Abstract
Aims
Vascular disease states are associated with endothelial dysfunction and increased production of reactive oxygen species derived from NADPH oxidases. However, it remains unclear whether a primary increase in superoxide production specifically in the endothelium alters the initiation or progression of atherosclerosis.
Methods and results
Mice overexpressing Nox2 specifically in the endothelium (Nox2-Tg) were crossed with ApoE−/− mice to produce Nox2-Tg ApoE−/− mice and ApoE−/− littermates. Endothelial overexpression of Nox2 in ApoE−/− mice did not alter blood pressure, but significantly increased vascular superoxide production compared with ApoE−/− littermates, measured using both lucigenin chemiluminescence and 2-hydroxyethidium production (ApoE−/−, 19.9 ± 6.3 vs. Nox2-Tg ApoE−/−, 47.0 ± 7.0 nmol 2-hydroxyethidium/aorta, P< 0.05). Increased endothelial superoxide production increased endothelial levels of vascular cell adhesion protein 1 and enhanced macrophage recruitment in early lesions in the aortic roots of 9-week-old mice, indicating increased atherosclerotic plaque initiation. However, endothelial-specific Nox2 overexpression did not alter native or angiotensin II-driven atherosclerosis in either the aortic root or the descending aorta.
Conclusion
Endothelial-targeted Nox2 overexpression in ApoE−/− mice is sufficient to increase vascular superoxide production and increase macrophage recruitment possible via activation of endothelial cells. However, this initial increase in macrophage recruitment did not alter the progression of atherosclerosis. These results indicate that Nox-mediated reactive oxygen species signalling has important cell-specific and distinct temporal roles in the initiation and progression of atherosclerosis.
Keywords: Nox2, NADPH oxidase, Atherosclerosis, Macrophages, VCAM-1
1. Introduction
Increased production of reactive oxygen species (ROS) in the vascular wall is a hallmark of vascular disease states including atherosclerosis, diabetes, and hypertension.1 Excessive generation of ROS, particularly superoxide, is thought to be an important mediator of atherosclerotic initiation by causing increased oxidative stress, recruitment of inflammatory cells, and endothelial dysfunction. However, very few studies to date have assessed whether increasing ROS production specifically in the endothelial cell layer is enough to accelerate the initiation and progression of atherosclerosis.
The NADPH oxidases are major sources of ROS in the vasculature, generating superoxide from molecular oxygen using NADPH as the electron donor. NADPH oxidases are multi-subunit, membrane-bound enzymes incorporating one of several homologues of the membrane-bound Nox catalytic subunit.2 Tissue-specific Nox expression is observed in the vasculature, with Nox2 and Nox4 being expressed in endothelial cells,3,4 whereas in vascular smooth muscle cells (VSMCs) Nox1 and Nox4 predominate.5,6 NADPH oxidase-derived ROS have important roles in vascular disease pathogenesis. Both Nox2 and Nox4 have been shown to be localized within the plaque of diseased human coronary arteries.7 However, only Nox2 has been shown to be up-regulated in response to atherosclerosis.8,9 Previous studies investigating the role of NADPH oxidase in the development of atherosclerosis have produced mixed results with initial studies of global Nox210 and p47Phox knockout mice11 showing no difference in atherosclerosis in the aortic sinus. More recent studies have shown that global knockout of p47phox results in a moderate decrease in aortic atherosclerosis in both chow and high-fat fed animals,12 and that global Nox2 deletion results in a significant decrease in aortic atherosclerotic burden.9 However, while all studies to date have investigated whether a decrease in superoxide production altered the progression of atherosclerosis, very few have assessed if increasing superoxide production specifically in the endothelial cell layer alters the progression of atherosclerosis.
Thus far, very few studies have addressed the relative importance of the cellular source(s) of ROS in the pathology of vascular diseases. Although total vascular superoxide production is associated with vascular disease risk factors and endothelial dysfunction,13,14 it is possible that superoxide production in specific cell types might have particular importance. For example, the up-regulation of endothelial Nox2 expression and NADPH oxidase-derived ROS reported in ApoE−/− mice might more likely favour endothelial dysfunction and atherosclerosis.9 Although some studies have already addressed the potential role of NADPH oxidase-dependent ROS production in VSMCs and inflammatory cells, it remains unclear whether increasing superoxide production specifically in the endothelium alone is sufficient to alter the initiation and progression of atherosclerosis. To address this question, we determined how endothelial cell-specific Nox2 overexpression would alter total vascular superoxide production in ApoE−/− mice, and the effects of endothelial cell-specific Nox2 overexpression on the initiation and progression of atherosclerosis.
