Abstract
Circulating activated platelets roll and make transient contacts before ultimately adhering to a substrate. However, despite the dynamic nature of platelet adhesion, most in vitro adhesion and activation studies have focused on establishing local cause and effect relationships. Here, we determined the effect of exposing platelets to immobilized upstream human fibrinogen on downstream adhesion and activation. Microcontact printing was used to prepare substrates that contained well defined fibrinogen priming regions. Washed platelets were perfused over the substrates and adhesion and activation in a downstream capture region were compared with samples that did not contain a fibrinogen priming region. It was found that samples containing an upstream priming region resulted in higher adhesion, platelet spreading areas and aggregation than samples that lacked the priming region. Also, when the priming region was selectively blocked with a polyclonal anti-fibrinogen antibody, the platelet response was attenuated. To characterize this phenomenon further, flow cytometry was used to assess bulk platelet activation following fibrinogen priming. The expression of two activation markers, PAC-1 and P-selectin were quantified. Expression of both activation markers was found to be higher after perfusion over fibrinogen versus albumin-coated substrates.
Keywords: Upstream platelet activation, Platelet adhesion, Surface fibrinogen, Microcontact printing
1. Introduction
Platelet adhesion and activation on the surface of synthetic blood contacting biomaterials continues to be a challenge for vascular devices. Activation stimulates the local activation of plasma coagulation factors and eventually leads to formation of a fibrin clot. An adverse platelet response to vascular implants can lead to many complications including occlusion, neointimal hyperplasia, and embolism. Consequently, a considerable amount of effort has been devoted to developing materials that minimize the platelet response [1-4].
The general methodology used to study the blood compatibility of a biomaterial is to examine the direct local effects of a material property on platelet adhesion and activation [5-7]. However, surface induced platelet adhesion and activation is a dynamic process. Platelets attach/detach and roll [8-10], before ultimately forming stable adhesive interactions. In fact, most platelet-surface contacts are transient [10]. Even though transient interactions do not result in local platelet adhesion and aggregate formation, it is unlikely that they leave the activation state of the platelet unaffected. With each surface contact there is the opportunity to interact with adsorbed plasma protein agonists such as fibrinogen and vWf, through specific interactions with integrin αIIbβ3 and GPIb-IX-V membrane receptors respectively [11-13]. Also, platelets may interact with exposed subendothelial collagen due to injury at the anastamoses of the implanted vascular device [14]. Furthermore, previous studies have found that the platelet-surface response is changed when the upstream environment is varied [15]. Taken together, upstream platelet-surface interactions may affect downstream adhesion and activation. Specifically, upstream interactions with protein agonists may “prime” platelets for downstream adhesion and activation.
In this study, we characterized the effect of upstream platelet–fibrinogen interactions on downstream adhesion and activation. Microcontact printing (μCP) was used to covalently immobilize fibrinogen priming regions onto chemically reactive substrates and the downstream platelet response was observed. Adhesion, activation and aggregation were found to be significantly higher on samples containing a fibrinogen priming region compared with control samples. Also, the increase in downstream adhesion was attenuated when the priming region was blocked with a polyclonal antibody for fibrinogen suggesting fibrinogen is, in fact, capable of inducing a downstream response. The effect of transient platelet-surface contacts on bulk platelet activation was assessed by quantifying P-selectin and active αIIbβ3 using flow cytometry. An increase in bulk platelet activation was observed after perfusion over samples prepared with covalently immobilized fibrinogen versus albumin. These results suggest that platelets are capable of being primed for downstream adhesion and activation by upstream immobilized protein agonists. These findings have implications for both the design of vascular devices as well as the design of in vitro platelet adhesion and activation assays.
2. Methods
2.1. Preparation of polydimethylsiloxane (PDMS) stamps for μCP
PDMS stamps were prepared from masks with randomly distributed mm-sized features that were defined to cover 85% of the stamp surface area (Fig. 1A). Mask patterns were developed by generating a 500 × 500 array of randomly distributed black and white pixels using Mathematica (Wolfram). Patterns were transferred to chromium coated silica wafers using conventional photolithography. First, the pattern was uploaded into a mask making software, L-Edit (Tanner), where each pixel was defined to be 25 μm × 25 μm. An Electromask MM250 (Interserv Technology) pattern generator was used to produce the first mask (Amask = 1.25 cm × 1.25 cm and Apixel = 25 μm × 25 μm). This was followed by two 5× image reductions and one repeat step to produce a final mask with a 20 × 20 pattern array of randomly distributed micron sized features (Amask = 1 cm × 1 cm and Apixel = 1 μm × 1 μm). Sylgard 184 silicone elastomer (Dow Corning) was mixed with curing agent in a 10:1 ratio and poured over the patterned mask. PDMS was degassed by placing the samples under vacuum for 30 min. The samples were cured for 30 min at 100 °C and carefully peeled away from the mask to remove the patterned stamps. This was followed by an additional 60 min of curing at 60 °C. After curing the stamps were incubated overnight in hexane to remove any polymer that did not crosslink. To eliminate swelling that occurred after hexane incubation, stamps were rinsed for 30 min in a 95% ethanol solution, 30 min in Milli-Q water, and dried for 30 min at 60 °C.
