Abstract
Work in this laboratory demonstrated a role for rapid eye movement sleep (REMS) in critical period (CP), postnatal days (P)17-30, synaptic plasticity in visual cortex. Studies in adolescent rats showed that REMS deprivation (REMSD) reinitiates a developmentally regulated form of synaptic plasticity that otherwise is observed only in CP animals. Subsequent work showed that REMSD affects inhibitory mechanisms that are thought to be involved in terminating the CP. Neurotrophins are implicated in the synaptic plasticity that underlies CP maturation and also final closure of the CP in visual cortex. Expression of brain-derived neurotrophic factor (BDNF) is dependent upon neuronal activity, and REMSD may block BDNF expression. We propose that REMS contributes to the maturation of visual cortex through regulation of BDNF expression and consequent, downstream increase in cortical inhibitory tone.
In this study, osmotic minipumps delivered BDNF into visual cortex on one side of brain. The opposite hemisphere was not implanted and served as an internal control. We tested the hypothesis that BDNF is blocked by REMSD in late-adolescent rats and investigated whether replacing BDNF prevents induction of LTPWM-III by theta burst stimulation (TBS). We also assessed relative inhibitory tone in visual cortex with paired-pulse stimulation (PPS) in animals that were similarly REMSD- and BDNF-infused. After REMSD, both hemispheres were prepared in parallel for in vitro synaptic plasticity studies (LTPWM-III or PPS).
In visual cortex of REMSD rats on the side receiving BDNF infusions (8 of 8 animals), TBS consistently failed to induce LTPWM-III. In contrast, LTPWM-III was obtained (5 of 5 animals) in the matched non-infused hemisphere, as expected in rats of this age. REMSD animals that were unilaterally infused with saline produced LTPWM-III in both hemispheres. PPS studies in another group of REMSD animals that were unilaterally BDNF-infused displayed age-appropriate inhibition of the second response on the BDNF-infused side (5/5), whereas on the non-infused side facilitation was observed (3/3).
Intracortical infusion of BDNF in REMSD adolescent rats appears to restore neurochemical processes necessary for termination of the CP for developmentally regulated synaptic plasticity in visual cortex. The results suggest that REMSD blocks BDNF expression and also maturation of inhibitory processes in adolescent visual cortex. These data support REMS’ function in brain development.
Keywords: paired-pulse, visual cortex, synaptic plasticity, rats, multiple-pedestals
Introduction
The brain is most plastic during the developmental period. For example, during an early-life critical period (CP) of development in the visual system, typically spanning postnatal days P17–P30, synaptic connectivity is strongly affected by sensory input, whereas later in life this plasticity is available only under a limited set of conditions (c.f.,19). The classical experiments demonstrating the so called “ocular dominance shift” revealed that when vision in one eye is blocked for a short time in CP animals, visual system synaptic connections are remodeled and a response bias, or a shift towards input from the open eye, is observed18. Blocking visual input to one eye in adults does not typically produce shifts in ocular dominance (but see19). Despite an expanding literature describing the mechanisms underlying this ocular dominance shift, our understanding is not complete. Many of the known mechanisms have been reported, however, to have commonalities with certain forms of experimentally produced, in vitro models of synaptic plasticity such as long-term potentiation (LTP), long-term depression (LTD), and paired-pulse stimulation (PPS) 16,31. Several in vitro forms of LTP can be produced at synapses in visual cortex3,20. An age-dependent type21 can usually be evoked only during the developmental CP of the visual system21. This form of LTP (LTPWM-III) is produced in visual cortex layers II/III by fast frequency, theta-burst stimulation (TBS) of the white matter (WM) below V1 that carries visual information from lateral geniculate nucleus (LGN) to cortical processing areas. Shifts in LTPWM-III production and ocular dominance following arrested visual input during the CP appear to depend upon NMDA receptors2, metabotropic glutamate receptors39, and several neurotrophic factors, including brain-derived neurotrophic factor (BDNF)17,22,26.
