Background: This study was designed to compare the role of Snail and Slug in pancreatic cancer.
Results: Snail and Slug have differential effects on three-dimensional scattering, motility, adhesion, and Rho signaling.
Conclusion: Snail, but not Slug, promotes motility and scattering in three-dimensional collagen of pancreatic cancer cells.
Significance: Understanding how Snail and Slug modulate pancreatic cancer progression may identify therapeutic targets for this deadly disease.
Keywords: Cell Invasion, Collagen, EMT, ERK, Matrix Metalloproteinase (MMP), Rac1, Rho GTPases, Slug, Snail
Abstract
The Snail family of transcription factors has been implicated in pancreatic cancer progression. We recently showed that Snail (Snai1) promotes membrane-type 1 matrix metalloproteinase (MT1-MMP)- and ERK1/2-dependent scattering of pancreatic cancer cells in three-dimensional type I collagen. In this study, we examine the role of Slug (Snai2) in regulating pancreatic cancer cell scattering in three-dimensional type I collagen. Although Slug increased MT1-MMP expression and ERK1/2 activity, Slug-expressing cells failed to scatter in three-dimensional collagen. Moreover, in contrast to Snail-expressing cells, Slug-expressing cells did not demonstrate increased collagen I binding, collagen I-driven motility, or α2β1-integrin expression. Significantly, inhibiting β1-integrin function decreased migration and scattering of Snail-expressing cells in three-dimensional collagen. As Rho GTPases have been implicated in invasion and migration, we also analyzed the contribution of Rac1 and Rho signaling to the differential migration and scattering of pancreatic cancer cells. Snail-induced migration and scattering were attenuated by Rac1 inhibition. In contrast, inhibiting Rho-associated kinase ROCK1/2 increased migration and scattering of Slug-expressing cells in three-dimensional collagen and thus phenocopied the effects of Snail in pancreatic cancer cells. Additionally, the increased migration and scattering in three-dimensional collagen of Slug-expressing cells following ROCK1/2 inhibition was dependent on β1-integrin function. Overall, these results demonstrate differential effects of Snail and Slug in pancreatic cancer and identify the interplay between Rho signaling and β1-integrin that functions to regulate the differential scattering and migration of Snail- and Slug-expressing pancreatic cancer cells.
Introduction
Pancreatic cancer, which is one of the deadliest of human malignancies (1–3), is associated with a pronounced collagen I-rich stromal reaction (4–6). Although collagen I can function as a barrier to invasion, increased collagen I expression in the gene signatures of several epithelial cancers is in fact associated with increased risk of metastasis (7, 8). Moreover, collagen fibers can facilitate metastasis by directing cancer cells to the vasculature (9, 10). We have previously shown that pancreatic cancer cells on encountering collagen I up-regulate membrane-type 1 matrix metalloproteinase (MT1-MMP,3 a.k.a. MMP-14) (11–14), a key proteinase that promotes growth and invasion in the three-dimensional collagen microenvironment (15, 16). Recently, we have found that pancreatic cancer cells on encountering collagen I also up-regulate the transcription factor Snail (Snai1) (12), one of the main regulators of epithelial-mesenchymal transition (17, 18). Snail increases MT1-MMP expression in pancreatic cancer cells, mediated partly through increased ERK1/2 signaling, to promote scattering and invasion of cancer cells in the collagen microenvironment (12). Significantly, both Snail and MT1-MMP are overexpressed in human pancreatic tumor specimens (11, 12, 18, 19).
Members of the Rho family of small GTPases have been shown to be key regulators of cellular invasion and migration by their action on actin, microtubules, and actomyosin contractility (20, 21). For example, Rac1 enhances cellular migration by promoting lamellipodia formation (22), whereas RhoA signals to Rho-associated kinases ROCK1/2 to induce actomyosin contractility (23, 24). There is also significant cross-talk between the different Rho-GTPase family members. Specifically, activation of Rac1 signaling can inhibit RhoA function, whereas activation of ROCK1/2 can inhibit Rac1 function (25). The cytoskeletal changes induced by Rho-GTPases can also in turn affect the function of integrins (26, 27). More importantly, the Rho proteins are deregulated in tumors and correlate with disease progression (28). Gene expression profiles have shown that Rac1 is up-regulated in human pancreatic tumor specimens (29) and that deletion of Rac1 in a mouse model of pancreatic cancer prevents tumor development (30). Interestingly, Rho-GTPases have also been shown to modulate the expression and function of Snail family members (31, 32).
Although the Snail-related protein Slug (Snai2) is also overexpressed in human pancreatic tumors (33), it is not known the extent to which Slug functions to modulate the behavior of pancreatic cancer cells in the collagen-rich tumor microenvironment. Both Snail and Slug can increase matrix metalloproteinase (MMP) expression and promote migration and invasion (34). Slug can increase MMP-9 expression in pancreatic cancer cells and oral squamous cell cancer cells to promote invasion (35, 36). Unlike Snail, which causes single cell migration of oral cancer cells (37), we have previously shown that Slug does not cause single cell migration but rather is involved in cohort migration of oral squamous cancer cells (36). Slug, but not Snail, also promotes cohort migration of breast cancer cells (38), suggesting that Snail and Slug can have differing effects on the behavior of cancer cells.
In this study, we examine the effect of Slug on MT1-MMP expression and on scattering of pancreatic cancer cells in three-dimensional collagen gels. Similar to Snail, we have found that Slug increases MT1-MMP expression and ERK1/2 phosphorylation; however, Slug does not increase scattering of pancreatic cancer cells in three-dimensional collagen. Also in contrast to Snail, we show that Slug does not increase single cell migration of pancreatic cancer cells, nor increase α2β1-integrin expression to enhance migration and scattering in three-dimensional collagen. We show that Snail-induced migration and scattering are mediated by Rac1, whereas Rho-associated kinase ROCK1/2 functions to block migration and scattering of Slug-expressing cells, suggesting that Snail and Slug can differentially modulate Rho GTPases to effect single cell migration and scattering in three-dimensional collagen.
EXPERIMENTAL PROCEDURES
Materials
General tissue culture materials were obtained from VWR International (West Chester, PA). Antibodies against Snail (3879) and phospo-ERK1/2 (9101) and the MEK1/2 inhibitor U0126 (9903) were purchased from Cell Signaling (Danvers, MA). Antibodies against Slug (sc-1539), α-tubulin (sc-8035), ROCK2 (sc-5561), β1-integrin (P5D2, sc-13590 and M106, sc-8978), and α3-integrin (I-19, sc-6592) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rac1 (05-389), TIMP-2 (IM11L), α2-integrin (MAB1936 and MAB1950), α3-integrin (MAB1952), and β1-integrin-blocking (MAB1987z, P4C10 clone) antibodies were obtained from Millipore. Transferrin receptor (BD612124) and ROCK1 (BD611136) antibodies were obtained from BD Biosciences. MT1-MMP (ab38971) and HRP-conjugated secondary antibodies were obtained from Abcam (Cambridge, MA) and Sigma, respectively. Type I collagen (354249), diluted to 2 mg/ml according to manufacturer's protocol, was purchased from BD Biosciences. MMP inhibitor GM6001 (561206), ROCK1/2 inhibitor Y27632 (688001), and Rac1 inhibitor NSC23766 were purchased from Calbiochem and used at a final concentration of 10 μm. Nucleofector electroporation kit (VCA-1001) was purchased from Lonza. Collagenase (4196) was purchased from Worthington Biochemical.
