Abstract
Background
Dental caries is one of the most common diseases in the world. However, our understanding of how the microbial community composition changes in vivo as caries develops is lacking.
Objective
An in vivo model was used in a longitudinal cohort study to investigate shifts in the microbial community composition associated with the development of enamel caries.
Design
White spot lesions were generated in vivo on human teeth predetermined to be extracted for orthodontic reasons. The bacterial microbiota on sound enamel and on developing carious lesions were identified using the Human Oral Microbe Identification Microarray (HOMIM), which permits the detection of about 300 of the approximate 600 predominant bacterial species in the oral cavity.
Results
After only seven weeks, 75% of targeted teeth developed white spot lesions (8 individuals, 16 teeth). The microbial community composition of the plaque over white spot lesions differed significantly as compared to sound enamel. Twenty-five bacterial taxa, including Streptococcus mutans, Atopobium parvulum, Dialister invisus, and species of Prevotella and Scardovia, were significantly associated with initial enamel lesions. In contrast, 14 bacterial taxa, including species of Fusobacterium, Campylobacter, Kingella, and Capnocytophaga, were significantly associated with sound enamel.
Conclusions
The bacterial community composition associated with the progression of enamel lesions is specific and much more complex than previously believed. This investigation represents one of the first longitudinally-derived studies for caries progression and supports microbial data from previous cross-sectional studies on the development of the disease. Thus, the in vivo experiments of generating lesions on teeth destined for extraction in conjunction with HOMIM analyses represent a valid model to study succession of supragingival microbial communities associated with caries development and to study efficacy of prophylactic and restorative treatments.
Keywords: white spot lesions, caries, HOMIM, molecular microbiology
Enamel caries, one of the most common human diseases, is considered a multi-factorial disease involving interactions among the oral microbiota, host immune system, diet, saliva and properties of the tooth surface. The ecological plaque hypothesis (and its many variations) (1–5) stressed the importance of changes in local environmental conditions as causes for shifts in the proportions of species within a mixed plaque population. The ecological plaque hypothesis also states that potentially cariogenic bacteria may be present in clinically low levels in dental health. The implications are that caries could be controlled not only by targeting cariogenic bacteria but also by interfering with local factors responsible for driving the harmful shifts in the microbiota. Culture-independent methods based on molecular analysis of 16S rRNA genes have led to an expanded list of caries-associated species in addition to Streptococcus mutans, including species of Actinomyces, Abiotrophia, Atopobium, Bifidobacterium, Lactobacillus, Olsenella, Pseudoramibacter, Scardovia, Selenomonas and Veillonella (6–11). In particular, recent molecular studies (10) have associated Actinomyces gerencseriae, A. naeslundii and A. israelii with initial enamel lesions also known as white spot lesions (WSLs). Furthermore, many studies support the view that caries can develop in the absence of S. mutans (5, 10) (12). Consequently, several bacterial species, either alone or as a group, other than S. mutans may also play major roles in caries development (1, 5, 13, 14).
In this study, WSLs were artificially induced in situ on the buccal surface of premolars intended for extraction due to orthodontic treatment. The microbial communities were followed longitudinally in each subject for seven weeks and the communities of sound enamel were compared with those of WSLs by using the Human Oral Microbe Identification Microarray (HOMIM), which is a high-throughput method capable of simultaneously detecting about 300 oral predominant bacterial species including those that have not yet been cultivated (11, 15) (http://mim.forsyth.org).
Material and methods
Subject population
The subject population consisted of five male and three female individuals of Scandinavian origin, chosen from an orthodontic clinic for the study. All were 10 to 14 years old and had premolars scheduled for extraction due to orthodontic reasons. Only subjects with little to no past caries experience were included in the study. A total of 23 premolars were used and the premolars had no signs of clinical WSLs at baseline. For all scheduled study visits, participants did not brush their teeth the morning prior to sampling and had no breakfast within an hour before sampling. All appointments were scheduled between 9 am and 12 pm. While enrolled in the study, participants brushed their teeth with the non-fluoride toothpaste Solidox® (Lilleborg AS, Oslo, Norway) and did not use any fluoride or antimicrobials, such as chlorhexidine or triclosane. The study was approved by the Regional Ethics Committee (REK sør-øst, PB 1130, Blindern, Oslo, Norway). The subjects and their guardians signed an informed consent form before the start of the study.