After P30, WM stimulation alone seldom evokes LTPWM-III21,30. Rearing in complete darkness extends the usual duration of the CP for production of LTPWM-III21. Studies in our laboratory demonstrated that suppression of rapid eye movement sleep (REMS), starting just before the end of the CP and lasting up to ten days, promotes a similar extension of the CP for LTPWM-III36. We subsequently found that REMS deprivation (REMSD), initiated shortly after the expected end-point of the CP in early adolescent rats (P35-45), seems to reinstate the conditions for producing LTPWM-III35. These findings and the dark-rearing studies from Bear’s laboratory21 suggest that endogenous neural activation during REMS as well as exogenous sensory stimulation of visual system contribute to configuring synaptic connectivity in the developing brain despite acting in different circadian phases. REMS-generated activation of the visual system synapses on many of the same LGN cells that also receive inputs from retinal ganglion cells9,32. Accordingly, sequential activation of visual neurons by waking sensory input and REMS-generated activity appears necessary for normal termination of CP synaptic plasticity. Conversely, removal of either of these sources of activation is sufficient to delay the end of the CP21,36.
Although the specific mechanisms that operate to terminate the CP for LTPWM-III are incompletely established, neurotrophic factors appear to be critical to visual system development17,33. Though not the sole regulator of synaptic plasticity, BDNF and its tyrosine kinase receptors likely influence synaptic plasticity in developing visual cortex5. When the postnatal rise of BDNF was accelerated in transgenic mice, precocious development of visual acuity and earlier termination of the CP for ocular-dominance was observed14,17. These mice additionally exhibit accelerated maturation of cortical gamma-aminobutyric acid (GABAergic) inhibition and age-dependent decline of cortical LTPWM-III14,17. Recent data strengthen BDNF’s influence on the GABAergic system in visual cortex during the CP to bring about a developmental shift from a largely excitatory initial effect to a later, almost exclusively inhibitory, effect14,17,25. BDNF facilitates developing GABA inhibitory processes, thereby contributing to closure of the CP15,17.
Other lines of evidence suggest that BDNF also plays a key role in maturation of GABAergic mechanisms and closure of the CP in visual cortex. Visual activity differentially modulates BNDF mRNA expression in visual cortex, which is low in dark-reared rats and elevates after exposure to light33. GABAergic transmission in visual cortex slices from dark-reared, CP rats is threefold lower than in normally reared animals28. Further, mice lacking the enzyme to synthesize an isoform of a GABA synthetic enzyme, glutamic acid decarboxylase (GAD65), show unremitting delay of CP onset, but injection of GABAergic agonists initiates the CP11.
We have observed that REMSD alters the balance between inhibitory and excitatory mechanisms in the developing, CP visual cortex34. Taken together with REMSD’s extension of the period in which LTPWM-III can be produced36, we hypothesized that REMSD suppresses development of inhibitory processes in visual cortex. We examine here whether REMSD affects developmental synaptic plasticity in visual cortex via a BDNF pathway. We explore this possibility by infusing BDNF into REMSD, adolescent rat visual cortex. We then tested for LTPWM-III in both the infused and non-infused sides of brain. The influence of REMSD and BDNF on inhibitory mechanisms in the adolescent rat is gauged by the relative maturity of inhibitory mechanisms in visual cortex.
Methods
All procedures were approved by the University of Mississippi Medical Center (UMMC) IACUC and comply with regulatory guidelines. Long-Evans Hooded rats (P28) from timed-pregnant dams (Harland Laboratories, Inc.) were housed in the animal facilities for one week. Each animal was then implanted with a cannula aimed at the binocular area of visual cortex on the left side of brain (A: 2.0, L: 2.0, and H: 1.0 – 2.0 mm below skull29). The cannula was connected to an osmotic minipump (Alzet, Model 2002 or 1007D, 0.5 μl/h) filled with either BDNF (0.5 mg/ml, in PBS with 1% BSA, gift of Regeneron Pharmaceuticals, Inc.) or 0.9% saline (n= 8 and 2, respectively), and placed subcutaneously on the animal’s back. The minipump was connected to the brain by polyethelene tubing front-loaded with sufficient saline to prevent drug delivery (i.e., saline delivery only) during the three-day postoperative recovery period plus first two days of REMSD. An air bubble between saline and drug prevented diffusion. Delivery of BDNF to visual cortex in the treated group did not start until the third (final) day of pedestal REMSD. The rationale for this delay was to permit time for establishment of the REMSD effect on synaptic plasticity34,36 before determining whether exogenous BDNF reverses the effect. The opposite hemisphere was not implanted and served as a within-animal control. Minipump infusions of BDNF do not spread to the opposite hemisphere13,23.