Generation of Pancreatic Cancer Cells Inducibly Expressing Snail and Slug
The Snail and Slug genes were subcloned into pRetroX-Tight-Pur vector, and viral particles were generated as previously published (12, 36). A similar protocol was used to generate viral particles for pTet-On advanced vector (Clontech). Parental Panc1 and AsPC1 cells were first infected with pTet-On, then stable cells resistant to G418 were selected, followed by infection with pTight-Snail, pTight-Slug, or pTight-Luc, and stable cell lines resistant to both G418 and puromycin were selected. The stable cell lines were routinely maintained in DMEM with Tet system approved FBS (Clontech), puromycin, and G418. For Snail and Slug induction, doxycycline at a final concentration of 2 μg/ml was added to the growth media.
Down-regulation of Rac1 and ROCK1/2
Rac1 expression was transiently down-regulated using a pool of three sequences specific siRNAs (Rac1si) from Santa Cruz Biotechnology (sc-44325). ROCK1 was down-regulated using the duplex siRNA (ROCK1Si) forward 5-GCCAAUGACUUACUUAGGAdTdT-3 and reverse 5-UCCUAAGUAAGUCAUUGGCdTdT-3, whereas ROCK2 was down-regulated using the duplex siRNA (ROCK2Si) forward 5-GCAAAUCUGUUAAUACUCGdTdT-3 and reverse 5-CGAGUAUUAACAGAUUUGCdTdT-3 (39). Pancreatic cancer cells were transfected with siRNA for the gene of interest or control siRNA using the Nucleofector kit R (Amaxa/Lonza) (12).
Embedding and Examination of Cells in Three-dimensional Type I Collagen Gels
Collagen mixture (2 mg/ml) was made by adding the appropriate volumes of sterile water, 10× DMEM, and NaOH and kept on ice until needed (40). Cells were then suspended in the collagen solution and allowed to gel for 20 min at 37 °C. For RNA extraction, the gel containing cells was processed using the Qiagen RNeasy extraction kit (74106) to extract RNA for quantitative RT-PCR analysis. For protein analyses, cells were extracted out of the gels using collagenase and lysed. For morphological examination of cells, 5 × 103 cells were suspended in collagen; the resulting cell colonies were examined using a Zeiss Axiovert 40 CFL microscope, and pictures were taken with a Nikon Coolpix 4500 camera. The percentage of colony scattering was quantified by counting the average number of scattered colonies, (loosely arranged, elongated with projections) per field, from a minimum of five different fields at 100× magnification.
Motility Assay
Haptotactic motility was assessed as described previously with some modification (11, 36, 41). One thousand cells were plated onto thin-layer type I collagen overlaid with colloidal gold. Cells were allowed to migrate, and phagokinetic tracks were monitored by visual examination using a Zeiss microscope and photographed using a Nikon camera.
Adhesion Assay
Cells were seeded onto tissue culture plates precoated with type I collagen. Cells were allowed to adhere at 37 °C for 10 min, washed once with PBS, and then fixed and stained (40, 41). Cells were imaged using a Zeiss microscope, photographed using a Nikon camera, and counted using the ImageJ software.
DQ-Collagen I Assay
Glass bottom culture dishes (P35GC-1.5-14-C, MatTek Corp., Ashland, MA) were coated with 50 μl of Matrigel containing 25 μg/ml DQ-collagen I (D12060, Molecular Probes) at 37 °C for 20 min, and then pancreatic cancer cells were plated onto the coated surfaces. The cells were then imaged 24 h later using Zeiss LSM 510 META confocal microscope (42).
Flow Cytometric Analysis
Cells (3 × 105) were treated with monoclonal anti α2-, α3-, or β1-integrin (1:100) for 1 h at room temperature with gentle shaking. Cells were stained with secondary antibody conjugated to Alexa Fluor 488 (1:200) for 30 min at room temperature, washed twice with PBS, and resuspended in PBS for analysis with Summit software 4.3 on a Beckman Coulter fluorescence-activated cell sorter (39).
Cell Surface Biotinylation
Doxycycline-treated pancreatic cancer cells were grown to confluence in a 6-well plate, washed with ice-cold phosphate-buffered saline, and incubated at 4 °C for 30 min with 0.5 mg/ml cell-impermeable Sulfo-NHS-LC-Biotin in ice-cold phosphate-buffered saline followed by washing with 100 mm glycine to quench free biotin (39, 43–45). To isolate biotinylated cell surface proteins, equal amounts of protein from each sample were incubated with streptavidin beads at 4 °C for 14 h followed by centrifugation. After boiling in Laemmli sample dilution buffer to dissociate streptavidin bead-biotin complexes, the biotin-labeled samples were analyzed by SDS-PAGE (7.5% gels) and immunoblotted for integrins.
Quantitative Real Time-PCR Analysis
Reverse transcription of RNA to cDNA was performed using TaqMan reverse transcription reagents (N808-0234) from Applied Biosystems. Quantitative gene expression was performed for TIMP-2 (Hs00234278_m1) and GAPDH (Hs99999905_m1) with gene-specific TaqMan probes, TaqMan universal PCR master mix (4324018) and the 7500 fast real time PCR system from Applied Biosystems. The data were then quantified with the comparative CT method for relative gene expression (11, 37, 46).
Immunoblotting
Immunoblotting was done as described previously (43, 44), and integrins were detected by enhanced chemiluminescence using Western blotting reagents (Pierce Biotechnology).
Statistical Analysis
All statistical analyses were done using Microsoft Excel.
RESULTS
Slug Increases MT1-MMP but Does Not Induce Scattering in Three-dimensional Collagen
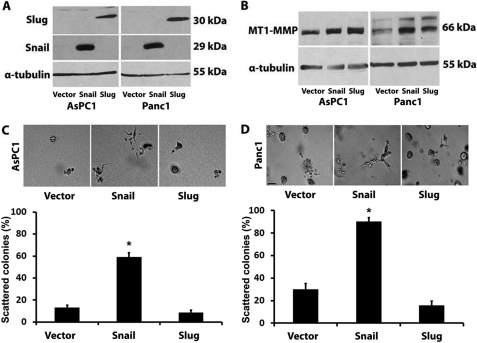
We recently showed that Snail induces MT1-MMP in pancreatic cancer cells to promote scattering in three-dimensional collagen gels (12). Although the Snail-related protein Slug is up-regulated in human pancreatic cancer and contributes to tumor progression (33, 35), Snail and Slug can have differing effects on the behavior of cancer cells. Thus, to better understand the role of Snail and Slug in pancreatic cancer, we created Panc1 and AsPC1 cells expressing Snail or Slug protein using a doxycycline-inducible system. Treatment with doxycycline resulted in robust expression of Snail and Slug in both AsPC1 and Panc1 cells (Fig. 1A). Initially, we examined the effect of Snail and Slug on MT1-MMP, a key proteinase that is required for invasion of cancer cells in the collagen-rich tumor microenvironment. As we recently showed that Snail increases MT1-MMP in Panc1 cells (12), Snail also increases MT1-MMP in AsPC1 cells (Fig. 1B). We also found that Slug increases MT1-MMP to levels comparable with those induced by Snail in both AsPC1 and Panc1 cells (Fig. 1B). We next examined the effect of expressing Snail and Slug on the behavior of AsPC1 and Panc1 cells grown in three-dimensional collagen gels. Expression of Snail, which we have shown causes a scattering phenotype in Panc1 cells (12), also causes scattering of AsPC1 cells in three-dimensional collagen (Fig. 1C). However, Slug failed to induce a scattering phenotype in either AsPC1 or Panc1 cells (Fig. 1, C and D). These results suggest that up-regulation of MT1-MMP is not sufficient to induce scattering of Slug-expressing cells in three-dimensional collagen.