2. Methods
2.1. Animals
Endothelial-targeted Nox2 transgenic mice (Nox2-Tg), overexpressing human Nox2 under the control of the Tie2 promoter/enhancer,15 were crossed with ApoE−/− mice (Jackson laboratories, USA) to generate matched litters of Nox2-Tg ApoE−/− and ApoE−/− mice. Animals were housed in individually ventilated cages with 12 h light/dark cycle and controlled temperature (20–22°C). Standard chow (B&K Ltd, UK) and water were available ab libitum. Genotyping of experimental mice was performed by standard PCR techniques to confirm the presence or absence of the ApoE gene and human Nox2 transgene. Tissue for primary cell cultures and assays was isolated from mice after culling with an overdose of anaesthetic (isoflurane >20%) and confirmation of death by either cervical dislocation or exsanguination. All animal procedures were approved and carried out in accordance with the University of Oxford Ethics Committee and the UK Home Office Animals (Scientific Procedures) Act 1986. All procedures conformed with the Directive 2010/63/EU of the European Parliament.
2.2. Quantitative real-time RT–PCR
Primary cells and whole thoracic aortas were obtained from 16-week-old Nox2-Tg ApoE−/− mice and their ApoE−/− littermates. Primary endothelial cells were isolated from lungs by immunoselection with CD31 antibody (BD Biosciences, UK)-coated magnetic Dynabeads (Invitrogen, UK) as described previously.15 VSMCs were obtained from the thoracic aorta by established culture techniques.16 Resident peritoneal macrophages were obtained after peritoneal lavage and plastic selection. Total RNA was obtained by Trizol extraction. Reverse transcription was carried out using the QuantiTect reverse transcription kit (Qiagen, UK) on 1 μg total cell RNA. Quantitative real-time RT–PCR was performed with an iCycler IQ real-time detection system (BioRad Laboratories, USA) using primers and probes from the TaqMan Gene Expression Assay system (Applied Biosystems, UK).
2.3. Western blotting
Western blotting was carried out on lung homogenates (15 µg protein) using standard techniques and anti-Nox2, (BD Transduction Laboratories, USA) and GAPDH (Chemicon, USA) antibodies.
2.4. Quantification of superoxide production
Superoxide production from thoracic aortas of 24-week-old mice was measured by quantifying the accumulation of 2-hydroxyethidium by HPLC as previously described.17 Briefly, aortas were incubated at 37°C in Krebs-HEPES buffer containing dihydroethidium (25 µM; DHE) and NADPH (300 µmol/L) for 30 min. Separation of DHE, 2-hydroxyethidium, and ethidium was performed using a gradient HPLC system (Jasco, UK) with an ODS3 reverse phase column (250, 4.5 mm; Hichrom, UK), and quantified using a fluorescence detector set at 510 nm (excitation) and 595 nm (emission).
Superoxide production in the aortic root of 9-week-old mice was assessed using DHE in frozen aortic root sections (10 µm) incubated with DHE (2 µmol/L; Molecular Probes) at 37°C for 20 min. 2-hydroxyethidum production was assessed on a Zeiss scanning confocal microscope with a ×63 oil immersion objective. Staining was quantified in images throughout the aortic root and specifically in the endothelium by quantifying fluorescence on the luminal side of the internal elastic lamina using Image Pro Plus (MediaCybernetics, USA).
Superoxide generation was also measured by lucigenin-enhanced chemiluminescence, as previously described.14,18 Chemiluminescence of freshly harvested intact aortas (9–18 weeks) was measured in a FB12 luminometer (Berthold Detection Systems, Germany) at 37°C under basal conditions and after addition of NADPH (300 μmol/L), followed by the NADPH oxidase flavoprotein inhibitor diphenyleneiodonium (DPI; 10 μmol/L).
2.5. Blood pressure and pulse measurements in conscious mice
The heart rate and the systolic blood pressure were measured using an automated computerized tail-cuff system in conscious mice following five consecutive training periods (Visitech BP2000, Visitech Systems, Inc., USA). All recordings were performed between 0800 and 1200 h, on 9- and 20–24-week-old mice. To ensure accuracy, 20 preliminary recordings were taken followed by 15 actual recordings for each animal. The mean of these 15 recordings was used for blood pressure and heart rate analysis.
2.6. Lipid and lipoprotein analysis
Biochemical analyses of plasma lipids were performed on heparinized blood plasma using an ABX Pentra Clinical Chemistry bench-top analyser (Horiba ABX, France). Oxidized LDL (OxLDL) was measured in heparinized blood plasma using a murine OxLDL sandwich ELISA (USCN, China).
2.7. Histology and immunohistochemistry of atherosclerotic aortic root sections
Lesion size was assessed in paraffin-embedded aortic root sections stained with Masson-Goldner trichrome (Merck, Germany). The average lesion size was calculated from three sections taken at 100 µm intervals starting from the section showing all three aortic cusps. The infiltration of macrophages into aortic lesions was analysed using anti-Mac-3 (BD Pharmingen, UK) immunostaining. Plaque smooth muscle cell content was assessed using α-smooth muscle actin (Sigma-Aldrich, UK) immunostaining. Apoptosis was assessed using the ApopTag Peroxidase In Situ Apoptosis Detection kit (Millipore, USA). The collagen content of atherosclerosic plaques was analysed using Masson-Goldner stained sections (Merck, Germany). Vascular cell adhesion molecule 1 (VCAM-1) and CD68 expression in aortic roots was assessed in acetone-fixed frozen sections using anti-VCAM-1 (Southern Biotech, UK) and anti-CD68 (AbD Serotec, UK). Aortic lipid deposition was assessed in fixed aortas stained with Oil red O. The lesion area, and Mac-3, CD68, and VCAM-1 positive areas were quantified from digitized microscopic images using Image-Pro Plus.