Fig. 1.
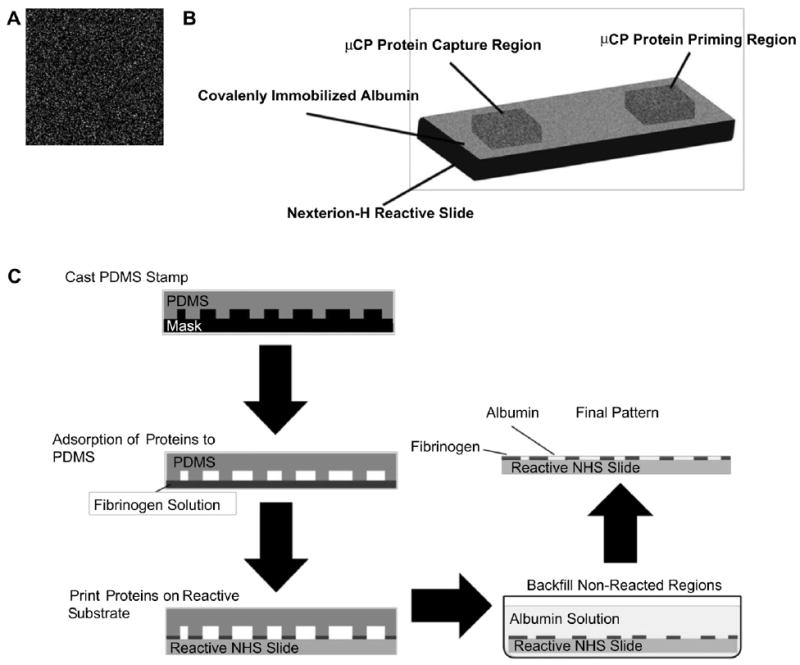
Sample preparation using μCP: (A) Image of a random pattern used to prepare PDMS stamps with an 85% relative coverage area. (B) Nexterion-H samples were prepared with covalently immobilized protein patterns using μCP. (C) A schematic representation of the μCP process. First PDMS stamps are cast and cured in patterned masks. The stamps are transferred to a protein solution where they are “inked” by allowing the protein to adsorb to the surface. The protein coated stamp is placed in contact with the reactive surface allowing the protein transfer to occur. On Nexterion-H substrates, the printed surface was incubated in an albumin solution to passivate the unpatterned regions.
2.2. Covalent immobilization of fibrinogen to reactive surfaces
PDMS Stamps were “inked” with human fibrinogen in PBS (cFgn = 1 mg/ml, pH 8.5) for 15 min, rinsed in Milli-Q water and dried with N2 gas. The fibrinogen coated stamps were placed in contact with commercially available Nexterion-H (Schott) reactive slides with 490 Pa of pressure applied evenly using a 5 g weight. These slides contain a reactive coating in which a cross-linked poly(ethylene glycol) PEG layer is functionalized with NHS esters providing means for covalent protein immobilization through the terminal amine group [16]. A fibrinogen “priming” region was printed in the upstream region on test samples and a platelet capture region was printed 10 mm downstream of the priming region (Fig. 1B). Fibrinogen coated stamps were allowed to react with the surface for 1 h. After fibrinogen was printed, the nonreacted regions were passivated by incubating the samples in an albumin solution in PBS (cHSA = 1 mg/ml, pH 8.5) for 30 min (Fig. 1C). Albumin was chosen over small molecules such as ethanolamine for background passivation because it demonstrated an improved ability to eliminate platelet adhesion (data not shown). Following protein immobilization, samples were vigorously rinsed in a 1% Tween solution to remove any protein not covalently immobilized on the surface. Samples were then rinsed thoroughly with Milli-Q water, dried with N2 gas and stored under vacuum until use.
2.3. Surface characterization
The printed fibrinogen patterns were previously characterized [17]. Briefly, the integrity of the protein patterns was characterized visually using fluorescence microscopy. In order to visualize immobilized protein patterns, fibrinogen was labeled with Alexa Fluor 488 succinimidyl ester (Invitrogen) following the manufacturers protocols. The protein transfer efficiency was quantitatively characterized using lateral force microscopy (LFM). LFM measurements were obtained in air on an Explorer AFM (TopoMetrix) with silicon cantilevers (Mikromasch) having a force constant of 0.03 N/m and a radius of curvature < 10 nm. These measurements (n = 10) were used to compare the actual printed protein area with target values.
2.4. Blocking immobilized fibrinogen priming regions
Control samples were prepared by blocking the upstream fibrinogen region with a rabbit α-polyclonal antibody raised against human fibrinogen (Calbiochem). Blocking was achieved by selectively incubating the priming region with 1:100 dilution of anti-fibrinogen in 0.1 M PBS (pH 7.4) for 30 min. The samples were rinsed three times in Milli-Q water following blocking, and immediately used for experiments.