FIGURE 1.
Slug increases MT1-MMP, but does not induce scattering in three-dimensional collagen gels. PDAC (AsPC1 and Panc1) cells were transfected with pTet-On (Clontech) vector and co-transfected with pTight-Luc, pTight-Snail, or pTight-Slug vector, and cell lines resistant to both G418 and puromycin were selected (PDAC-Vector, PDAC-Snail, PDAC-Slug). A and B, an equal number of PDAC-Vector, PDAC-Snail, and PDAC-Slug cells were plated on plastic and treated with doxycycline (2 μg/ml). The expression of Snail and Slug was analyzed at 16 h by Western blot analysis using α-tubulin as normalization control (A). MT1-MMP expression was analyzed at 24 h by Western blotting (B). C and D, stable PDAC cells were embedded in three-dimensional collagen gels (2 mg/ml), and doxycycline-containing media were changed every 2 days for 4–6 days. The effect of Snail and Slug on colony morphology was examined by phase contrast microscopy at 4 days for AsPC1 cells and 6 days for Panc1 cells (top), and the percentage of scattered colonies per field was quantified as detailed under “Experimental Procedures.” (bottom). The results are representative of at least four independent experiments. Error bars in C and D indicate S.E. *, p < 0.05.
Effect of Snail and Slug on TIMP-2 Levels and ERK1/2 Phosphorylation
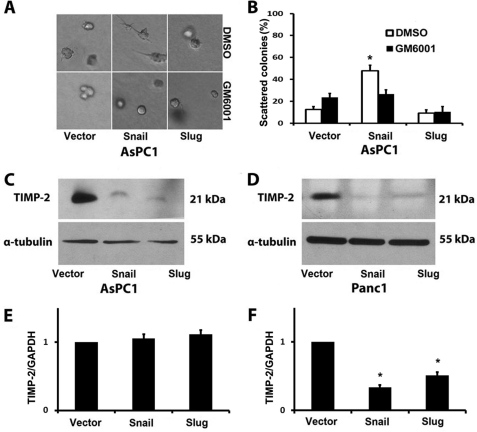
Because the Snail-induced scattering phenotype of Panc1 cells requires MMP activity (12), we examined whether the phenotype in AsPC1 cells was also dependent on MMP activity. AsPC1 cells in three-dimensional collagen were grown in the presence of the broad spectrum MMP inhibitor GM6001 or DMSO as vehicle control. As shown in Fig. 2, A and B, Snail-induced scattering of AsPC1 cells also requires MMP activity. We next evaluated the effect of Snail and Slug on TIMP-2, an endogenous inhibitor of MT1-MMP activity that blocks Snail-induced scattering in three-dimensional collagen (12). Snail and Slug cells were grown in three-dimensional collagen, and TIMP-2 protein levels in the conditioned media surrounding the cells in three-dimensional collagen were examined by Western blotting. Expression of Snail and Slug reduced TIMP-2 protein levels in the conditioned media from both AsPC1 and Panc1 cells (Fig. 2, C and D). We also examined the effect of Snail and Slug on TIMP-2 mRNA levels by real time PCR. Snail and Slug did not affect TIMP-2 mRNA levels in AsPC1 cells (Fig. 2E), but decreased TIMP-2 mRNA levels to a similar extent in Panc1 cells (Fig. 2F). Thus, the difference in the scattering phenotype between Snail- and Slug-expressing pancreatic cancer cells in three-dimensional collagen cannot be due to changes in TIMP-2 levels. In addition, we examined the effect of Snail and Slug on pericellular proteolytic activity using DQ-collagen I substrate and found no difference in the collagen proteolytic activity between Snail- and Slug-expressing pancreatic cancer cells (supplemental Fig. S1).
FIGURE 2.
Effect of Snail and Slug on TIMP-2 levels. A and B, stable PDAC cells were grown in three-dimensional collagen gels (2 mg/ml) and doxycycline together with DMSO (vehicle control) or GM6001 (10 μm) added every 2 days for 4–6 days. The effect on colony morphology was examined by phase contrast microscopy (A), and the percentage of scattered colonies per field was quantified (B). Stable PDAC cells were embedded in three-dimensional collagen gels (2 mg/ml), and doxycycline-containing media were changed every 2 days for 4–6 days. C and D, the conditioned media surrounding the cells in the collagen gel were collected, concentrated, and analyzed for TIMP-2 levels by Western blotting, and the lysates of cells in the collagen gel were analyzed for α-tubulin by Western blotting. E and F, the effect of Snail and Slug on TIMP-2 mRNA expression was analyzed by quantitative RT-PCR using GAPDH as normalization control. Error bars in E and F indicate S.E. *, p < 0.05. The results are representative of at least three independent experiments.
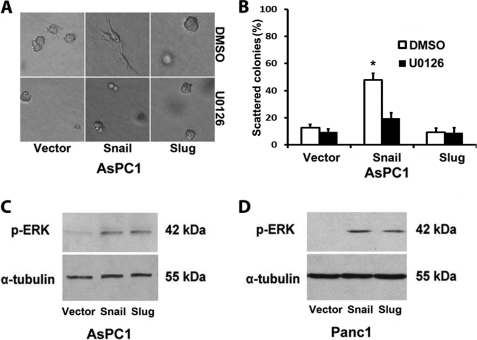
Because Snail-induced scattering of Panc1 cells in three-dimensional collagen requires ERK1/2 activity (12), we examined whether the increased scattering of AsPC1-Snail cells also requires ERK1/2 and whether there is differential activation of ERK1/2 signaling by Snail and Slug in pancreatic cancer cells. As shown in Fig. 3, A and B, Snail-induced scattering of AsPC1 cells in three-dimensional collagen requires ERK1/2 signaling. However, there is no difference in the relative activation of ERK1/2 signaling in the Snail- and Slug-expressing AsPC1 and Panc1 cells (Fig. 3, C and D). Moreover, there was no difference in the expression of ERK1/2 in the Snail- and Slug-expressing AsPC1 and Panc1 cells (supplemental Fig. S2A). These results suggest that despite the fact that Slug increases ERK1/2 activity, it is not sufficient to induce scattering of Slug-expressing cells in three-dimensional collagen.
FIGURE 3.
Snail and Slug increase ERK1/2 phosphorylation in PDAC cells. A and B, AsPC1-vector, AsPC1-Snail, and AsPC1-Slug cells were suspended in three-dimensional collagen gel (2 mg/ml), and fresh serum-containing medium supplemented with doxycycline and either DMSO or U0126 (10 μm) was added every 2 days for 4 days. The effect on colony morphology was examined by phase contrast microscopy (A), and the percentage of scattered colonies per field was quantified (B). C and D, stable PDAC cells were serum-starved for 24 h and induced with doxycycline (2 μg/ml) for 12 h, and the lysates were analyzed for phospho-ERK1/2 (p-ERK) and α-tubulin expression by Western blotting. The results are representative of at least three independent experiments. Error bars in B indicate S.E. *, p < 0.05.