Clinical procedures
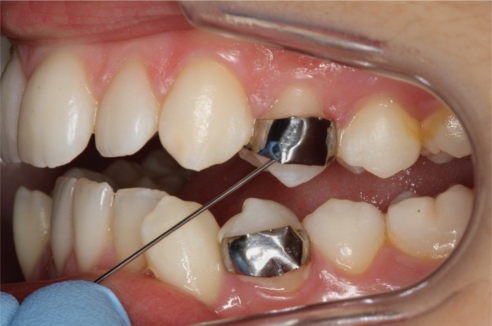
Orthodontic bands with two metal posts (0.8 mm thick) welded onto the inner buccal surface specifically designed for plaque accumulation (16), were banded on premolars destined for extraction during the first visit (Fig. 1). The buccal surface of the premolars was visually inspected before the banding. No signs of previous or current caries activity were detected. Temp Bond NE (Kerr Corporation, Orange, CA, USA) was used to cement the bands in place (17). Any residual cement between the buccal tooth surface and the band was removed. Plaque samples were taken from premolars at baseline (immediately before banding) and at the final visit 7 weeks after banding.
Fig.1.
Orthodontic bands designed for plaque retention. The bands were placed in vivo on premolars.
Care was taken not to touch the premolar area prior to plaque sampling. A lip spreader was used to isolate the premolars and the buccal surfaces were allowed to air dry before sample collection. The same examiner collected all samples with a sterile orthodontic wire (Fig. 1) and immediately suspended each sample in 300 µl TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.4). The samples were transported to the laboratory using the NalgeneTM Labtop Cooler (−20°C), before placing them at −80°C until further processing. For baseline sampling, the buccal enamel surface where the band would be placed was sampled with a probe. At the final visit, the probe was inserted into the space between the band and the buccal tooth surface and the plaque sample was collected from the enamel surface.
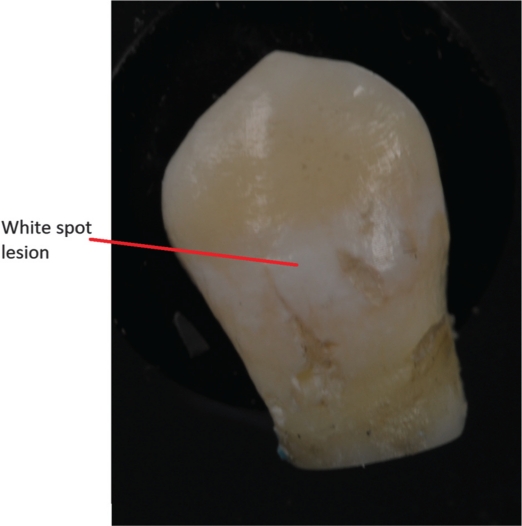
After collecting the sample at the final visit, the examiner extracted the premolars and the orthodontic treatment was continued as planned by the orthodontist. The buccal surface of the extracted premolars was carefully cleaned with water, air dried and then the buccal lesion was given a score in the following manner (Fig. 2): no WSLs=1, minor WSLs=2 and severe WSLs=3 (18).
Fig. 2.
Resulting white spot lesion after 7 weeks of plaque retention. The photograph was taken after the removal of an orthodontic band.
DNA isolation and HOMIM protocol
DNA was isolated from clinical samples using a Ready-Lyse™ Lysozyme Solution (Epicentre) for overnight incubation before using a MasterPure DNA Purification Kit (Epicentre) according to the manufacturer's directions. Purified DNA samples were analyzed using HOMIM, which was developed to simultaneously detect ∼300 of the most prevalent oral bacterial species. The method is described in detail elsewhere (15). Briefly, 16S rRNA-based, reverse-capture oligonucleotide probes (typically 18–20 bases) were printed on aldehyde-coated glass slides. 16S rRNA genes were PCR amplified from DNA extracts using 16S rRNA universal forward and reverse primers and labeled via incorporation of Cy3-dCTP in a second nested PCR. The labeled 16S rRNA gene amplicons were hybridized overnight with probes on the slides. After washing, the microarray slides were scanned using an Axon 4000B scanner and crude data were extracted using GenePix Pro software.