2.5. Platelet adhesion studies
Fresh whole blood was collected from healthy human donors in a 1:7 ACD solution. The blood was centrifuged for 15 min at 1500 rpm to separate platelet rich plasma (PRP). The PRP supernatant was aspirated off using a transfer pipette. Prostaglandin E1 (PGE1, 300 nM) was added to the PRP to inhibit aggregation during preparation [18]. PRP was then centrifuged for another 15 min at 2100 rpm to isolate the platelet pellet. The platelet poor plasma (PPP) supernatant was carefully discarded and the platelet pellet was gently re-suspended in prewarmed Tyrodes-HEPES buffer (37 °C, pH 7.4) [19]. Washed platelets were counted using a hemocytometer and the concentration was adjusted to 2.5 × 107 platelets/ml.
Platelet perfusion studies were conducted in a parallel plate flow cell as described previously [15]. Briefly, washed platelets were perfused for 5 min at a flow rate of 20 ml/h (γ ~ 100 s−1) in a parallel plate flow cell (w = 0.5 cm, h = 0.025 cm). Adhesion was quantified by averaging samples (n = 30) downstream of the priming region. In studies where Nexterion-H reactive substrates were used, average platelet spreading area and the number of aggregates per sample were also calculated. The platelet spreading area was calculated by taking the average area of 100 spreading platelets in the downstream region. Platelet aggregates were defined as a cluster of 3 or more platelets and the average number per sample was quantified (n = 30). Significance between data sets was established using unpaired t-tests.
2.6. Flow cytometry
Flow cytometry was used to measure levels of expressed P-selectin and the active conformation of integrin αIIbβ3 on the membranes of platelets. Platelets were perfused over substrates in which the entire sample area contained either immobilized albumin or fibrinogen. The flow cells used for these studies were designed to maintain similar conditions as described previously [15] however the length of the channel was increased by using a serpentine flow channel pattern versus a rectangular pattern. Following perfusion, a 100 μl aliquot of the platelet supernatant was collected and incubated for 30 min with either anti-CD62P (BD Biosciences) or PAC-1 (BD Biosciences) to label P-selectin and active αIIbβ3, respectively (c = 1 μg/ml, BD Biosciences). Also, two 100 μl aliquots were obtained and labeled prior to perfusion. One sample was stimulated by addition of thrombin immediately prior to labeling (c = 0.1 units/ml) and the other was left unstimulated to serve as positive and negative controls, respectively. In order to ensure platelets were properly identified, they were labeled with CD41b (BD Biosciences) which binds to the αIIb subunit of integrin αIIbβ3 regardless of the activation state of the receptor. Rat monoclonal anti-mouse IgG and IgM served as a negative control for P-selectin and PAC-1 test samples, respectively. Following labeling, platelets were fixed in 1% para-formaldehyde and stored at 4 °C. Analysis of 10,000 events was conducted on a FACScan (BD Biosciences) analyzer.
3. Results
3.1. Reactive surface and fibrinogen pattern characterization
Fig. 2 provides a representative image for fibrinogen printed surfaces. As previously shown [17], fluorescence images of 85% printed protein micropatterns were acquired to qualitatively assess the integrity of printed fibrinogen patterns and ensure no major defects were present. These images were also acquired after samples were vigorously rinsed in a 1% Tween solution suggesting the protein present on the surface was, in fact, covalently immobilized.
Fig. 2.
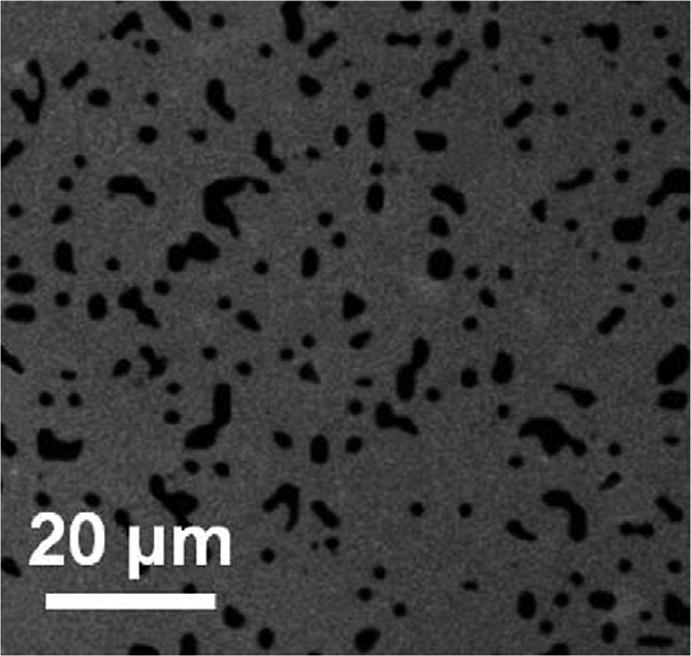
A representative fluorescence image of an 85% printed fibrinogen pattern where the grey represents Alexa Fluor 488 labeled fibrinogen. Images were acquired after a vigorous surfactant rinse.