Snail, but Not Slug, Increases Pancreatic Cancer Cell Collagen I Binding, Collagen I-driven Motility, and α2β1-Integrin Expression
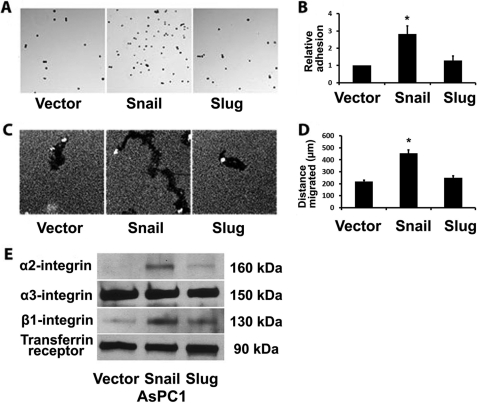
Because the interaction of the cells with the surrounding extracellular matrix can also affect the ability of cells to invade and scatter (47), we examined the effect of Snail and Slug on adhesion of pancreatic cancer cells to type I collagen. AsPC1 cells were treated with doxycycline for 24 h to induce Snail and Slug expression, trypsinized, and plated onto collagen-coated tissue culture plates for 10 min for the cells to adhere and then washed with PBS to remove nonadherent cells. As shown in Fig. 4, A and B, Snail-expressing cells demonstrate increased adhesion to collagen. We also examined the effect of Snail and Slug on single cell migration of AsPC1 cells using a colloidal gold assay. As shown in Fig. 4, C and D, AsPC1 cells migrate on collagen-coated plates as demonstrated by the tracks generated by individual cells, with AsPC1-Snail cells demonstrating increased migration when compared with AsPC1-vector or AsPC1-Slug cells. Similar to the increased scattering of AsPC1-Snail cells in three-dimensional collagen (Fig. 3), the increased migration of AsPC1-Snail cells also required ERK1/2 signaling (supplemental Fig. S3).
FIGURE 4.
Snail, but not Slug, increases pancreatic cancer cell collagen I binding, collagen I-driven motility, and α2β1-integrin expression. A and B, stable AsPC1 cells were induced with doxycycline (2 μg/ml) for 24 h and then plated onto tissue culture plates coated with thin-layer collagen, allowed to adhere at 37 °C for 10 min, washed, and then stained. Adherent cells were photographed (A) and counted (B). C and D, AsPC1 cells were induced with doxycycline (2 μg/ml) for 24 h, plated onto thin-layer type I collagen matrix overlaid with colloidal gold, and allowed to migrate for 24 h, and the tracks were photographed (C) and quantified (D). E, equal numbers of AsPC1 cells were induced with doxycycline for 24 h, surface-labeled with biotin, and lysed, and cell lysates were immunoprecipitated with streptavidin and analyzed for α2-, α3-, and β1-integrin and transferrin receptor (loading control) by Western blotting. Error bars in B and D indicate S.E. The results are representative of at least three independent experiments. *, p < 0.05.
We next evaluated the effect of Snail and Slug on the expression of α2- and β1-integrins, which mediate binding to collagen I (48, 49). As a control, we also examined the effect of Snail and Slug on α3-integrin expression. AsPC1 cells were treated with doxycycline for 24 h to induce Snail and Slug expression and surface-labeled with biotin. The lysates were then immunoprecipitated with streptavidin, and the effect on integrin expression was determined by Western blotting. As shown in Fig. 4E, Snail significantly increases cell surface α2- and β1-integrin expression without affecting cell surface α3-integin levels. Moreover, flow cytometric analysis revealed that Snail also increases α2- and β1-integrin expression in Panc1 cells (supplemental Fig. S4).
β1-Integrin Mediates Snail-driven Three-dimensional Scattering and Collagen I-driven Motility
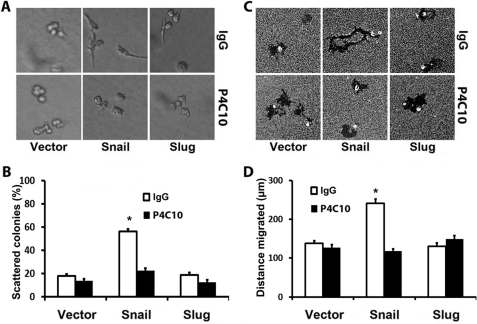
We next examined whether the β1-integrin expression mediates three-dimensional scattering and motility of AsPC1 cells. AsPC1 cells were treated with doxycycline for 24 h to induce Snail and Slug expression, trypsinized, and preincubated with control IgG or function-blocking β1-integrin antibody (clone P4C10) and then suspended in three-dimensional collagen gels or plated onto thin-layer type I collagen matrix overlaid with colloidal gold. As shown in Fig. 5, A and B, Snail-expressing cells demonstrate a scattered phenotype in three-dimensional collagen, which is significantly attenuated in the presence of the β1-integrin-blocking antibody. Similarly, the β1-integrin-blocking antibody also significantly attenuates Snail-induced motility of AsPC1 cells (Fig. 5, C and D).
FIGURE 5.
β1-Integrin mediates Snail-driven three-dimensional scattering and collagen-driven motility. AsPC1-vector, AsPC1-Snail, and AsPC1-Slug cells were induced with doxycycline (2 μg/ml) for 24 h, trypsinized, and incubated with a function-blocking β1-integrin antibody (P4C10) or isotype-matched control IgG antibody for 30 min. A and B, the cells were then suspended in three-dimensional collagen gel (2 mg/ml), and fresh serum-containing medium supplemented with doxycycline and β1-integrin-blocking antibody or control antibody was added every 2 days for 4 days. The effect on colony morphology was examined by phase contrast microscopy (A), and the percentage of scattered colonies per field was quantified (B). C and D, cells were also plated onto thin-layer type I collagen matrix overlaid with colloidal gold and allowed to migrate for 24 h, and the tracks were photographed (C) and quantified (D). Error bars in B and D indicate S.E. The results are representative of at least four independent experiments. *, p < 0.05.
Rac1 Mediates Snail-induced Scattering and Motility, whereas ROCK1/2 Blocks Scattering and Motility of Slug Cells
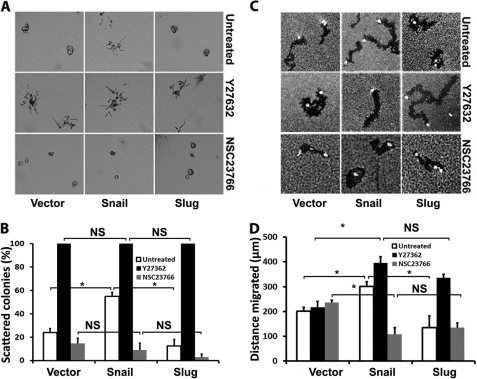
Members of the Rho family of GTPases have been implicated in migration and invasion in three-dimensional collagen matrices, with Rac1 and RhoA-ROCK1/2 signaling having differing effects on migration and invasion (25). Thus, we examined the effect of blocking Rac1 or ROCK1/2 signaling in AsPC1-Snail and AsPC1-Slug cells using well defined small molecule inhibitors of Rac1 and ROCK1/2. Stable AsPC1 cells were suspended in three-dimensional collagen and treated with the Rac1 inhibitor NSC23766 or the ROCK1/2 inhibitor Y27632 (50, 51). Although Snail and Slug did not affect expression of Rac1 or ROCK1/2 (supplemental Fig. S2, B and C), the Rac1 inhibitor significantly decreased the number of scattered colonies seen in AsPC1-Snail cells (Fig. 6, A and B). In contrast, the ROCK1/2 inhibitor caused the generation of scattered colonies in all AsPC1 cells. We also examined the effect of the Rac1 and ROCK1/2 inhibitors on collagen-driven motility. As shown in Fig. 6, C and D, inhibiting Rac1 decreases the motility of AsPC1-Snail cells, whereas inhibiting ROCK1/2 increases motility of AsPC1-Slug cells.