2.8. In vivo angiotensin II infusion
ApoE−/− and Nox2-Tg ApoE−/− mice (20 weeks old) were anaesthetized by inhalation of 2% isoflurane, 95% oxygen. The depth of anaesthesia was continually monitored by assessing reflexes, respiration, and heart rate. Osmotic minipumps (Alza Corp, USA) containing AngII (0.4 or 0.8 mg/kg/day for 4 weeks) were implanted subcutaneously. Mice were recovered and maintained for 4 weeks prior to sacrifice.
2.9. Statistical analysis
Data are presented as mean ± SEM. Groups were compared using the Mann–Whitney U test for non-parametric data or Student's t-test for parametric data. When comparing multiple groups, data were analysed by analysis of variance (ANOVA) with the Newman–Keuls post hoc test for parametric data or the Kruskal–Wallis test with the Dunns post hoc test for non-parametric data. A value of P< 0.05 was considered statistically significant.
3. Results
3.1. Expression of endothelial-specific NADPH oxidases in Nox2-Tg ApoE−/− mice
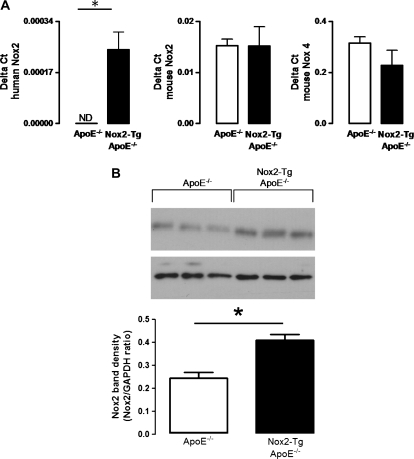
We first aimed to assess whether endothelial expression of human Nox2 altered the expression of the endogenously expressed endothelial-specific NADPH oxidases. There was no difference in expression of either mouse Nox2 or mouse Nox4 in aortas from Nox2-Tg ApoE−/− mice compared with their ApoE−/− littermates. As expected, expression of the human Nox2 transgene was observed only in aortas from Nox2-Tg ApoE−/− mice (Figure 1A). Western blot analysis indicated that endothelial-specific expression of human Nox2 resulted in an increase in total Nox2 protein expression (Figure 1B).
Figure 1.
Characterization of NADPH oxidase expression in the Nox2-Tg ApoE−/− mouse. (A) mRNA expression of human Nox2, mouse Nox2 and mouse Nox4 in whole aortas from Nox2-Tg ApoE−/− and ApoE−/− mice. Data were quantified using the Delta ct method and GAPDH used as the house-keeping gene. (B) Western blot of global Nox2 in lung homogenates from Nox2-Tg ApoE−/− and ApoE−/− mice. Data are expressed as the mean ± SEM of n= 3–6 mice per group. *P< 0.05; ND, not detectable.
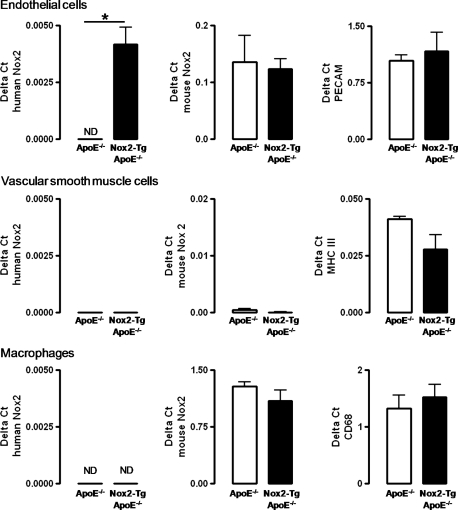
As NADPH oxidase activity in inflammatory cells and VSMCs has been shown to play a role in the progression of atherosclerosis, we aimed to confirm the cell specificity of transgenic human Nox2 expression in Nox2-Tg ApoE−/− mice. Importantly, transgenic human Nox2 was expressed exclusively in endothelial cells from Nox2-Tg ApoE−/− mice and was not detectable in macrophages or VSMCs from these animals, nor in any cell types from ApoE−/− mice, indicating that expression of the transgene was restricted to the endothelial cell layer (Figure 2).
Figure 2.
Characterization of NADPH oxidase expression in primary vascular cells from the Nox2-Tg ApoE−/− mouse. Human Nox2, mouse Nox2 and cell-specific markers in primary endothelial cells, VSMCs and primary macrophages from Nox2-Tg ApoE−/− and ApoE−/− mice. Tissue-specific markers were PECAM for primary endothelial cells, myosin heavy chain (MHC) III for primary VSMCs, and CD68 for resident macrophages. Data were quantified using the Delta ct method, and GAPDH used as the house-keeping gene. Data are expressed as the mean ± SEM of n= 3–6 mice per group. *P< 0.05. ND, not detectable.