In order to determine the efficiency of protein transfer, samples were scanned with LFM. This technique measures deflections of a cantilever tip that arise from variations in friction over heterogeneous surfaces providing contrast between the printed and nonprinted regions. For these studies, PDMS stamps with a target protein coverage area of 85% were used for printing. Previously, actual printed coverage area was quantified for each LFM sample (n = 10) and found to be 85.5% +/−0.9%, suggesting an accurate protein transfer efficiency [17].
3.2. Effect of upstream immobilized fibrinogen on the downstream platelet response
Here, samples were prepared with a covalently immobilized fibrinogen priming and capture region as described above with 85% coverage area stamps. The background was passivated with covalently immobilized albumin. The effect of fibrinogen priming on the downstream platelet response was quantified by comparing average adhesion, spreading area, and aggregate formation in the downstream capture region with controls that did not contain a priming region (Fig. 3). Aggregates were defined as 3 or more platelets in a single cluster. In all three cases, adhesion, spreading area, and aggregation were significantly higher on samples with the fibrinogen priming region (p < 0.01), suggesting that upstream platelet–protein interactions are capable of eliciting a downstream response. Platelet adhesion was observed in the fibrinogen priming region, however overall values were lower than the capture region (data not shown).
Fig. 3.
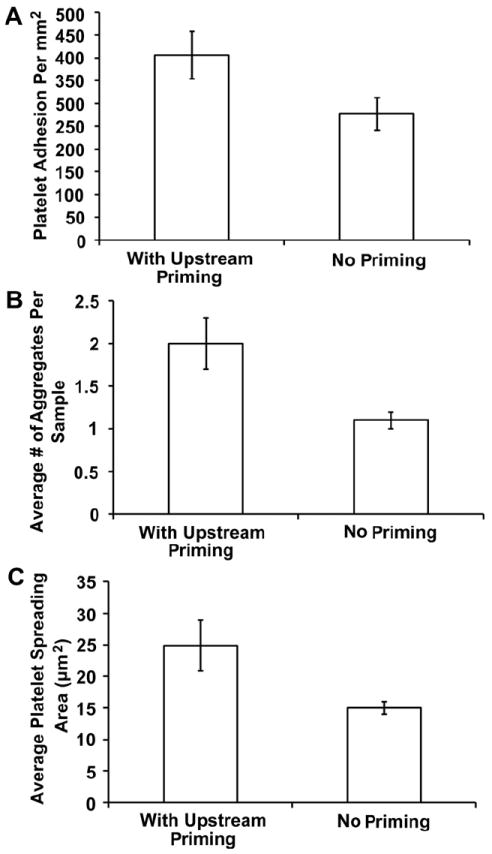
Effect of an upstream immobilized fibrinogen priming region on downstream (A) adhesion, (B) aggregation, and (C) spreading area on Nexterion-H substrates. Samples (n = 30) were acquired 10–15 mm downstream of the priming region. The error bars represent the standard error of the mean with a 95% confidence interval.
To explicitly confirm that the presence of fibrinogen priming region is amplifying the downstream platelet response, a test sample was prepared with the upstream fibrinogen priming region blocked by a polyclonal antibody (for fibrinogen). Average adhesion in the downstream capture region was compared with adhesion values on samples with and without an upstream fibrinogen priming region (Fig. 4).
Fig. 4.
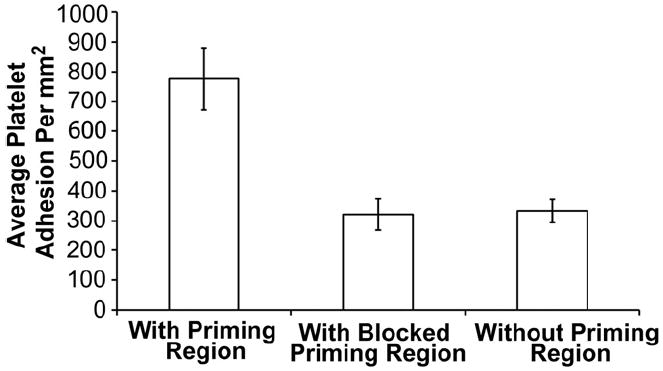
Blocking fibrinogen priming region with an anti-fibrinogen polyclonal antibody attenuates the downstream adhesion response. The effect of priming platelets with immobilized upstream fibrinogen on downstream adhesion was compared with samples containing no priming region and a priming region blocked with an anti-fibrinogen polyclonal antibody. Samples (n = 30) in the downstream capture region were averaged and error bars represent the standard error of the mean with a 95% confidence interval.