FIGURE 6.
Rac1 inhibitor blocks Snail-induced scattering and motility, whereas ROCK1/2 inhibitor promotes scattering and motility of Slug cells. AsPC1-vector, AsPC1-Snail, and AsPC1-Slug cells were induced with doxycycline (2 μg/ml) for 24 h. A and B, the cells were then suspended in three-dimensional collagen gel (2 mg/ml), and fresh serum-containing medium supplemented with doxycycline and ROCK1/2 inhibitor Y27632 (10 μm) or Rac1 inhibitor NSC23766 (10 μm) was added every 2 days for 4 days. The effect on colony morphology was examined by phase contrast microscopy (A), and the percentage of scattered colonies per field was quantified (B). C and D, cells were also plated onto thin-layer type I collagen matrix overlaid with colloidal gold and allowed to migrate for 24 h, and the tracks were photographed (C) and quantified (D). Error bars in B and D indicate S.E. The results are representative of at least three independent experiments. *, p < 0.05. NS, not significant.
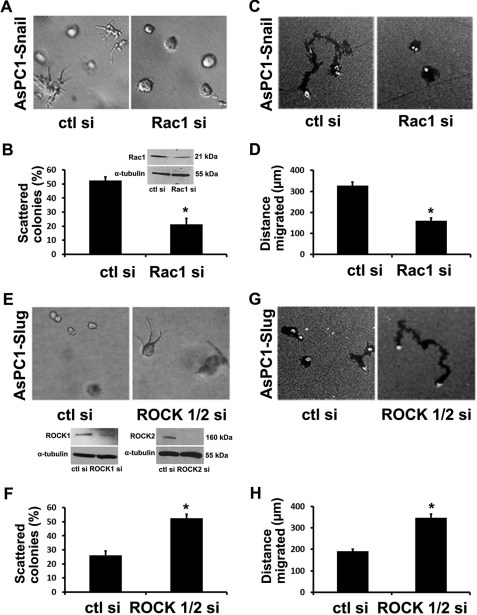
To further demonstrate the role of Rac1 in regulating Snail-induced scattering phenotype and motility, AsPC1-Snail cells were transfected with control siRNA or siRNA against Rac1. The cells were then suspended in three-dimensional collagen gels or plated onto collagen matrix overlaid with colloidal gold. The Rac1 siRNA significantly decreased Rac1 protein expression (Fig. 7B, inset), decreased the number of scattered colonies (Fig. 7, A and B), and attenuated the collagen-driven motility (Fig. 7, C and D). Similarly, the effect of siRNA against ROCK1/2 on the behavior of Slug cells was also examined. The siRNA successfully decreased ROCK1 and ROCK2 protein expression (Fig. 7F, inset), resulting in increased scattering in three-dimensional collagen (Fig. 7, E and F) and increased collagen-driven motility of Slug cells (Fig. 7, G and H).
FIGURE 7.
Rac1 siRNA blocks Snail-induced scattering and motility, whereas ROCK1/2 siRNA promotes scattering and motility of Slug cells. A–D, AsPC1-Snail cells transfected with control siRNA (ctl si) or Rac1 siRNA (Rac1 si) were suspended in three-dimensional collagen gels and allowed to grow for 3 days in media supplemented with doxycycline (A) or plated onto thin-layer type I collagen matrix overlaid with colloidal gold and allowed to migrate over 24 h (C). The percentage of scattered colonies per field (B) and the tracks generated by motile cells were quantified (D). Rac1 expression was analyzed by Western blotting using α-tubulin as loading control (B, inset). E–H, AsPC1-Slug cells transfected with control siRNA or a mixture of ROCK1 and ROCK2 siRNA were suspended in three-dimensional collagen gels and allowed to grow for 3 days (E) or plated onto thin-layer type I collagen matrix overlaid with colloidal gold and allowed to migrate over 24 h (G). The percentage of scattered colonies per field (F) and the tracks generated by motile cells were quantified (H). ROCK1 and ROCK2 expression was analyzed by Western blotting using α-tubulin as loading control (F, inset). Error bars in B, D, F, and H indicate S.E. The results are representative of at least three independent experiments. *, p < 0.05.
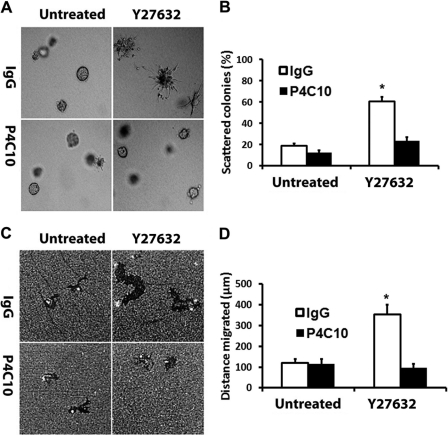
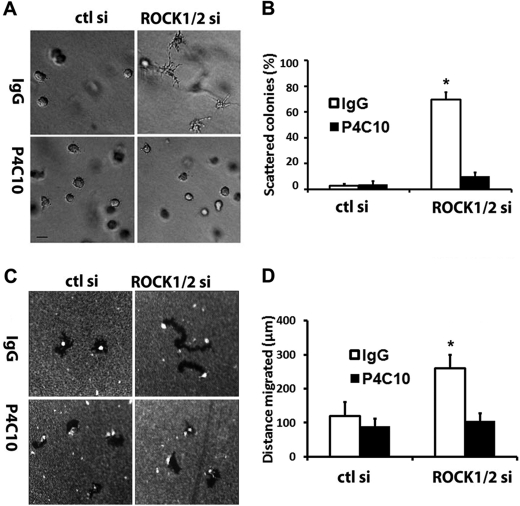
Y27632- and ROCK1/2 siRNA-driven Scattering and Motility of Slug Cells Are Mediated by β1-Integrin
Because Snail-induced scattering and motility are dependent on β1-integrin (Fig. 5), we next examined whether the increased scattering and motility of Slug cells following Y27632 treatment were also dependent on β1-integrin. AsPC1-Slug cells were treated with doxycycline for 24 h, trypsinized, and incubated with control IgG antibody or β1-integrin-blocking antibody for 30 min. The cells were then suspended in three-dimensional collagen gels or plated atop collagen matrix overlaid with colloidal gold. As shown previously in Fig. 6A, AsPC1-Slug cells plated in collagen and treated with Y27632 demonstrate increased projection in the presence of control IgG antibody. In contrast, the function-blocking β1-integrin antibody blocks Y27632-induced phenotype of AsPC1-Slug cells in three-dimensional collagen (Fig. 8, A and B). Similarly, the β1-integrin antibody blocks Y27632-induced motility of AsPC1-Slug cells (Fig. 8, C and D). We also examined whether the ROCK1/2 siRNA-induced scattering and motility of AsPC1-Slug cells could be blocked with the function-blocking β1-integrin antibody. AsPC1-Slug cells were transfected with control siRNA or siRNAs against ROCK1 and ROCK2, allowed to recover overnight, trypsinized, and incubated with control IgG antibody or β1-integrin-blocking antibody for 30 min. The cells were then grown in three-dimensional collagen gels or plated atop collagen matrix overlaid with colloidal gold. As shown in Fig. 9, A and B, the function-blocking β1-integrin antibody blocks ROCK1/2 siRNA-induced phenotype of AsPC1-Slug cells in three-dimensional collagen. The β1-integrin antibody also blocks ROCK1/2 siRNA-induced motility of AsPC1-Slug cells (Fig. 9, C and D). Overall, these results demonstrate the interplay between Rho signaling and β1-integrin that functions to control scattering and motility of Snail- and Slug-expressing pancreatic cancer cells.