3.2. Determination of vascular superoxide production in Nox2-Tg ApoE−/− mice
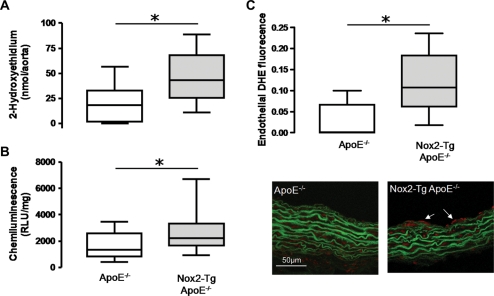
Previous studies from our group have established that on a wild-type background, expression of human Nox2 transgene results in an endothelial-specific increase in total vascular superoxide production. To confirm that total vascular superoxide production was still increased in Nox2-Tg mice on a hyperlipidaemic ApoE−/− background, we measured vascular superoxide production using 2-hydroxyethidium quantification and lucigenin-enhanced chemiluminescence. In thoracic aortas both methods of measuring NADPH-stimulated superoxide production (2-hydroxyethidium accumulation and lucigenin-enhanced chemiluminescence; Figure 3A and B) showed that superoxide production was significantly increased in the aortas from Nox2-Tg ApoE−/− mice compared with their ApoE−/− littermates (ApoE−/−, 19.9 ± 6.3 vs. Nox2-Tg ApoE−/−, 47.0 ± 7.0 nmol 2-hydroxyethidium/aorta, P< 0.05). Superoxide production was inhibited by >90% by diphenylene iodonium (10 µM; data not shown).
Figure 3.
Quantification of aortic superoxide production in Nox2-Tg ApoE−/− and ApoE−/− mice. Quantification of NADPH-dependent thoracic aortic superoxide production by (A) 2-hydroxyethidium fluorescence HPLC showed that superoxide production was significantly increased in aortas from 24-week-old Nox2-Tg ApoE−/− mice vs. ApoE−/− mice (*P< 0.05, n= 9–13). Data are presented as box plots of the tiron-inhibitable fraction of 2-hydroxyethidium production. (B) Lucigenin-enhanced chemiluminescence showed that superoxide production was significantly increased in aortas from 9 to 18-week-old Nox2-Tg ApoE−/− mice vs. ApoE−/− mice (*P< 0.05, n= 13–17). (C) 2-Hydroxyethidium fluorescence in frozen sections of the aortic root from 9-week-old Nox2-Tg ApoE−/− mice vs. ApoE−/− mice demonstrated that endothelial superoxide production was significantly increased in aortic roots from 9-week-old Nox2-Tg ApoE−/− mice vs. ApoE−/− mice (*P< 0.05, n= 8–5). Data are presented as box plots. Area of 2-hydroxyethidium fluorescence in frozen sections was normalized to vessel length.
To confirm that the increase in total aortic superoxide production was due to increased endothelial superoxide production, we assessed endothelial superoxide production by DHE fluorescence in frozen aortic root sections. Nox2-Tg ApoE−/− mice had significantly greater endothelial superoxide production compared with ApoE−/− littermates (9-week-old mice, P< 0.05; Figure 3C). As expected, due to the expression profile of the transgene, no difference in medial superoxide production was observed between the two groups (data not shown).
3.3. Effects of endothelial Nox2 overexpression on endothelial cell activation and macrophage recruitment in ApoE−/− mice
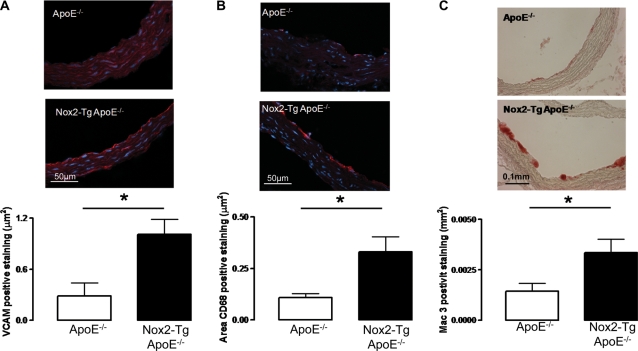
We next aimed to assess the effect of increased Nox2-derived endothelial superoxide production on endothelial cell activation. VCAM-1 expression is known to be a marker of endothelial cell activation in ApoE−/− mice.19 VCAM-1 levels were significantly increased by greater than three-fold in aortic roots from 9-week-old Nox2-Tg ApoE−/− mice compared with ApoE−/− (1.00 ± 0.178 vs. 0.288 ± 0.149 mm2, n= 5, P< 0.05; Figure 4A).
Figure 4.