Platelet adhesion on samples primed with upstream fibrinogen was again significantly higher (~775 platelets/mm2) than samples that did not possess an upstream priming region (~325 platelets/ mm2). Also, when the upstream priming region was blocked with an antihuman fibrinogen polyclonal antibody the downstream platelet adhesive response was attenuated (~345 platelets/mm2) and there was no significant difference with unprimed samples (p < 0.01). These results further confirmed that the presence of upstream immobilized protein agonists primed platelets for downstream adhesion.
3.3. Assessment of bulk platelet activation using flow cytometry
In previous experiments, we found that upstream immobilized fibrinogen was capable of increasing surface induced platelet adhesion and activation downstream. To further investigate this phenomenon, the bulk platelet response to surface immobilized fibrinogen was characterized using flow cytometry. Two activation markers (PAC-1 and P-selectin) were quantified after washed platelet perfusion over either a fibrinogen or an albumin-coated substrate. The percent of expression events out of 10,000 (n = 4) for each receptor was recorded and compared with expression prior to perfusion (negative control) and expression after thrombin stimulation (positive control) (Fig. 5). Significant increases (p < 0.01) in both PAC-1 and P-selectin expression on platelets perfused over fibrinogen substrates were observed compared with albumin substrates. For PAC-1, average percent of platelets expressing the active conformation if integrin αIIbβ3 was 40.8% and 54.9% for albumin and fibrinogen substrates respectively (Fig. 5A). The average percent of platelets expressing the P-selectin membrane receptor was 2.9% and 9.1% for albumin and fibrinogen substrates respectively (Fig. 5B).
Fig. 5.
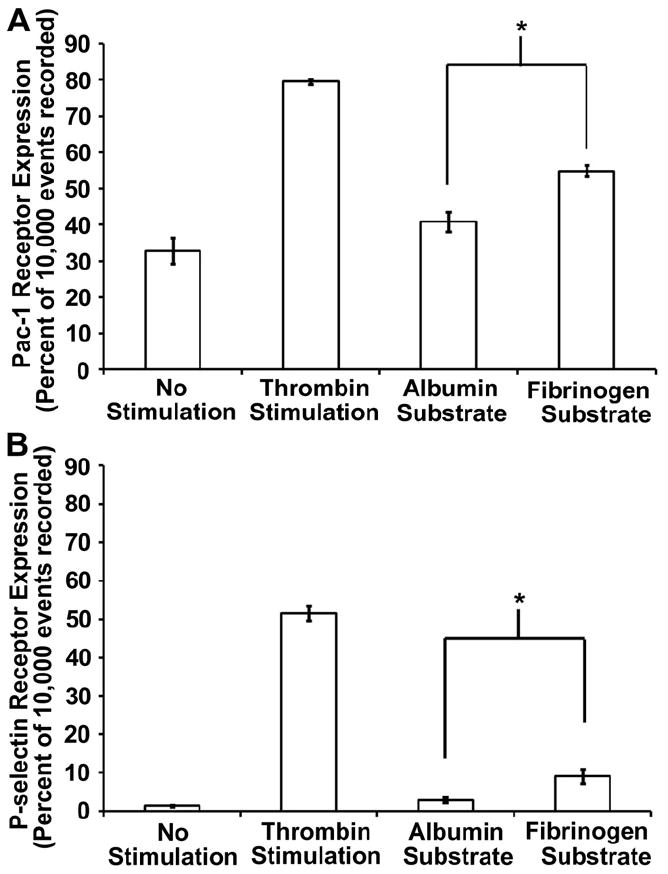
Flow cytometry analysis of bulk platelet activation. (A) Pac-1 and (B) P-selectin expression were quantified on washed platelets following perfusion over both an albumin and a fibrinogen substrate. These samples were also compared with unstimulated (negative control) and thrombin stimulated (positive control) samples collected prior to perfusion. Samples (n = 4) represent the average percent of platelets expressing each receptor out of out of 10,000 recorded events. The error bars represent the standard error of the mean calculated from a 95% confidence interval.
4. Discussion
In this study, the effect of exposing platelets to immobilized upstream fibrinogen on downstream adhesion and activation was characterized. Fibrinogen was chosen as a model protein since it is the primary adhesive ligand for platelets and required for aggregation [20-22]. Overall adhesion (Fig. 3A) was found to be significantly higher when flowing platelets were primed with upstream immobilized fibrinogen. Platelet spreading area and aggregate formation are two variables directly correlated to the activation state of the platelet. Here, both were found to be significantly higher (Fig. 3B and C) when platelets were primed. It should be noted that, in its native state, fibrinogen is relatively inert to quiescent platelets; it is only when it is adsorbed and unfolds that adhesion is supported [23] due to the exposure of epitopes capable of interacting with integrin αIIbβ3. Although the conformation of surface immobilized fibrinogen was not specifically elucidated, the difference in the downstream platelet response between samples with and without a priming region suggests it is in fact fibrinogen, activating platelets. Also, when platelets were perfused over samples where the fibrinogen priming region was blocked, the downstream response was statistically similar to perfusion over samples that did not contain a priming region, but were coated with albumin (Fig. 4). This further confirms the observed downstream platelet response is, in fact, due to fibrinogen priming and that upstream platelet interactions with the substrate can elicit a downstream response.