FIGURE 8.
Y27632-driven scattering and motility of Slug cells is mediated by β1-integrin. AsPC1-Slug cells were induced with doxycycline (2 μg/ml) for 24 h, trypsinized, and incubated with control IgG antibody or function-blocking β1-integrin antibody (P4C10) for 30 min. A and B, the cells were then suspended in three-dimensional collagen gel (2 mg/ml), and fresh serum-containing medium supplemented with doxycycline and ROCK1/2 inhibitor Y27632 was added for 2 days. The effect on colony morphology was examined by phase contrast microscopy (A), and the percentage of scattered colonies per field was quantified (B). C and D, cells were also plated onto thin-layer type I collagen matrix overlaid with colloidal gold and allowed to migrate for 24 h, and the tracks were photographed (C) and quantified (D). Error bars in B and D indicate S.E. The results are representative of at least three independent experiments. *, p < 0.05.
FIGURE 9.
ROCK1/2 siRNA-driven scattering and motility of Slug cells is mediated by β1-integrin. AsPC1-Slug cells transfected with control siRNA (ctl si) or a mixture of ROCK1 and ROCK2 siRNA (ROCK1/2 si), allowed to recover for 24 h, trypsinized, and incubated with control IgG antibody or function-blocking β1-integrin antibody (P4C10) for 30 min. A and B, the cells were then suspended in three-dimensional collagen gel (2 mg/ml). The effect on colony morphology was examined by phase contrast microscopy (A), and the percentage of scattered colonies per field was quantified (B). C and D, cells were also plated onto thin-layer type I collagen matrix overlaid with colloidal gold and allowed to migrate for 24 h, and the tracks were photographed (C) and quantified (D). Error bars in B and D indicate S.D. The results are representative of at least three independent experiments. *, p < 0.05.
DISCUSSION
There is increasing evidence that members of the Snail family of transcription factors can have both similar and differing roles in cancer progression (17). Both Snail and Slug have been associated with proteinase expression and invasion (52, 53). We have previously shown that both proteins increase expression of MMP-9 in oral cancer cells (36, 37), and in this study, we show that both Snail and Slug both increase MT1-MMP in pancreatic cancer cells. We also show that Snail and Slug both repress TIMP-2 protein levels in pancreatic cancer cells. It has previously been shown that Snail and Slug can increase invasion of breast, squamous, and pancreatic cancer cells (35–38). We have found that Snail also increases invasion and scattering of pancreatic cancer cells in three-dimensional collagen (12); however, expression of Slug in pancreatic cancer cells does not result in increased scattering of pancreatic cancer cells in three-dimensional collagen. We also show that Snail and Slug have differing effects on single cell migration of pancreatic cancer cells. This is consistent with what we have previously found in oral cancer cells. Snail enhanced single cell migration of oral cancer cells, whereas Slug promoted cohort migration of oral cancer cells (36, 37). Moreover, Slug-expressing breast tumors appear to invade as a cohesive group of cells, whereas Snail-expressing tumors show more individual invasion of cancerous cells into the surrounding stroma (38).
We also show that Snail and Slug have differing effects on cell adhesion and integrin expression. In contrast to Slug, Snail increases adhesion and enhances expression of collagen-binding α2β1integrins to promote motility. Blocking β1-integrin attenuates Snail-driven motility and invasion of pancreatic cancer cells. Snail has previously been shown to modulate integrin expression. Snail increases expression of α5-integrin and represses α3-, α6-, and β4-integrin expression in Madin-Darby canine kidney cells and A431 cells (54). Thus, Snail increases binding of Madin-Darby canine kidney cells and A431 cells to fibronectin and decreases attachment to basement membrane proteins (54). However, Snail was recently shown to repress α5-, α2-, and β1-integrin expression in ARCaP and LNCaP prostate cancer cells and consequently was associated with decreased adhesion to fibronectin and collagen (55). Although we found that Slug does not affect α2-, α3-, or β1-integrin expression in pancreatic cancer cells, Slug represses α3-, β1-, and β4-integrin expression in keratinocytes to decrease attachment to laminin-5 matrix (56). These results suggest that the effects of Snail and Slug on integrin expression depend on the type and the nature of tumor cells.
We show that Snail and Slug have a similar effect on MT1-MMP and TIMP-2 levels and on pericellular proteolytic activity. Interestingly, the effect of Snail and Slug on TIMP-2 is primarily at the protein level instead of at the mRNA level. It has previously been shown that TIMP-2 can be regulated at the post-transcriptional level by an MT1-MMP-dependent endocytic degradation process (43, 44, 57, 58). TIMP-2 is recruited to the cell surface by MT1-MMP, endocytosed via an MT1-MMP-dependent process, and subsequently degraded in the lysosomes (43, 44, 57, 58). Although we have previously shown that MT1-MMP is required for Snail-mediated invasion and scattering in three-dimensional collagen (12), our results based on the differential effect of Snail and Slug on two-dimensional collagen migration and scattering in three-dimensional collagen suggest that MT1-MMP induction is not sufficient for invasion of Slug-expressing cells in three-dimensional collagen. This is in agreement with findings in cervical cancer cells where expression of MT1-MMP was also not sufficient to promote invasion (59). The MT1-MMP-expressing cervical cells became invasive only following treatment with epidermal growth factor (59). Previously, it was shown that MT1-MMP and β1-integrins coordinately regulate migration and invasion in three-dimensional collagen. MT1-MMP participates and cooperates with β1-integrin to regulate migration of endothelial cells on collagen and fibronectin (60). Fibrosarcoma and breast cancer cells have been shown to coordinately utilize MT1-MMP and β1-integrin to remodel collagen necessary for migration and invasion in three-dimensional collagen (61, 62). We also show that Snail-induced migration and scattering are regulated by β1-integrin, which suggests that MT1-MMP and β1-integrin likely cooperate in the collagen microenvironment to promote migration and invasion of pancreatic cancer cells.
It is now well established that cell migration involves Rho GTPases, which can control the interplay between integrins and the cytoskeleton to regulate migration and invasion (20, 21). Significantly, our results suggest that Snail and Slug can have differing effects on Rac and Rho signaling to regulate migration and invasion. We show that inhibiting Rac1 attenuates Snail-driven motility and scattering, whereas inhibiting ROCK1/2 signaling promotes Slug-driven motility and invasion. It has also been shown that inhibiting Rho signaling using a dominant negative Rho mutant or ROCK1/2 inhibitors enhances migration of squamous cell cancer cells on collagen I (63). ROCK1/2 inhibitors also enhance invasion of gastric cancer cells through Matrigel (64). Rac1 and ROCK1/2 signaling have also been demonstrated to have an antagonistic effect on each other's cellular behavior (65). For example, mesenchymal cell movement, which is characterized by elongated cellular morphology, is driven by activation of Rac1 and decreased Rho signaling mediated by Rac1 activation of WAVE2 (25). Conversely, Rho-kinase signaling can activate the Rac GAP ARHGAP22 to suppress mesenchymal movement by inactivating Rac (25). The increased invasion of gastric cancer cells following treatment with ROCK1/2 inhibitors can be blocked with a Rac1 inhibitor (64), further demonstrating significant cross-talk between Rac1 and ROCK1/2 signaling. Overall, our results demonstrate the differential effects of Snail and Slug in pancreatic cancer and identify the interplay between Rho signaling and β1-integrin that functions to regulate scattering and migration of pancreatic cancer cells by Snail and Slug.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R01CA126888 (to H. G. M.) from the NCI, a Merit grant from the Department of Veterans Affairs (to H. G. M.), and the Baseball Charities Foundation (to M. A. S.).