REMSD was accomplished by the multiple-small-pedestals-over-water technique, which is highly selective in reducing REMS27,36,37. Inasmuch as previous studies showed that this REMSD method reliably induced adolescent animals to display LTPWM-III, sleep was not recorded35. Animals were on a 12/12 L/D schedule and had free access to food and potable water. At the end of the third day of REMSD, animals were sacrificed (by guillotine under isoflurane), and whole cortical slices were prepared for electrophysiological study. Following published protocols, the brain was rapidly retrieved, immersed in ice-cold, cutting buffer (artificial cerebral-splinal fluid, ACSF; in mM concentrations: 124 NaCl, 5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, 10 dextrose and 1 kynurenate), and cut on a Vibratome (Ted Pella, Inc.) into whole coronal sections (400 μm) of V121,36. The sections containing Infused and non-infused tissue were prepared for in vitro electrophysiological studies, and placed in the interface chamber (Fine Science Tools, Inc.) to equilibrate in a warmed (31° C), oxygenated (95%O2/5%CO2) environment and bathed in ACSF without kynurenate (1–1.5 ml/min) for one hour prior to electrode placement. A glass pipette recording electrode (1–2mΩ, 1M NaCl) was placed in layer II–III of V1 of each hemisphere. On the treated side, it was positioned away from the infusion site to avoid the surrounding, presumably injured, tissue13. The electrode still resided in tissue affected by exogenous BDNF (~1–2 mm from the visible cannula track). Attempts to record nearer to the infusion site typically resulted in unstable or absent responses. On the non-infused side, the electrode approximated the placement on the treated side. Stimulating electrodes were positioned at the WM/layer VI interface just slightly off center in a radial line with the recording electrodes.
Electrophysiological experiments in pairs of treated and non-treated brain-slices were conducted in parallel on separate recording channels. Responses were monitored on an oscilloscope, digitized and stored on a computer. A full input-output curve determined the single-stimulation intensity that yielded a half-maximal response, which was used throughout. A 20min baseline was attempted, utilizing square-wave stimuli (10–150 μA) of 0.2ms duration, delivered every 30s (S88, Grass Instruments, Quincy, MA, USA). Baseline responses with more than ± 10% variability were rejected, and the experiment restarted. After a stable baseline, five, 2s episodes, each 10s apart, of TBS (12-stimulus trains at 6 Hz, each train containing four pulses at 100 Hz) were delivered. The amplified (P511, Grass Instruments, Quincy, MA, USA), extracellular field potentials were digitized at 20 kHz (DAP 2400e/4, Microstar Laboratories, WA, USA) and later analyzed to extract the amplitude (CPA, Data Wave Technologies, CO, USA). Induction of LTP was considered successful if, after TBS, the response amplitude reliably increased over baseline, as determined, according to previously published methods4,36 by an F-test of the ratio of the variances for the reduced versus the full model. We examined whether LTP induction was more likely to be produced in infused or non-infused tissue with the Fisher exact t-test (p<0.05).
Three, immature, CP rats (P22-25) were implanted with minipumps containing the same concentration of BDNF as in the older animals. The younger animals were not REMS-deprived and were at an age (P23-25) when LTPWM-III is readily produced30. This group served to control for the possibility that BDNF might block LTPWM-III at any point in development.
