B., Joos S., Kokocinski F., Benner A., Stange D. E., Neben K., Fiegler H., Carter N. P., Reifenberger G., Korshunov A., Lichter P. (2005) Genomic and protein expression profiling identifies CDK6 as novel independent prognostic marker in medulloblastoma. J. Clin. Oncol. 23, 8853–8862 [DOI] [PubMed] [Google Scholar]
- 33. Lopez-Beltran A., Ordóñez J. L., Otero A. P., Blanca A., Sevillano V., Sanchez-Carbayo M., Muñoz E., Cheng L., Montironi R., de Alava E. (2010) Cyclin D3 gene amplification in bladder carcinoma in situ. Virchows Arch. 457, 555–561 [DOI] [PubMed] [Google Scholar]
- 34. Park S. J., Lee T. J., Chang I. H. (2011) Role of the mTOR pathway in the progression and recurrence of bladder cancer. An immunohistochemical tissue microarray study. Korean J. Urol. 52, 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.