This article contains supplemental Figs. S1–S4.
- MT1-MMP
- membrane-type 1 matrix metalloproteinase
- ROCK
- Rho-associated kinase
- DMSO
- dimethyl sulfoxide
- PDAC
- pancreatic ductal adenocarcinoma.
REFERENCES
- 1. Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90 [DOI] [PubMed] [Google Scholar]
- 2. Hidalgo M. (2010) Pancreatic cancer. N. Engl. J. Med. 362, 1605–1617 [DOI] [PubMed] [Google Scholar]
- 3. Vincent A., Herman J., Schulick R., Hruban R. H., Goggins M. (2011) Pancreatic cancer. Lancet 378, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maitra A., Hruban R. H. (2008) Pancreatic cancer. Annu. Rev. Pathol. 3, 157–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahadevan D., Von Hoff D. D. (2007) Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 6, 1186–1197 [DOI] [PubMed] [Google Scholar]
- 6. Shields M. A., Dangi-Garimella S., Redig A. J., Munshi H. G. (2012) Biochemical role of the collagen-rich tumor microenvironment in pancreatic cancer progression. Biochem. J. 441, 541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tavazoie S. F., Alarcón C., Oskarsson T., Padua D., Wang Q., Bos P. D., Gerald W. L., Massagué J. (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramaswamy S., Ross K. N., Lander E. S., Golub T. R. (2003) A molecular signature of metastasis in primary solid tumors. Nat. Genet. 33, 49–54 [DOI] [PubMed] [Google Scholar]
- 9. Condeelis J., Segall J. E. (2003) Intravital imaging of cell movement in tumors. Nat. Rev. Cancer 3, 921–930 [DOI] [PubMed] [Google Scholar]
- 10. Provenzano P. P., Eliceiri K. W., Campbell J. M., Inman D. R., White J. G., Keely P. J. (2006) Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ottaviano A. J., Sun L., Ananthanarayanan V., Munshi H. G. (2006) Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-β1. Cancer Res. 66, 7032–7040 [DOI] [PubMed] [Google Scholar]
- 12. Shields M. A., Dangi-Garimella S., Krantz S. B., Bentrem D. J., Munshi H. G. (2011) Pancreatic cancer cells respond to type I collagen by inducing Snail expression to promote membrane-type 1 matrix metalloproteinase-dependent collagen invasion. J. Biol. Chem. 286, 10495–10504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dangi-Garimella S., Strouch M. J., Grippo P. J., Bentrem D. J., Munshi H. G. (2011) Collagen regulation of let-7 in pancreatic cancer involves TGF-β1-mediated membrane-type 1 matrix metalloproteinase expression. Oncogene 30, 1002–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dangi-Garimella S., Krantz S. B., Barron M. R., Shields M. A., Heiferman M. J., Grippo P. J., Bentrem D. J., Munshi H. G. (2011) Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 71, 1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hotary K. B., Allen E. D., Brooks P. C., Datta N. S., Long M. W., Weiss S. J. (2003) Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114, 33–45 [DOI] [PubMed] [Google Scholar]
- 16. Sabeh F., Ota I., Holmbeck K., Birkedal-Hansen H., Soloway P., Balbin M., Lopez-Otin C., Shapiro S., Inada M., Krane S., Allen E., Chung D., Weiss S. J. (2004) Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 167, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thiery J. P., Acloque H., Huang R. Y., Nieto M. A. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 [DOI] [PubMed] [Google Scholar]
- 18. Krantz S. B., Shields M. A., Dangi-Garimella S., Bentrem D. J., Munshi H. G. (2010) Contribution of epithelial-mesenchymal transition to pancreatic cancer progression. Cancers 2, 2084–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iacobuzio-Donahue C. A., Ryu B., Hruban R. H., Kern S. E. (2002) Exploring the host desmoplastic response to pancreatic carcinoma: gene expression of stromal and neoplastic cells at the site of primary invasion. Am. J. Pathol. 160, 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ridley A. J. (2001) Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471–477 [DOI] [PubMed] [Google Scholar]
- 21. Ridley A. J. (2001) Rho GTPases and cell migration. J. Cell Sci. 114, 2713–2722 [DOI] [PubMed] [Google Scholar]
- 22. Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 [DOI] [PubMed] [Google Scholar]
- 23. Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. (1997) Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275, 1308–1311 [DOI] [PubMed] [Google Scholar]
- 24. Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science 273, 245–248 [DOI] [PubMed] [Google Scholar]
- 25. Sanz-Moreno V., Gadea G., Ahn J., Paterson H., Marra P., Pinner S., Sahai E., Marshall C. J. (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135, 510–523 [DOI] [PubMed] [Google Scholar]
- 26. Rottner K., Hall A., Small J. V. (1999) Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9, 640–648 [DOI] [PubMed] [Google Scholar]
- 27. Zamir E., Geiger B. (2001) Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 114, 3583–3590 [DOI] [PubMed] [Google Scholar]
- 28. Sahai E., Marshall C. J. (2002) RHO-GTPases and cancer. Nat. Rev. Cancer 2, 133–142 [DOI] [PubMed] [Google Scholar]
- 29. Crnogorac-Jurcevic T., Efthimiou E., Capelli P., Blaveri E., Baron A., Terris B., Jones M., Tyson K., Bassi C., Scarpa A., Lemoine N. R. (2001) Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene 20, 7437–7446 [DOI] [PubMed] [Google Scholar]
- 30. Heid I., Lubeseder-Martellato C., Sipos B., Mazur P. K., Lesina M., Schmid R. M., Siveke J. T. (2011) Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology 141, 719–730 [DOI] [PubMed] [Google Scholar]
- 31. Radisky D. C., Levy D. D., Littlepage L. E., Liu H., Nelson C. M., Fata J. E., Leake D., Godden E. L., Albertson D. G., Nieto M. A., Werb Z., Bissell M. J. (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lester R. D., Jo M., Montel V., Takimoto S., Gonias S. L. (2007) uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J. Cell Biol. 178, 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hotz B., Arndt M., Dullat S., Bhargava S., Buhr H. J., Hotz H. G. (2007) Epithelial to mesenchymal transition: expression of the regulators Snail, Slug, and Twist in pancreatic cancer. Clin. Cancer Res. 13, 4769–4776 [DOI] [PubMed] [Google Scholar]
- 34. Munshi H. G., Stack M. S. (2006) Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 25, 45–56 [DOI] [PubMed] [Google Scholar]
- 35. Zhang K., Chen D., Jiao X., Zhang S., Liu X., Cao J., Wu L., Wang D. (2011) Slug enhances invasion ability of pancreatic cancer cells through up-regulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab. Invest. 91, 426–438 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36. Joseph M. J., Dangi-Garimella S., Shields M. A., Diamond M. E., Sun L., Koblinski J. E., Munshi H. G. (2009) Slug is a downstream mediator of transforming growth factor-β1-induced matrix metalloproteinase-9 expression and invasion of oral cancer cells. J. Cell. Biochem. 108, 726–736 [DOI] [PubMed] [Google Scholar]