Quantification of aortic root VCAM-1 and macrophage levels in Nox2-Tg ApoE−/− and ApoE−/− mice at 9 weeks of age. (A) Aortic root VCAM-1 staining (VCAM-1 positive cells stain red) was significantly greater in aortic roots from Nox2-Tg ApoE−/− mice compared with ApoE−/− littermates (P< 0.05, n= 5). (B) Aortic root macrophage content was quantified by immunohistochemical staining in frozen sections for CD68 (macrophages stained red); Nox2-Tg ApoE−/− mice had significantly greater macrophage content compared with ApoE−/− littermates (P< 0.05, n= 5–8). (C) Aortic root macrophage content was quantified by immunohistochemical staining in paraffin sections for Mac-3 (macrophages stained red); Nox2-Tg ApoE−/− mice had significantly greater macrophage recruitment compared with ApoE−/− littermates (P< 0.05, n= 5–8). For quantification of VCAM-1 and CD68 staining, the area of positive staining was normalized to vessel length.
Previous studies have shown that at 9 weeks of age, ApoE−/− mice on a chow diet start to show the development of fatty streaks in the aortic roots driven by monocyte recruitment and retention in the sub-endothelial layer.20 We next sought to determine whether the observed increase in VCAM-1 levels observed in Nox2-Tg ApoE−/− mice altered macrophage recruitment to the aortic root using the macrophage marker CD68. Aortic roots from 9-week-old Nox2-Tg ApoE−/− mice had significantly greater CD68 immunostaining compared with ApoE−/− littermates (0.332 ± 0.073 vs. 0.108 ± 0.018 mm2, n= 5–8, P< 0.05; Figure 4B). This finding was confirmed using a second macrophage stain (Mac-3) in paraffin sections; Mac-3 staining was significantly greater in aortic roots from 9-week-old Nox2-Tg ApoE−/− mice vs. ApoE−/− mice (Figure 4C). Macrophage staining was localized to the luminal surface of the aortic root in fatty streaks; very few macrophages were observed in the medial cell layer. As expected, these early fatty streaks stained almost exclusively positive for macrophages. These findings indicate that endothelial cell activation and the recruitment of macrophages to fatty streaks are increased by endothelial-specific Nox2 overexpression. To eliminate the possibility of apoptotic changes due to increased levels of endothelial ROS, we performed TUNEL staining in the aortic roots of 9- and 24-week-old mice. No difference was observed between the two groups in either endothelial cell or plaque apoptosis (Supplementary material online, Figure S1).
To ensure that the difference in endothelial cell activation and macrophage recruitment was not due to any difference in lipid levels, we assessed lipid levels in the plasma of 9- and 24-week-old mice. No difference in plasma OxLDL was observed between the two groups, indicating that increasing endothelial superoxide did not alter the circulating levels of OxLDL. In addition, there was no difference in total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides between Nox2-Tg ApoE−/− and ApoE−/− littermates. As observed on a wild-type background, endothelial-specific overexpression of Nox2 on a hyperlipademic ApoE−/− background did not alter body weight, basal systolic blood pressure, or pulse rate at either 9 or 24 weeks of age (Supplementary material online, Table S1).
3.4. Effects of endothelial Nox2 overexpression on atherosclerotic lesion formation in ApoE−/− mice
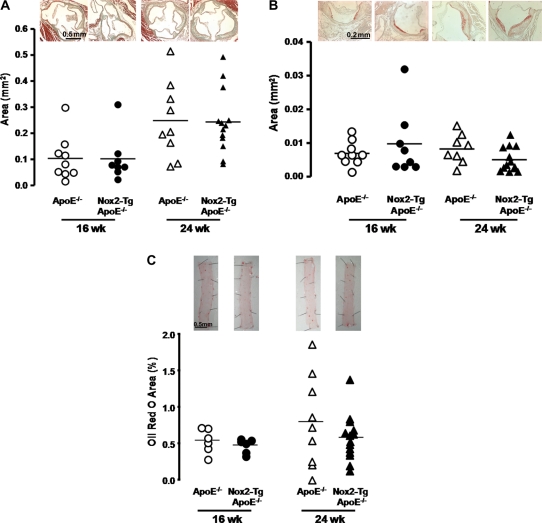
To determine how the observed increases in endothelial cell activation and macrophage recruitment altered progression of atherosclerosis, aortic root plaque formation was quantified in Nox2-Tg ApoE−/− mice and ApoE−/− littermates at 16 and 24 weeks of age. A significant time-dependent increase in aortic root atherosclerosis was observed from 16 to 24 weeks of age (Figure 5A, P< 0.05). However, there was no difference in either atherosclerotic plaque area or in plaque progression between Nox2-Tg ApoE−/− and ApoE−/− mice (Figure 5A). Immunostaining for Mac-3 revealed that the macrophage content in these advanced lesions was similar between the two groups (P< 0.05; Figure 5B). In addition, there was no difference in the composition of the plaque between the two groups, with both collagen content and plaque smooth muscle cell content similar between groups as assessed by immunohistochemistry (Supplementary material online, Table S2).