In this study, platelet perfusion was conducted at a shear rate of γ = 100 s−1, representative of venous flow. The choice of a lower shear rate was ideal for this system in order to minimize shear-induced integrin αIIbβ3 activation [24]. If shear rate is increased, the convective transport of platelets to the surface also increases thus it can be expected that surface induced platelet responses will also be amplified.
Platelet-material interactions do not always end in an adhesive event. Platelets may contact the substrate transiently and return to the bulk solution. To characterize downstream bulk platelet activation, flow cytometry was used to quantify the expression of two membrane receptors indicative of activation. Upon activation, integrin αIIbβ3 undergoes a conformational change from its low affinity state to its high affinity state and RGD ligands such as fibrinogen are capable of inducing this activation [25]. PAC-1 is an antibody that recognizes the active conformation of integrin αIIbβ3 and was found to be expressed at significantly higher levels on platelets perfused over fibrinogen substrates versus albumin substrates (Fig. 5A). Following platelet activation, platelets undergo release reactions called degranulation. During this process, P-selectin is translocated from the inner membranes of platelet granules to the outer membrane [26]. It was also determined that presence of P-selectin receptors on platelets perfused over fibrinogen substrates was significantly higher than on platelets perfused over albumin substrates (Fig. 5B). Both these findings suggest that surface immobilized fibrinogen is capable of activating platelets in the bulk which will affect the downstream platelet response. It should be noted that the increase in expression for both markers on fibrinogen substrates was low when compared to thrombin activated positive controls. By considering the number of platelets that actually interact with the surface compared to the total bulk platelet concentration, this can be explained.
According to the Von Smoluchowski–Levich equation, the platelet flux to the surface in a parallel plate flow cell can be modeled by the following equation [27]:
where j* = the instantaneous platelet flux [platelets/cm2 sec] to the interface, Dplt = platelet diffusion coefficient, c = bulk platelet concentration (~2.5 × 107 platelets/ml), b = ½ the height of the flow cell (~0.13 cm), Vm = mean velocity (~0.7 cm/s), and x = position in the flow cell. This equation assumes the flow is laminar and fully developed. According to this model, the deposition rate changes with, x, the distance from the flow inlet due to the presence of a hydrodynamic boundary later. When this function is integrated with respect to the time it takes for the sample volume of platelets to travel through the flow cell, one obtains the overall deposition rate as a function of distance from the flow inlet per area. Dplt can be estimated to be ~10 −13 m2/s using the Stokes–Einstein relationship. When the flux equation is integrated over the length of the flow channel, the overall flux of platelets to the surface is ~830 platelets/cm sec. When this value is multiplied by the width of the flow channel and the time it takes for one sample volume (0.2 ml) to travel over the surface area of the flow channel (t = 36 s, w = 0.3 cm) the theoretical number of platelets contacting the flow chamber walls in each sample is ~18,000 platelets. This only represents approximately 0.36% of the total platelets present in the bulk solution (~5,000,000). Consequently, it is not surprising that increases in bulk activation due to platelet–fibrinogen interactions are small when compared to the thrombin activated positive controls (Fig. 5). It is also important to note that platelet and coagulation mechanisms are inherently designed to be amplified in the physiological environment so even a small amount of activation can be significant. It also should be noted that this approximation does not take into account external forces on platelets including gravity, buoyancy, and any surface forces.
Traditionally, blood compatibility studies focus on establishing the local platelet response to the biomaterial. However, since platelet-surface interactions are often transient [10], these studies may not be sufficient to understand the whole picture. Platelet membrane receptor interactions with surface immobilized proteins, such as vWf-GPIb-IX-V and fibrinogen-αIIbβ3, are capable of activating platelets [11-13,23]. It is unlikely that these transient contacts leave the phenotype unchanged. For example, upstream platelet activation on devices such as prosthetic heart valves and stents have been shown to cause downstream thromboembolic complications [28,29]. Also, it has been hypothesized that transient platelet-surface interactions trigger platelet microparticle formation at the site of contact [30]. Platelet microparticles have pro-coagulant surface properties and may have serious blood compatibility implications in the absence of stable platelet adhesion [31,32]. It is evident that the “history” of transient platelet-substrate contacts is an important consideration in the design of in vitro blood compatibility studies.
Characterizing the effect of transient interactions on the downstream platelet response is also an important aspect in the development of cardiovascular devices. When a device such as a vascular graft or heart valve is implanted there exists a region in which the native vessel is joined with the synthetic material. This region, also known as the anastomotic region, is characterized by high rates of stenosis (narrowing) and subsequently, higher fluid shear rates [33]. The anastomosis is also characterized by adsorption of pro-coagulant proteins such as fibrinogen due to damage of the vessel endothelium at this location [34]. This presents an ideal environment for platelet activation to occur and may have serious consequences for the blood compatibility of the downstream biomaterial. Another implication is in the use of grafts or shunts in hemodialysis patients. Traditionally, the most common cause of arteriovenous graft failure is neointimal hyperplasia at the graft-venous anastomoses [35,36]. The exact mechanism is not yet understood. However, it has been suggested that the physiology of the venous endothelial layer may play a role [36]. It is possible, however, that the upstream artery-graft anastomoses may be pre-activating platelets for downstream adhesion since this region is characterized by both high shear forces as well as a damaged endothelial layer. In order to better understand the role of platelet activation in device failure we must take into account the transient nature of adhesion and activation. This will, in turn, help us develop more hemocompatible biomaterials.