To establish the relative maturity and CP-closing capacity of the inhibitory GABAergic mechanisms in cortical layer IV20, we conducted paired-pulse stimulation (PPS) in another group of REMSD- and BDNF-infused adolescent rats (n=5). As in the LTP-studies, PPS trials were carried out on matched pairs of BDNF-infused- and non-infused coronal V1 slices from the same animal. Stimulating electrodes were placed sequentially in layer IV and at the WM/layer VI interface. In every experiment the half-maximum voltage was the same. After a stable baseline, paired stimulations were presented at 20, 40, 60 and 80ms intervals, each of the stimulations 30s apart. In each animal, five sets of presentations were completed at every interval at both sites and averaged. Paired-pulse ratios were the amplitude of the second response relative to the first. Repeated measure ANOVAs examined differences between treatments at the four IPIs. Inhibition is indicated by a < 1 and facilitation by a > 1 ratio. At IPIs longer than 20ms, facilitation is only observed in normal animals younger than 30-days31.
Results
As expected in adolescent rats after a period of REMSD35, TBS of WM on the non-infused side uniformly induced LTPWM-III (5/5, Figure 1A). In contrast, induction of LTPWM-III failed in hemispheres receiving BDNF infusions (8/8 animals, Figure 1B). As such, BDNF-infused visual cortex in REMSD, adolescent rats showed significantly less LTPWM-III production (no post-TBS increase in response amplitude) than observed in the same animals’ non-infused cortex (Fishers exact t-test, p < 0.001). In the unilaterally saline-infused, REMS-deprived rats (n=2), LTPWM-III was obtained in both hemispheres, the infused (Figure 1C) and non-infused (data not shown).
Figure 1.
Effects of cortical infusion of BDNF on LTPWM-III in visual cortex in REMSD adolescent rats. A. LTP in layer III of V1 after theta burst stimulation (TBS) of the white matter (LTPWM-III) is robustly induced on the non-infused side of brain in REMSD rats. B. On the opposite side of the same coronal section, infusion of BDNF to upper visual cortex prevents TBS of the white matter from producing LTP. C. TBS of white matter produces robust LTPWM-III in tissue slices from two REMSD animals receiving only saline infusions on the recorded side of brain. In each panel the 1-min averaged, responses are plotted, normalized to each animal’s own baseline (± SEM). The up-arrow indicates the timing of the TBS. The responses shown in insets for each panel are from an individual animal in that group. Plotted are averages of five responses taken from the middle of the baseline and the post-TBS phases of each experiment.
LTPWM-III was observed in two of the three BDNF-infused, normally sleeping CP pups. In the third animal, LTP was not induced at either the WM or layer IV stimulation site, suggesting that the tissue might have been compromised (c.f. 36). The two animals had a 114% mean increase in LTPWM-III above their baseline levels (data not shown, see30,36,38). Ability of BDNF to block LTPWM-III at any age was not supported.
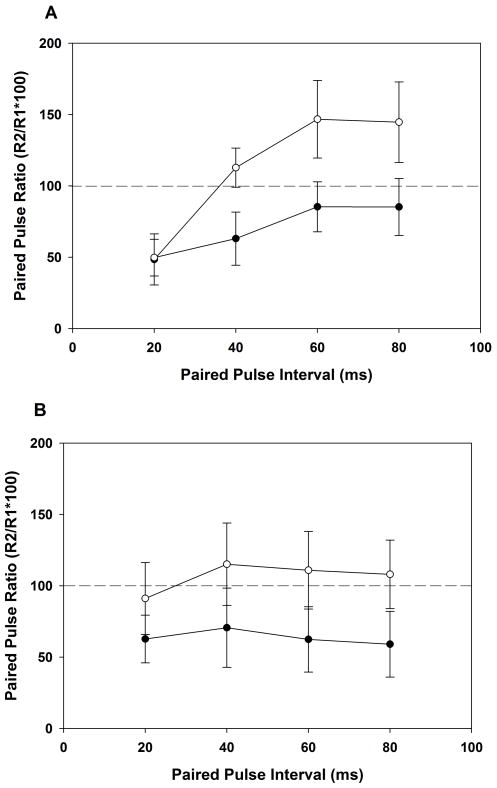
REMSD adolescent rats in the PPS trials showed facilitation of the second response to each stimulus pair directed at WM except at 20-ms IPI on the BDNF non-infused side of visual cortex (Figure 2A). When PPS was directed at layer IV on the same side, the second response was also facilitated at IPIs longer than 20-ms (Figure 2B). The magnitude of facilitation in layer IV at each of the three longer inter-pulse intervals tended, however, to be less than with WM stimulation (F=4.802, p=0.06). In contrast, on the BDNF-infused side, inhibition of the second response was observed at all four IPIs and at both, the WM and layer IV stimulation sites.