- 37. Sun L., Diamond M. E., Ottaviano A. J., Joseph M. J., Ananthanarayan V., Munshi H. G. (2008) Transforming growth factor-β1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through Snail expression. Mol. Cancer Res. 6, 10–20 [DOI] [PubMed] [Google Scholar]
- 38. Côme C., Magnino F., Bibeau F., De Santa Barbara P., Becker K. F., Theillet C., Savagner P. (2006) Snail and Slug play distinct roles during breast carcinoma progression. Clin. Cancer Res. 12, 5395–5402 [DOI] [PubMed] [Google Scholar]
- 39. Dangi-Garimella S., Redig A. J., Shields M. A., Siddiqui M. A., Munshi H. G. (2010) Rho-ROCK-myosin signaling mediates membrane-type 1 matrix metalloproteinase-induced cellular aggregation of keratinocytes. J. Biol. Chem. 285, 28363–28372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munshi H. G., Stack M. S. (2002) Analysis of matrix degradation. Methods Cell Biol. 69, 195–205 [DOI] [PubMed] [Google Scholar]
- 41. Ward S. T., Dangi-Garimella S., Shields M. A., Collander B. A., Siddiqui M. A., Krantz S. B., Munshi H. G. (2011) Ethanol differentially regulates Snail family of transcription factors and invasion of premalignant and malignant pancreatic ductal cells. J. Cell Biochem. 112, 2966–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jedeszko C., Sameni M., Olive M. B., Moin K., Sloane B. F. (2008) Visualizing protease activity in living cells: from two dimensions to four dimensions. Curr. Protoc. Cell Biol. Chapter 4, Unit 4.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Munshi H. G., Wu Y. I., Ariztia E. V., Stack M. S. (2002) Calcium regulation of matrix metalloproteinase-mediated migration in oral squamous cell carcinoma cells. J. Biol. Chem. 277, 41480–41488 [DOI] [PubMed] [Google Scholar]
- 44. Munshi H. G., Wu Y. I., Mukhopadhyay S., Ottaviano A. J., Sassano A., Koblinski J. E., Platanias L. C., Stack M. S. (2004) Differential regulation of membrane-type 1 matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-β1-induced pericellular collagenolysis. J. Biol. Chem. 279, 39042–39050 [DOI] [PubMed] [Google Scholar]
- 45. Munshi H. G., Ghosh S., Mukhopadhyay S., Wu Y. I., Sen R., Green K. J., Stack M. S. (2002) Proteinase suppression by E-cadherin-mediated cell-cell attachment in premalignant oral keratinocytes. J. Biol. Chem. 277, 38159–38167 [DOI] [PubMed] [Google Scholar]
- 46. Diamond M. E., Sun L., Ottaviano A. J., Joseph M. J., Munshi H. G. (2008) Differential growth factor regulation of N-cadherin expression and motility in normal and malignant oral epithelium. J. Cell Sci. 121, 2197–2207 [DOI] [PubMed] [Google Scholar]
- 47. Lauffenburger D. A., Horwitz A. F. (1996) Cell migration: a physically integrated molecular process. Cell 84, 359–369 [DOI] [PubMed] [Google Scholar]
- 48. Kadler K. E., Baldock C., Bella J., Boot-Handford R. P. (2007) Collagens at a glance. J. Cell Sci. 120, 1955–1958 [DOI] [PubMed] [Google Scholar]
- 49. Leitinger B., Hohenester E. (2007) Mammalian collagen receptors. Matrix Biol. 26, 146–155 [DOI] [PubMed] [Google Scholar]
- 50. Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 [DOI] [PubMed] [Google Scholar]
- 51. Gao Y., Dickerson J. B., Guo F., Zheng J., Zheng Y. (2004) Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. U.S.A. 101, 7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olmeda D., Montes A., Moreno-Bueno G., Flores J. M., Portillo F., Cano A. (2008) Snai1 and Snai2 collaborate on tumor growth and metastasis properties of mouse skin carcinoma cell lines. Oncogene 27, 4690–4701 [DOI] [PubMed] [Google Scholar]
- 53. Moreno-Bueno G., Cubillo E., Sarrió D., Peinado H., Rodríguez-Pinilla S. M., Villa S., Bolós V., Jordá M., Fabra A., Portillo F., Palacios J., Cano A. (2006) Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 66, 9543–9556 [DOI] [PubMed] [Google Scholar]
- 54. Haraguchi M., Okubo T., Miyashita Y., Miyamoto Y., Hayashi M., Crotti T. N., McHugh K. P., Ozawa M. (2008) Snail regulates cell-matrix adhesion by regulation of the expression of integrins and basement membrane proteins. J. Biol. Chem. 283, 23514–23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Neal C. L., Mckeithen D., Odero-Marah V. A. (2011) Snail negatively regulates cell adhesion to extracellular matrix and integrin expression via the MAPK pathway in prostate cancer cells. Cell Adh. Migr. 5, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turner F. E., Broad S., Khanim F. L., Jeanes A., Talma S., Hughes S., Tselepis C., Hotchin N. A. (2006) Slug regulates integrin expression and cell proliferation in human epidermal keratinocytes. J. Biol. Chem. 281, 21321–21331 [DOI] [PubMed] [Google Scholar]
- 57. Maquoi E., Frankenne F., Baramova E., Munaut C., Sounni N. E., Remacle A., Noël A., Murphy G., Foidart J. M. (2000) Membrane-type 1 matrix metalloproteinase-associated degradation of tissue inhibitor of metalloproteinase 2 in human tumor cell lines. J. Biol. Chem. 275, 11368–11378 [DOI] [PubMed] [Google Scholar]
- 58. Shofuda K., Moriyama K., Nishihashi A., Higashi S., Mizushima H., Yasumitsu H., Miki K., Sato H., Seiki M., Miyazaki K. (1998) Role of tissue inhibitor of metalloproteinases-2 (TIMP-2) in regulation of pro-gelatinase A activation catalyzed by membrane-type matrix metalloproteinase-1 (MT1-MMP) in human cancer cells. J. Biochem. 124, 462–470 [DOI] [PubMed] [Google Scholar]
- 59. Zhai Y., Hotary K. B., Nan B., Bosch F. X., Muñoz N., Weiss S. J., Cho K. R. (2005) Expression of membrane-type 1 matrix metalloproteinase is associated with cervical carcinoma progression and invasion. Cancer Res. 65, 6543–6550 [DOI] [PubMed] [Google Scholar]
- 60. Gálvez B. G., Matías-Román S., Yáñez-Mó M., Sánchez-Madrid F., Arroyo A. G. (2002) ECM regulates MT1-MMP localization with β1- or αvβ3-integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J. Cell Biol. 159, 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolf K., Friedl P. (2009) Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin. Exp. Metastasis 26, 289–298 [DOI] [PubMed] [Google Scholar]
- 62. Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S., Friedl P. (2007) Multistep pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 9, 893–904 [DOI] [PubMed] [Google Scholar]
- 63. Zhou H., Kramer R. H. (2005) Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J. Biol. Chem. 280, 10624–10635 [DOI] [PubMed] [Google Scholar]
- 64. Matsuoka T., Yashiro M., Kato Y., Shinto O., Kashiwagi S., Hirakawa K. (2011) RhoA/ROCK signaling mediates plasticity of scirrhous gastric carcinoma motility. Clin. Exp. Metastasis 28, 627–636 [DOI] [PubMed] [Google Scholar]
- 65. Friedl P., Alexander S. (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.