Figure 5.
Quantification of aortic root and aortic atherosclerosis in Nox2-Tg ApoE−/− and ApoE−/− mice at 16 and 24 weeks of age. (A) Aortic root plaque area was quantified using Masson–Goldner staining of aortic root sections. No difference in plaque area was observed between Nox2-Tg ApoE−/− and ApoE−/− mice at 16 or 24 weeks of age (P> 0.05). (B) Aortic root macrophage content was quantified by immunohistochemical staining for Mac-3 (macrophages stained red). No difference in macrophage content was observed between Nox2-Tg ApoE−/− mice and there ApoE−/− littermates (P> 0.05). (C) Aortic atherosclerosis was assessed by en face analysis of Oil red O staining in the aorta of Nox2-Tg ApoE−/− mice and their ApoE−/− littermates. No difference in aortic atherosclerosis was observed between Nox2-Tg ApoE−/− mice and ApoE−/− mice (P> 0.05). A time-dependent increase in aortic root plaque area, macrophage content, and aortic plaque area was observed in both groups (P< 0.05). Each point is representative of one animal.
To determine whether endothelial-specific Nox2 overexpression altered lipid deposition in the descending aorta, excised aortas were stained with Oil red O (Figure 5C). As observed in the aortic root, a time-dependent increase in lipid deposition was observed in both groups (P< 0.05).
However, there was no difference in lipid deposition in the descending aorta between Nox2-Tg ApoE−/− mice and ApoE−/− littermates (P> 0.05).
3.5. Effect of angiotensin II on blood pressure and atherosclerotic plaque progression
Recent studies have indicated that Nox2 is important for AngII signalling. AngII treatment results in hypertension and accelerated plaque progression in ApoE−/− mice, and hence we sought to determine whether endothelial-specific expression of human Nox2 would alter these responses to AngII. AngII treatment (0.4 or 0.8 mg/kg/day), delivered for 4 weeks by osmotic minipumps, resulted in a significant increase in systolic blood pressure in both Nox2-Tg ApoE−/− mice and ApoE−/− littermates (Nox2-Tg ApoE−/− 107.6 ± 2.6 vs. 130.2 ± 10.5 mmHg, ApoE−/− 101.2 ± 3.3 vs. 136.8 ± 9.3 mmHg, P< 0.001 for both; Supplementary material online, Table S1). However, there was no difference between Nox2-Tg ApoE−/− and ApoE−/− mice in either absolute blood pressure or the rate of increase in blood pressure after AngII treatment (P= 0.745, Supplementary material online, Table S1).
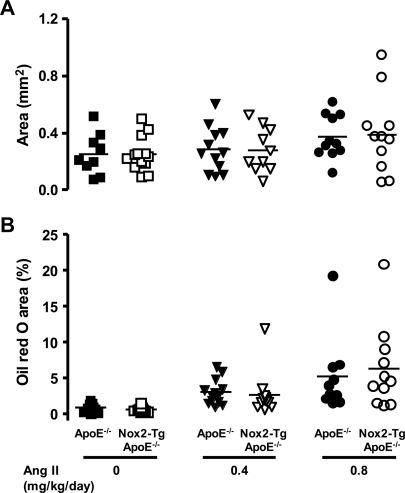
To test the effects of AngII on atherosclerosis, we treated mice with 0.4 or 0.8 mg/kg/day AngII for 4 weeks. There was a dose-dependent increase in atherosclerosis in both the aortic root and the thoracic descending aorta (Figure 6, P< 0.05). However, progression of AngII-induced atherosclerosis was not different between Nox2-Tg ApoE−/− and ApoE−/− mice (P< 0.05). In addition there was no difference in the composition of the plaques between the two groups, with macrophage, collagen, and smooth muscle cell content being similar (Supplementary material online, Table S2).
Figure 6.
AngII-induced atherosclerosis in the aortic root and descending aorta of Nox2-Tg ApoE−/− and ApoE−/− mice. Atherosclerosis was assessed in the aortic root and descending aorta of Nox2-Tg ApoE−/− and ApoE−/− littermates at 24 weeks of age after 4 weeks of treatment with either 0.4 or 0.8 mg/kg/day AngII or no treatment. (A) Aortic root plaque area was quantified in Masson-Goldner stained sections. No difference in plaque size was observed between Nox2-Tg ApoE−/− and ApoE−/− mice (P> 0.05). (B) Aortic atherosclerosis was assessed by en face analysis of Oil red O staining. No difference in aortic atherosclerosis was observed between Nox2-Tg ApoE−/− and ApoE−/− mice (P> 0.05). A significant dose-dependent increase in aortic root plaque area and aortic atherosclerosis was observed in both groups (P< 0.05). Each point is representative of one animal.