Analysis of microbial community profiles
Microbial community profiles were generated from image files of scanned HOMIM arrays using a HOMIM online analysis tool (available at: http://bioinformatics.forsyth.org/homim/). The detection of a particular taxon in a sample was determined by the presence of a fluorescent spot for that unique probe. A mean intensity for each taxon was calculated from the hybridization spots of the same probe and the signals were normalized and calculated as previously described (15). The range of signal levels was obtained by raising normalized signal intensities to the power of 0.3. Any original signal that was less than two times the background value was reset to 1 and was assigned to the signal level 0; indicating absence of a particular taxon in a sample. The remaining signals, those greater than 1, were categorized into scores from 1 to 5; corresponding to different signal levels. To determine how bacterial community composition varied across samples, we compared total hybridization profiles obtained by HOMIM arrays for each sample using correspondence analysis (CoA) in MeV 4.6 (19). Analysis was performed on the absolute intensity of HOMIM data (frequency of scores from 0 to 5) as well as binary data (presence/absence). The prevalence of each taxon was computed for each subject and averaged within groups. Wilcoxon Rank Sum test and t-test were used to identify statistically significant differences between groups (baseline and final visit; WSLs 1, 2 and 3; and plaque indices 0, 1 and 2). A p-value of <0.05 was considered significant. False discovery rate (FDR) using Benjamini-Hochberg correction was used to control for multiple hypothesis. For the cluster analysis, Pearson correlation was used as a distance metric selection. JMP 9.0 (www.jmp.com/) and R Statistical Package (www.r-project.org/) were used in statistical analyses.
Results
Succession of microbial communities
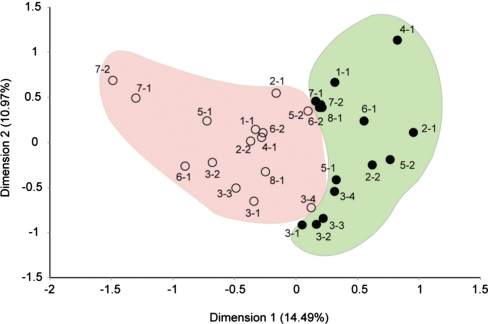
At the end of seven weeks at the final visit, 75% of the premolars had developed WSLs on their buccal surfaces underneath the retention band (Fig. 2). There was a clear shift of the microbiota from plaque found on sound enamel (baseline) as compared to plaque retained after seven weeks (final visit). Microbial communities at baseline were found to be significantly different (p<0.001; t test) from the microbial communities at the final visit (Figs. 3 and 4). For each visit, samples had a bacterial ‘signature’ for each individual; samples from the same individual, in general, were more similar to one another than when taken from two different individuals (Fig. 3).
Fig. 3.
Plot of correspondence analysis (CA) of the enamel bacterial community using binary data (presence/absence). Each circle represents one community from a single visit from an individual patient. Only communities with data for both the baseline and final visit are plotted and labeled by color according to visit. The baseline samples are labeled with solid circles; samples from the final visit (7 weeks later) are labeled with open circles. Numbers next to each circle indicate a patient and tooth number. The percentages of variation described by the correspondence analysis coordinates are shown in parentheses. Microbial communities from the baseline visit are significantly different (p<0.001; t test) from the final visit.
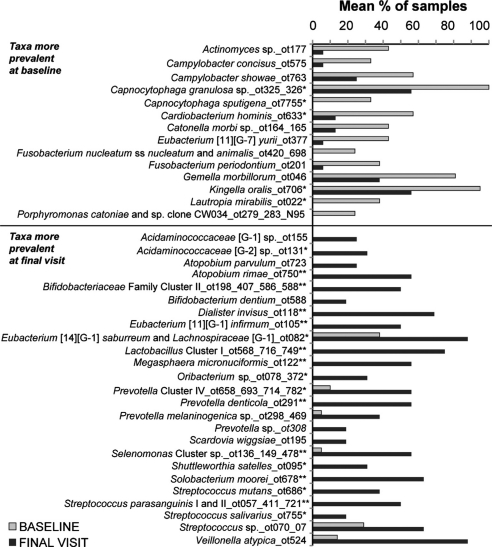
Fig. 4.
Mean frequencies of bacterial probes that were significantly different (p<0.05; Wilcoxon test) among subjects at baseline and final visit. *Indicates adjusted p (Benjamini/Hochberg) values p<0.01 and **p<0.001.
Microbial community of dental health (baseline)
Fourteen bacterial taxa were found to be prevalent in plaque covering sound enamel at baseline (p<0.01; Fig. 4). The most commonly detected species in greater than 80% of samples were Gemella morbillorum, Kingella oralis and Capnocytophaga granulosa. All species have been detected previously in supragingival and subgingival plaque (15). All but Campylobacter concisus and Campylobacter showae are capable of fermenting carbohydrates to acid, although C. concisus and C. showae can produce acetate and or succinate with formate and fumarate in the culture medium (20).