5. Conclusions
In this study, the effect of priming platelets with covalently immobilized fibrinogen on the downstream response was investigated. Priming regions were prepared using μCP on surfaces capable of protein covalent immobilization. The downstream platelet response on PEG based model substrates was quantified by comparing adhesion, spreading area, and aggregation values with control samples that did not contain an upstream priming region. The results presented suggest that immobilized fibrinogen is capable of priming platelets and their response may be transient. The effect on immobilized fibrinogen on the bulk platelet activation was also quantified by measuring expression levels of P-selectin and PAC-1 expression. Although slight, there was an increase in expression after perfusion over fibrinogen substrates. This has both clinical significance and will be an important consideration in the design of future in vitro studies and cardiovascular devices.
Acknowledgments
This work was supported by the National Institutes of Health (5RO1 HL84586) and the American Heart Association (10PRE4010047). The authors would also like to thank Dr. Andy Weyrich and his research group for generously providing human blood for our studies.
References
- 1.Andrade J, Hlady V. Protein adsorption and materials biocompatibility: a tutorial review and suggested hypotheses. Adv Polym Sci. 1986;79:1–63. [Google Scholar]
- 2.Andrade JD, Nagaoka S, Cooper S, Okano T, Kim SW. Surfaces and blood compatibility current hypotheses. ASAIO Trans. 1987;33:75. [Google Scholar]
- 3.Tanzi MC. Bioactive technologies for hemocompatibility. Expet Rev Med Dev. 2005;2:473–92. doi: 10.1586/17434440.2.4.473. [DOI] [PubMed] [Google Scholar]
- 4.Ratner B. The catastrophe revisited: blood compatibility in the 21st century. Biomaterials. 2007;28:5144–7. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li D, Chen H, Glenn McClung W, Brash JL. Lysine-PEG modified polyurethane as a fibrinolytic surface: effect of PEG chain length on protein interactions, platelet interactions and clot lysis. Acta Biomater. 2009;5:1864–71. doi: 10.1016/j.actbio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Yim EKF, Sefton MV. Amidine surface modification of poly(acrylonitrile-co-vinyl chloride) reduces platelet adhesion. J Biomed Mater Res A. 2009;89A:780–90. doi: 10.1002/jbm.a.32022. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Horbett TA. Tetraglyme coatings reduce fibrinogen and von Wille-brand factor adsorption and platelet adhesion under both static and flow conditions. J Biomed Mater Res A. 2009;89A:791–803. doi: 10.1002/jbm.a.32085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson SP, Mistry N, Yuan Y. Platelets and the injured vessel wall – “Rolling into action”: focus on glycoprotein Ib/V/IX and the platelet cytoskeleton. Trends Cardiovasc Med. 2000;10:192–7. doi: 10.1016/s1050-1738(00)00062-1. [DOI] [PubMed] [Google Scholar]
- 9.Savage B, Saldvar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 10.Godo MN, Sefton MV. Characterization of transient platelet contacts on a polyvinyl alcohol hydrogel by video microscopy. Biomaterials. 1999;20:1117–26. doi: 10.1016/s0142-9612(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 11.Grunkemeier JM, Tsai WB, McFarland CD, Horbett TA. The effect of adsorbed fibrinogen, filbronectin, von Willebrand factor and vitronectin on the pro-coagulant state of adherent platelets. Biomaterials. 2000;21:2243–52. doi: 10.1016/s0142-9612(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 12.Jackson SP, Schoenwaelder SM, Yuan Y, Rabinowitz I, Salem HH, Mitchell CA. Adhesion receptor activation of phosphatidylinositol 3-kinase. von Wille-brand factor stimulates the cytoskeletal association and activation of phosphatidylinositol 3-kinase and pp60c-src in human platelets. J Biol Chem. 1994;269:27093–9. [PubMed] [Google Scholar]
- 13.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ. Signaling through GP Ib-IX-V activates αIIbβ3 independently of other receptors. Blood. 2004;103:3403–11. doi: 10.1182/blood-2003-10-3664. [DOI] [PubMed] [Google Scholar]