4. Discussion
In this study, we have investigated the effect of increased endothelial superoxide production on vascular superoxide production and atherosclerotic plaque progression in ApoE−/− mice via targeted Nox2 overexpression in the endothelium. Our key findings are that increased endothelial superoxide production via endothelial cell-specific Nox2 overexpression significantly increases vascular superoxide production. Increased endothelial superoxide production resulted in increased VCAM-1 levels and enhanced macrophage recruitment at 9 weeks of age. However, this initial increase in macrophage recruitment did not result in altered plaque progression at 16 or 24 weeks of age. Nor did overexpression of Nox-2 alter AngII-driven atherosclerosis.
Vascular disease states, including atherosclerosis, are associated with a net increase in vascular superoxide, to which NADPH oxidases in general, and Nox2-containing NADPH oxidase in particular, are important contributors.13,21 However, it remains unclear which cellular sources of vascular superoxide are most important in the pathogenesis of atherosclerosis. In this study, we show that an endothelial-specific increase in Nox2-derived superoxide production is sufficient to alter macrophage recruitment and endothelial cell activation, key factors in the initiation of atherosclerosis in ApoE−/− mice. Previous studies have shown that, in ApoE−/− mice, fatty streaks consisting of lipid-laden macrophages start to appear from 9 weeks of age. Fatty streaks are initially observed to form a single row in the sub-endothelial space, later growing in size as the macrophages aggregate into large groups.16 This supports the finding in our study where macrophage staining was predominantly observed in the sub-endothelial space as either a single line, or, in more advanced fatty streaks, in large groups. We hypothesized that the increased macrophage recruitment observed in Nox2-Tg ApoE−/− mice was driven by endothelial cell activation. Previous studies have shown that VCAM-1 plays a major role in the initiation of atherosclerosis.19 In addition, Ang II treatment has previously been shown to increase VCAM-1 expression in endothelial cells.22 Our data confirm this finding, indicating that increased endothelial Nox2-derived superoxide production results in increased VCAM-1 expression, which is likely to bring about the observed increase in monocyte recruitment.
This novel finding is important because Nox2 has been previously implicated in atherosclerosis in both humans and animal models. Both Nox2 and Nox4 have been shown to be localized within the plaque of diseased human coronary arteries,21 and we have previously demonstrated that in diseased human coronary arteries mRNA expression of Nox2 correlated with NADPH oxidase activity.8 A direct relationship between Nox2 and atherosclerosis has also been observed in mice. ApoE−/− mice have increased expression of aortic Nox2 compared with WT mice, and ApoE−/− mice lacking Nox2 in all cells have reduced aortic atherosclerosis compared with ApoE−/− mice.9 Although a role for Nox2 in atherosclerosis has been previous shown, no study to date has investigated the relative importance of endothelial superoxide production in atherosclerosis. Our results indicate that endothelial Nox2 plays a role in the recruitment of macrophages to fatty streaks indicating a role for endothelial Nox2 in the initiation of atherosclerosis. However, our findings also indicate that once atherosclerotic plaque has been initiated, increased endothelial superoxide has a limited role in plaque progression. This is interesting as increased vascular superoxide in Nox2-Tg ApoE−/− mice was still observed at 24 weeks of age, indicating that the lack of effect was not due to an overwhelming level of vascular superoxide production from alternative sources, which might have masked the elevated superoxide production from the overexpression of Nox2. In our study, we have shown that an increase in endothelial-specific Nox2-derived superoxide production over and above the levels observed in an ApoE−/− mouse does not increase the progression of atherosclerosis. However, we cannot rule out the possibility of a threshold effect and that the capacity of endothelial-specific Nox2-derived superoxide production to accelerate disease is already saturated. Alternatively, it could be that although Nox2 is up-regulated in atherosclerosis9 and we have shown that increased endothelial Nox2-derived superoxide results in increased macrophage recruitment, Nox2-derived endothelial superoxide may not be critical in the development of atherosclerosis. Alternative endothelial sources of ROS such as uncoupled eNOS, lipid peroxides, or an alternative NADPH oxidase-derived source of ROS such as Nox4 may play a greater role. The role of Nox4 in the progression of atherosclerosis has yet to be fully elucidated. Nox4 has a greater relative abundance in the vasculature compared with Nox2.4 Unlike Nox2, Nox4 is now considered to be a hydrogen peroxide, not a superoxide-producing enzyme,23 and studies in ApoE−/− mice have shown a role for hydrogen peroxide in the pathology of atherosclerosis.24 Future studies would be required to fully elucidate the role of Nox4 in atherosclerosis.