Microbial community of the initial caries lesion developed after 7 weeks (final visit)
Twenty-five bacterial species were significantly increased in plaque behind the retaining bands after seven weeks (Fig. 4). Many of these species have been previously associated with caries development, such as Streptococcus mutans, other species of Streptococcus and species of Lactobacillus. Other species less known to be associated with caries development included Atopobium parvulum, Dialister invisus, and species of Prevotella and Scardovia.
Of note, 25% of teeth did not develop a lesion after seven weeks. Consequently, we compared the changes in microbiota on pairs of samples between baseline and for those samples that did not develop a lesion. For this small sample size, there were no taxa that were significantly more prevalent at baseline or at the final visit.
Microbial community with respect to plaque index
Communities with Plaque Index 2 are significantly different from plaque indices 0 and 1. The average number of taxa for Plaque Index 0, 1 and 2 was 38.50, 44.13 and 53.68, respectively. The Wilcoxon Signed-Rank Test was used for each pair; plaque indices 0 and 2 were compared: p-value=0.0014, and plaque indices 1 and 2: p-value=0.0247.
Microbial community of initial caries lesions with respect to severity of disease
There were differences in the prevalence of specific taxa in supragingival plaque taken from WSL 1 sites as compared to supragingival plaque from WSL 3 sites at the final visit. Four taxa, namely Gemella morbillorum, Selenomonas sp. OT136/OT148, Megasphaera micronuciformis, and Selenomonas sputigena, were more prevalent in WSL 1 samples (no lesions), (p<0.01). However, due to the low number of samples (n), the results were not significant (e.g. p>0.05) when adjusted for multiple comparisons.
Discussion
The results clearly showed an ecological shift in dental plaque over developing WSLs in enamel. Many bacterial species, including typical caries-associated species such as S. mutans and Lactobacillus spp., increased with the development of WSLs. Consequently, the bacterial community composition associated with the progression of WSL is specific and much more complex than previously believed and should be explored in future studies. Our results not only confirmed previous studies (6, 8, 10, 11), but additional species or phylotypes that were significantly associated with the longitudinal progression of caries in the secondary dentition were identified (Table 1) (10).
Table 1.
Comparison of statistically significant bacterial taxa associated with carious lesions in the secondary dentition
| This study | Aas et al. (10) | ||
|---|---|---|---|
| Species/phylotype | WSL | WSL | caries |
| Acidaminococcaceae sp. OT131 | + | ||
| Acidaminococcaceae sp. OT155 | + | ||
| Atopobium spp. | + | + | |
| Bifidobacterium spp. | + | ||
| Campylobacter gracilis | + | ||
| Dialister invisus | + | ||
| Eubacterium infirmum | + | ||
| Eubacterium sp. OT082 | + | ||
| Lactobacillus spp. | + | + | |
| Leptotrichia sp. GT018 | + | ||
| Megasphaera micronuciformis | + | ||
| Oribacterium sp. OT078 | + | ||
| Prevotella denticola | + | + | |
| Prevotella melaninogenica | + | ||
| Prevotella sp. OT308 | + | ||
| Propionibacterium sp. FMA5 | + | ||
| Scardovia wiggsiae | + | ||
| Selenomonas sp. OT136 | + | + | |
| Shuttleworthia satelles | + | ||
| Solobacterium moorei | + | ||
| Streptococcus parasanguinis | + | ||
| Streptococcus sp. OT070 | + | ||
| Streptococcus salivarius | + | + | + |
| Streptococcus mutans | + | + | |
| Veillonella spp. | + | + | + |
Many of the bacterial species found to be increased in plaque covering sound enamel and in plaque behind the plaque retaining bands are capable of producing acid. Bacteria capable of producing caries need to be both acidogenic (able to produce acid) and aciduric (able to survive in an acid environment) (21). It is noteworthy that non-mutans streptococci, such as those found in WSLs, are capable of acidogenesis at low pH and typically outnumber mutans streptococci. Consequently, several species of non-mutans Streptococcus have been implicated in caries production (13). Indeed, in this study several species or phylotypes were significantly more prevalent in WSLs (Fig. 4).
Our results provided strong evidence in support of the ecological plaque hypothesis, that is, enamel caries develops due to changes in local environmental conditions (plaque retaining bands), which disrupt the natural balance between plaque and the host, leading to enrichment of organisms that can potentially cause caries. In addition, our results showed that many bacterial species other than S. mutans and lactobacilli are associated with caries in vivo.