- 14.Farndale RW. Collagen-induced platelet activation. Blood Cells Mol Dis. 2006;36:162–5. doi: 10.1016/j.bcmd.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Corum LE, Hlady V. Screening platelet-surface interactions using negative surface charge gradients. Biomaterials. 2010;31:3148–55. doi: 10.1016/j.biomaterials.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi H, Emoto K, Dubey M, Castner DG, Grainger DW. Imaging surface immobilization chemistry: correlation with cell patterning on non-adhesive hydrogel thin films. Adv Funct Mater. 2008;18:2079–88. doi: 10.1002/adfm.200800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corum LE, Eichinger CD, Hsiao TW, Hlady V. Using microcontact printing of fibrinogen to control surface-induced platelet adhesion and activation. Langmuir. 2011;27:8316–22. doi: 10.1021/la201064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mustard JF, Packham MA. Factors influencing platelet function: adhesion, release and aggregation. Pharmacol Rev. 1970;22:97–187. [PubMed] [Google Scholar]
- 19.McNicol A. Platelets: a practical approach. New York: Oxford University Press; 2002. Platelet preparation and estimation of functional responses; p. 5. [Google Scholar]
- 20.Kwak D, Wu Y, Horbett TA. Fibrinogen and von Willebrand’s factor adsorption are both required for platelet adhesion from sheared suspensions to poly-ethylene preadsorbed with blood plasma. J Biomed Mater Res. 2005;74A:69–83. doi: 10.1002/jbm.a.30365. [DOI] [PubMed] [Google Scholar]
- 21.Marguerie GA, Plow EF, Edgington TS. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979;254:5357–63. [PubMed] [Google Scholar]
- 22.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 23.Salzman EW, Lindon J, McManama G, Ware JA. Role of fibrinogen in activation of platelets by artificial surfaces. Ann NY Acad Sci. 1987;516:184–95. doi: 10.1111/j.1749-6632.1987.tb33040.x. [DOI] [PubMed] [Google Scholar]
- 24.Nesbitt WS, Kulkarni S, Giuliano S, Gonclaves I, Dopheide SM, Yap CL, et al. Distinct glycoprotein Ib/V/IX and integrin αIIbβ3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem. 2002;277:2965–72. doi: 10.1074/jbc.M110070200. [DOI] [PubMed] [Google Scholar]
- 25.Du X, Plow EF, Frelinger AL, O’Toole TE, Loftus JC, Ginsberg MH. Ligands “activate” integrin αIIbβ3 (platelet GPIIb-IIIa) Cell. 1991;3:409–16. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- 26.Berman CL, Yeo EL, Wencel-Drake JD, Furie BC, Ginsberg MH, Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986;78:130–7. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dabroś T, van de Ven TGM. A direct method for studying particle deposition onto solid surfaces. Colloid Polym Sci. 1983;26:694–707. [Google Scholar]
- 28.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–41. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 29.Nedeltchev K, Remonda L, Do DD, Breckenfeld C, Ozdoba C, Arnold M, et al. Acute stenting and thrombaspiration in basilar artery occlusions due to embolism from the dominating vertebral artery. Neuroradiology. 2004;46:686–91. doi: 10.1007/s00234-004-1217-z. [DOI] [PubMed] [Google Scholar]
- 30.Ferraz N, Carlsson J, Hong J, Ott MK. Influence of nanoporesize on platelet adhesion and activation. J Mater Sci Mater Med. 2008;19:3115–21. doi: 10.1007/s10856-008-3449-7. [DOI] [PubMed] [Google Scholar]
- 31.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263:18205–12. [PubMed] [Google Scholar]
- 32.Sandberg H, Bode AP, Dombrose FA, Hoechli M, Lentz BR. Expression of coagulant activity in human platelets: release of membranous vesicles providing platelet factor 1 and platelet factor 3. Thromb Res. 1985;39:63–79. doi: 10.1016/0049-3848(85)90122-7. [DOI] [PubMed] [Google Scholar]
- 33.Shin HS, Park K, Kim JH, Kim JJ, Dong KH, Moon MW, et al. Biocompatible PEG grafting on DLC-coated nitinol alloy for vascular stents. J Bioact Compat Pol. 2009;24:316–28. [Google Scholar]
- 34.Johnson PC, Garrett KO, Brash JL, Cornelius RM, Kaplan SS, Warty V. Delivery of passivating proteins to sutures during passage through the vessel wall reduces subsequent platelet deposition by blocking fibrinogen adsorption. Arterioscler Thromb. 1992;12:727–35. doi: 10.1161/01.atv.12.6.727. [DOI] [PubMed] [Google Scholar]
- 35.Asif A, Gadalean FN, Merrill D, Cherla G, Cipleu CD, Epstein DL, et al. Inflow stenosis in arteriovenous fistulas and grafts: a multicenter, prospective study. Kidney Int. 2005;67:1986–92. doi: 10.1111/j.1523-1755.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 36.Kelly BS, Heffelfinger SC, Whiting JF, Miller MA, Reaves A, Armstrong J, et al. Aggressive venous neointimal hyperplasia in a pig model of arteriovenous graft stenosis. Kidney Int. 2002;62:2272–80. doi: 10.1046/j.1523-1755.2002.00684.x. [DOI] [PubMed] [Google Scholar]