Figure 2.
Paired pulse stimulation (PPS) studies in REMSD adolescent rats A. Studies with the stimulation electrode positioned in white matter and responses registered in layer II/III. Averaged paired-pulse ratio (± SEM) for each rat (n=5) is plotted at each interpulse interval (IPI). On the non-infused side (open circles), white matter stimulation facilitates the second response of each pair except at the 20-ms IPI. On the BDNF-infused side (filled circles), inhibition of the second response is observed at all IPIs. B. Studies where the stimulation electrode is positioned in layer IV and responses are registered in layer II/III. On the non-BDNF-infused side (open circles, n=4), the second response is facilitated when PPS is directed at layer IV at all IPIs greater than 20-ms. The magnitude of facilitation at each of the inter-pulse intervals tended to be less after layer IV as compared to white matter stimulation (F=4.802, p= 0.06). On the BDNF-infused side (filled circles), inhibition of the second response is observed at all IPIs.
Discussion
Infusion of BDNF for 24 hrs was sufficient to antagonize the effects of three days of REMSD on LTPWM-III production in post-CP adolescent rats. As such, BDNF-infusion appears to reverse REMSD-induced reestablishment of the CP for developmental synaptic plasticity in adolescent rats35. The expected action of inhibitory mechanisms considered partly responsible for closure of the CP at this age31 seems to be reinitiated in REMSD adolescent rats by BDNF infusion. The specificity of BDNF for this outcome is underscored by the finding that LTPWM-III is reliably obtained in cortical slices from the animals’ non-BDNF-infused hemispheres. Inasmuch as stress has been shown to inhibit LTP36, concern about stress affecting the data in REMSD studies was minimized in our studies insofar as non-infused cortex served as an internal control. Further, the saline-infused, REMSD animals were capable of LTPWM-III production bilaterally.
In contrast, BDNF did not completely block LTPWM-III in younger, CP, control animals, suggesting that acute administration of BDNF does not prevent this form of synaptic plasticity irrespective of age. LTPWM-III observed in the young CP-rats was not as robust as expected, however (114% above baseline)30,38. A graded response might be anticipated because precocial termination of the CP for LTPWM-III was demonstrated in transgenic mice having endogenously high levels of BDNF14. The single day of BDNF-infusion in these animals may have increased inhibitory tone and initiated a precocial decline of the CP for LTPWM-III.
Our PPS results also support the likelihood that BDNF infusions increase inhibitory tone in visual cortex and, conversely, REMSD reduces it (c.f.34). Inasmuch as facilitation is exclusively observed in untreated animals younger than 30-days31, REMSD seems to reverse maturational effects. This is congruent with previous data that REMSD in adolescent rats appears to interrupt and reverse maturation of inhibitory mechanisms in visual cortex34. The idea that termination of the CP depends upon maturation of GABAergic mechanisms20,31 supports our proposal that REMS-driven activation of BDNF is necessary and sufficient to promote maturation of these inhibitory mechanisms in visual cortex.
REMSD during the CP has been found to reduce inhibition34, delay maturation36, and extend the CP35 in visual cortex. Similar effects are reported after removal of visual input (i.e., dark-rearing) during the CP16,21,28,31. The similar effects of REMSD and visual-deprivation on visual pathways may be pivotal for the capacity of either treatment to extend the CP for LTPWM-III21,36. Blockade of input to LGN occurs with both manipulations and potentially leads to reduction in activity-dependent expression of neurotrophic factors7,8. Such findings, together with the present results, suggest that REMS contributes to closure of the CP for synaptic plasticity by activating neurotrophic (BDNF)- and downstream inhibitory (GABAergic) mechanisms. BDNF is expressed in brain in an activity-dependent manner1,40. Accordingly, REMS-related activation of LGN relay cells may prompt release of BDNF, lead to GABAergic maturation and increased inhibition in visual cortex, and precipitate CP closure12.