In summary, the bacterial diversity of the predominant oral microbiota associated with development of cariogenic lesions was determined using HOMIM in an in vivo model. Our results indicate that the microbiota on intact enamel was significantly different from that of the microbiota associated with WSLs developed for seven weeks under protected metal bands. The microbial communities in dental plaque associated with caries included species of the genera Acidaminococcaceae, Streptococcus, Lactobacillus, Veillonella, Prevotella, Solobacterium, Scardovia, and Atopobium. Some of these were associated with the increasing severity of WSLs such as S. wiggsiae, S. salivarius and V. atypica and might play important roles in the process of caries development. The in vivo model of microbial community succession provided novel insights into the microbial community shifts associated with the development of WSLs, and likely dental caries. Consequently, this model can be used to study the efficacy of treatment or perturbations, such as diet, antibiotics, and other prophylactic and restorative treatments, and may help design more effective interventions and preventions.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors. Funding by the Faculty of Dentistry, University of Oslo, Oslo, Norway.
References
- 1.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–81. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 2.Kleinberg I. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med. 2002;13:108–25. doi: 10.1177/154411130201300202. [DOI] [PubMed] [Google Scholar]
- 3.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–94. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 4.Beighton D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol. 2005;33:248–55. doi: 10.1111/j.1600-0528.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 2008;42:409–18. doi: 10.1159/000159604. [DOI] [PubMed] [Google Scholar]
- 6.van Ruyven FO, Lingström P, van Houte J, Kent R. Relationship among mutans streptococci, ‘low-pH’ bacteria, and iodophilic polysaccharide-producing bacteria in dental plaque and early enamel caries in humans. J Dent Res. 2000;79:778–84. doi: 10.1177/00220345000790021201. [DOI] [PubMed] [Google Scholar]
- 7.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–9. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munson MA, Banerjee A, Watson TF, Wade WG. Molecular analysis of the microflora associated with dental caries. J Clin Microbiol. 2004;42:3023–9. doi: 10.1128/JCM.42.7.3023-3029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chhour KL, Nadkarni MA, Byun R, Martin FE, Jacques NA, Hunter N. Molecular analysis of microbial diversity in advanced caries. J Clin Microbiol. 2005;43:843–9. doi: 10.1128/JCM.43.2.843-849.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–17. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preza D, Olsen I, Willumsen T, Boches SK, Cotton SL, Grinde B, et al. Microarray analysis of the microflora of root caries in elderly. Eur J Clin Microbiol Infect Dis. 2009;28:509–17. doi: 10.1007/s10096-008-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowden GH. Does assessment of microbial composition of plaque/saliva allow for diagnosis of disease activity of individuals? Community Dent Oral Epidemiol. 1997;25:76–81. doi: 10.1111/j.1600-0528.1997.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 13.Sansone C, Van Houte J, Joshipura K, Kent R, Margolis HC. The association of mutans streptococci and non-mutans streptococci capable of acidogenesis at a low pH with dental caries on enamel and root surfaces. J Dent Res. 1993;72:508–16. doi: 10.1177/00220345930720020701. [DOI] [PubMed] [Google Scholar]
- 14.van Houte J, Lopman J, Kent R. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res. 1996;75:1008–14. doi: 10.1177/00220345960750040201. [DOI] [PubMed] [Google Scholar]
- 15.Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–32. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Øgaard B, Rølla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94:68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 17.Daugela P, Oziunas R, Zekonis G. Antibacterial potential of contemporary dental luting cements. Stomatologija. 2008;10:16–21. [PubMed] [Google Scholar]
- 18.Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81:93–8. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 19.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, et al. TM4 microarray software suite. Methods Enzymol. 2006;411:134–93. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- 20.Vandamme P, Dewhirst FE, Paster BJ, Stephen LW, Genus I. Campylobacter, Sebald and Véron, , 907AL emend. Vandamme, Falsen, Rossau, Hosle, Segers, Tytgat and De Ley 1991a, 98. In: Garrity GM, Brenner DJ, Krieg NR, Stalye JT, editors. Bergey's manual of systematic bacteriology, vol. 2, part. C. The alpha-, beta-, delta-, and Epsilonproteobacteria. 2nd ed. New York: Springer; 2005; 1963. pp. 1147–60. [Google Scholar]
- 21.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]