Endothelial cells express both Nox2 and Nox4,3,4 and recent studies have indicated that Nox2 rather than Nox4 is more important for AngII-mediated signalling.25 Indeed, previous studies from our group have shown that Nox2-Tg mice on a wild-type background have an increased pressor response to Ang II compared with wild-type littermates.15 Hence, endothelial-specific Nox2-derived superoxide may have a greater role in AngII-driven vascular disease responses. It is well established that AngII treatment results in an enhanced vascular inflammation phenotype, resulting in accelerated atherosclerosis.26 In our study, treatment with AngII from 20 to 24 weeks of age significantly increased systolic blood pressure and resulted in a dose-dependent increase in atherosclerosis, demonstrating that AngII had the expected pathophysiological effects. However, endothelial-specific Nox2 overexpression did not alter these atherogenic responses with no changes in plaque size observed. This could indicate that Nox2-derived superoxide production in the endothelial cell layer does not drive accelerated AngII-mediated atherosclerosis. However, it could be that the relative contribution of endothelial-specific AngII-mediated superoxide production in these more advanced inflammatory plaques is minimal compared with the contribution from alternative cell types such as macrophages and VSMCs. This would confirm our previous findings that endothelial Nox2 was important in the early stages of atheroslcerotic plaque development. In this study, we have used Ang II treatment to accelerate atherosclerosis, as opposed to high-fat feeding. Both Ang II treatment and high-fat feeding lead to the formation of more advanced lesions; however, unlike high-fat feeding where lesion growth is stimulated by increased lipid levels, in the angiotensin II model plaque growth is stimulated by inflammatory cell influx. As we observed a phenotype in the early inflammatory cell recruitment phase, we chose to use the Ang II model in this study. We elected to use the ApoE−/− mouse as a model of atherosclerosis that develops atherosclerosis on a chow diet. However, atherosclerosis in the ApoE−/− is driven not only by the lipid environment but also by altered macrophage function which must also be considered in all studies of this type.
Compensatory changes in the expression of native Nox2 or another NADPH oxidase subtype might be diminishing the effects of increased endothelial Nox2-derived superoxide production on atherosclerosis plaque progression. Indeed, previous studies in human pulmonary endothelial cells have shown that knock-down of Nox2 using siRNA resulted in a compensatory increase in Nox4 mRNA expression.27 However, our study found no evidence for a compensatory change in the expression of either native Nox2 or Nox4 in whole aortas. Furthermore, there was no indication of ‘leaky’ expression of the transgene, since expression of human Nox2 was restricted to endothelial cells from Nox2-Tg ApoE−/− mice and was not observed in either VSMCs or macrophages from these animals. However, as demonstrated in our laboratory and by others, Nox4 is more abundantly expressed in the vasculature compared with Nox2, and thus Nox4-derived superoxide production may play a more important role in the progression of atherosclerosis.
In conclusion, we have shown that increasing Nox2-derived superoxide production specifically in endothelial cells in the ApoE−/− mouse is sufficient to increase vascular superoxide generation, and results in markedly increased endothelial cell activation and enhanced macrophage recruitment. However, this increase in recruitment of macrophages to fatty streaks within the vasculature did not result in increased plaque progression. These results indicate that Nox-mediated ROS signalling has important cell-specific and distinct temporal roles in the initiation and progression of atherosclerosis and add to our understanding of the specific role of endothelial-derived superoxide in these processes.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the British Heart Foundation (PG/05/141/20098., RG/07/003/23133). Funding to pay the Open Access publication charges for this article was provided by the British Heart Foundation.
Supplementary Material
Acknowledgements
We would like to thank Ms Lynn Nicholls for her kind help in the analysis of plaque apoptosis in this study.
Conflict of interest: None declared.
References
- 1.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 2.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, et al. NADPH Oxidases in Cardiovascular Health and Disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 3.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 5.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, et al. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 6.Ellmark SHM, Dusting GJ, Ng Tang Fui M, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, et al. Coronary artery superoxide production and Nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 9.Judkins CP, Diep H, Broughton BRS, Mast AE, Hooker EU, Miller AA, et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 10.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2000;20:1529–1535. doi: 10.1161/01.atv.20.6.1529. [DOI] [PubMed] [Google Scholar]
- 11.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, et al. Vascular effects following homozygous disruption of p47(phox) : an essential component of NADPH oxidase. Circulation. 2000;101:1234–1236. doi: 10.1161/01.cir.101.11.1234. [DOI] [PubMed] [Google Scholar]
- 12.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, et al. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzik TJ, West NEJ, Black E, McDonald D, Ratnatunga C, Pillai R, et al. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:e85–e90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 14.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 15.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 16.Coleman R, Hayek T, Keidar S, Aviram M. A mouse model for human atherosclerosis: long-term histopathological study of lesion development in the aortic arch of apolipoprotein E-deficient (E0) mice. Acta Histochemica. 2006;108:415–424. doi: 10.1016/j.acthis.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, et al. Quantitative regulation of intracellular endothelial nitric oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 18.Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, Rockett K, et al. Stoichiometric relationships between endothelial tetrahydrobiopterin, eNOS activity and eNOS coupling in vivo: Insights from transgenic mice with endothelial-targeted GTPCH and eNOS over-expression. Circ Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 19.Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, et al. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 21.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 22.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal J-F, Michel J-B. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-{kappa}B activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 23.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HHHW, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radical Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, et al. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 25.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and Nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 26.Daugherty A, Cassis L. Angiotensin II-mediated development of vascular diseases. Trends Cardiovasc Med. 2004;14:117–120. doi: 10.1016/j.tcm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JGN, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal. 2009;11:841–860. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.