In view of the preliminary nature of these proposals, studies are required to confirm the specific effects of REMS on BDNF expression as well as the putative role of BDNF in maturation of the “plasticity gate” (and associated GABAergic mechanisms) in visual cortex31. The current findings nevertheless support a developmental contribution by REMS to activation of BDNF and its role in closure of the CP in visual cortex.
Recent work demonstrates that REMSD in neonatal rats has relatively long-lasting effects upon brain maturation even after removal of REMS suppression24. Inasmuch as BDNF is implicated in development of several psychopathologies10, we speculate that early-life REMSD may eventuate in later neuropathologies6. Uncovering the CNS mechanisms affected by REMSD in neonates may lead to discovery of more efficient treatments for later-life, REMSD-related disorders.
Highlights.
Intracortical infusion of BDNF reverses synaptic plasticity effects of adolescent REMS deprivation.
REMS contributes to the effects of BDNF activation on closure of the critical period in visual cortex.
Adolescent REMS deprivation affects the development of visual cortical inhibitory mechanisms.
Acknowledgments
Research supported by: NIH/NINDS NS-31720 & UMMC Intramural Research Support Program).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Balkowiec A, Katz DM. Cellular Mechanisms Regulating Activity-Dependent Release of Native Brain-Derived Neurotrophic Factor from Hippocampal Neurons. Journal of Neuroscience. 2002;22:10399. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bear MF, Colman H. Binocular competition in the control of geniculate cell size depends upon visual cortical N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci U S A. 1990;87:9246–9249. doi: 10.1073/pnas.87.23.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bear MF, Kirkwood A. Neocortical long-term potentiation. Curr Opin Neurobiol. 1993;3:197–202. doi: 10.1016/0959-4388(93)90210-p. [DOI] [PubMed] [Google Scholar]
- 4.Bear MF, Press WA, Connors BW. Long-term potentiation in slices of kitten visual cortex and the effects of NMDA receptor blockade. J Neurophysiol. 1992;67:841–851. doi: 10.1152/jn.1992.67.4.841. [DOI] [PubMed] [Google Scholar]
- 5.Bracken BK, Turrigiano GG. Experience-dependent regulation of TrkB isoforms in rodent visual cortex. Devel Neurobio. 2009;69:267–278. doi: 10.1002/dneu.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: A link between stress and mood disorders. Psychoneuroendocrinology. 2009;34:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Capsoni S, Tongiorgi E, Cattaneo A, Domenici L. Dark rearing blocks the developmental down-regulation of brain-derived neurotrophic factor messenger RNA expression in layers IV and V of the rat visual cortex. Neuroscience. 1999;88:393–403. doi: 10.1016/s0306-4522(98)00250-4. [DOI] [PubMed] [Google Scholar]
- 8.Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lima AD, Singer W. The brainstem projection to the lateral geniculate nucleus in the cat: identification of cholinergic and monoaminergic elements. J Comp Neurol. 1987;259:92–121. doi: 10.1002/cne.902590107. [DOI] [PubMed] [Google Scholar]
- 10.Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17 (Suppl 3):306–310. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- 11.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino H, et al. GABAB Receptor Activation Triggers BDNF Release and Promotes the Maturation of GABAergic Synapses. Journal of Neuroscience. 2009;29:11650–11661. doi: 10.1523/JNEUROSCI.3587-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galuske RA, Kim DS, Castren E, Thoenen H, Singer W. Brain-derived neurotrophic factor reversed experience-dependent synaptic modifications in kitten visual cortex. Eur J Neurosci. 1996;8:1554–1559. doi: 10.1111/j.1460-9568.1996.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 14.Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:1–5. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. In: Pelt Jv., editor. Progress in Brain Research Development, Dynamics and Pathiology of Neuronal Networks: from Molecules to Functional Circuits. Elsevier; 2005. pp. 115–124. [DOI] [PubMed] [Google Scholar]
- 16.Heynen AJ, et al. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- 17.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 18.Hubel DH, Wiesel TN. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 19.Iny K, Heynen AJ, Sklar E, Bear MF. Bidirectional Modifications of Visual Acuity Induced by Monocular Deprivation in Juvenile and Adult Rats. Journal of Neuroscience. 2006;26:7368–7374. doi: 10.1523/JNEUROSCI.0124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- 22.Lein ES, Hohn A, Shatz CJ. Dynamic regulation of BDNF and NT-3 expression during visual system development. J Comp Neurol. 2000;420:1–18. doi: 10.1002/(sici)1096-9861(20000424)420:1<1::aid-cne1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Lodovichi C, Berardi N, Pizzorusso T, Maffei L. Effects of neurotrophins on cortical plasticity: same or different? J Neurosci. 2000;20:2155–2165. doi: 10.1523/JNEUROSCI.20-06-02155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez J, et al. Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience. 2008;153:44–53. doi: 10.1016/j.neuroscience.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto T, et al. Brain-derived neurotrophic factor-induced potentiation of glutamate and GABA release: Different dependency on signaling pathways and neuronal activity. Molecular and Cellular Neuroscience. 2006;31:70–84. doi: 10.1016/j.mcn.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 26.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 27.Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974;2:553–556. doi: 10.1016/0091-3057(74)90018-5. [DOI] [PubMed] [Google Scholar]
- 28.Morales B, Choi SY, Kirkwood A. Dark Rearing Alters the Development of GABAergic Transmission in Visual Cortex. Journal of Neuroscience. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sandiego: 1986. [Google Scholar]
- 30.Perkins AT, Teyler TJ. A critical period for long-term potentiation in the developing rat visual cortex. Brain Res. 1988;439:222–229. doi: 10.1016/0006-8993(88)91478-3. [DOI] [PubMed] [Google Scholar]
- 31.Rozas C, et al. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J Neurosci. 2001;21:6791–6801. doi: 10.1523/JNEUROSCI.21-17-06791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakai K. Anatomical and physiological basis of paradoxical sleep. In: McGinty DJ, et al., editors. Brain Mechanisms of Sleep. Raven Press; New York: 1985. pp. 111–137. [Google Scholar]
- 33.Schoups AA, Elliott RC, Friedman WJ, Black IB. NGF and BDNF are differentially modulated by visual experience in the developing geniculocortical pathway. Brain Res Dev Brain Res. 1995;86:326–334. doi: 10.1016/0165-3806(95)00043-d. [DOI] [PubMed] [Google Scholar]
- 34.Shaffery JP, Lopez J, Bissette G, Roffwarg HP. Rapid eye movement sleep deprivation in post-critical period, adolescent rats alters the balance between inhibitory and excitatory mechanisms in visual cortex. Neurosci Lett. 2006;393:131–135. doi: 10.1016/j.neulet.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 35.Shaffery JP, Lopez J, Bissette G, Roffwarg HP. Rapid eye movement sleep deprivation revives a form of developmentally regulated synaptic plasticity in the visual cortex of post-critical period rats. Neurosci Lett. 2006;391:96–101. doi: 10.1016/j.neulet.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Shaffery JP, Sinton CM, Bisset G, Roffwarg HP, Marks GA. Rapid Eye Movement Sleep Deprivation Modifies Expression of Long-term Potentiation in Visual Cortex of Immature Rats. Neuroscience. 2002;110:431–443. doi: 10.1016/s0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- 37.Suchecki D, Duarte PB, Tufik S. Sleep rebound in animals deprived of paradoxical sleep by the modified multiple platform method. Brain Res. 2000;875:14–22. doi: 10.1016/s0006-8993(00)02531-2. [DOI] [PubMed] [Google Scholar]
- 38.Teyler TJ, Perkins AT, Harris KM. The development of long-term potentiation in hippocampus and neocortex. Neuropsychologia. 1989;27:31–39. doi: 10.1016/0028-3932(89)90088-2. [DOI] [PubMed] [Google Scholar]
- 39.Wang XF, Daw NW. Long term potentiation varies with layer in rat visual cortex. Brain Res. 2003;989:26–34. doi: 10.1016/s0006-8993(03)03321-3. [DOI] [PubMed] [Google Scholar]
- 40.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]