Abstract
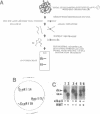
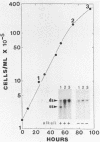
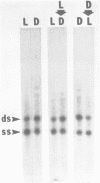
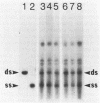
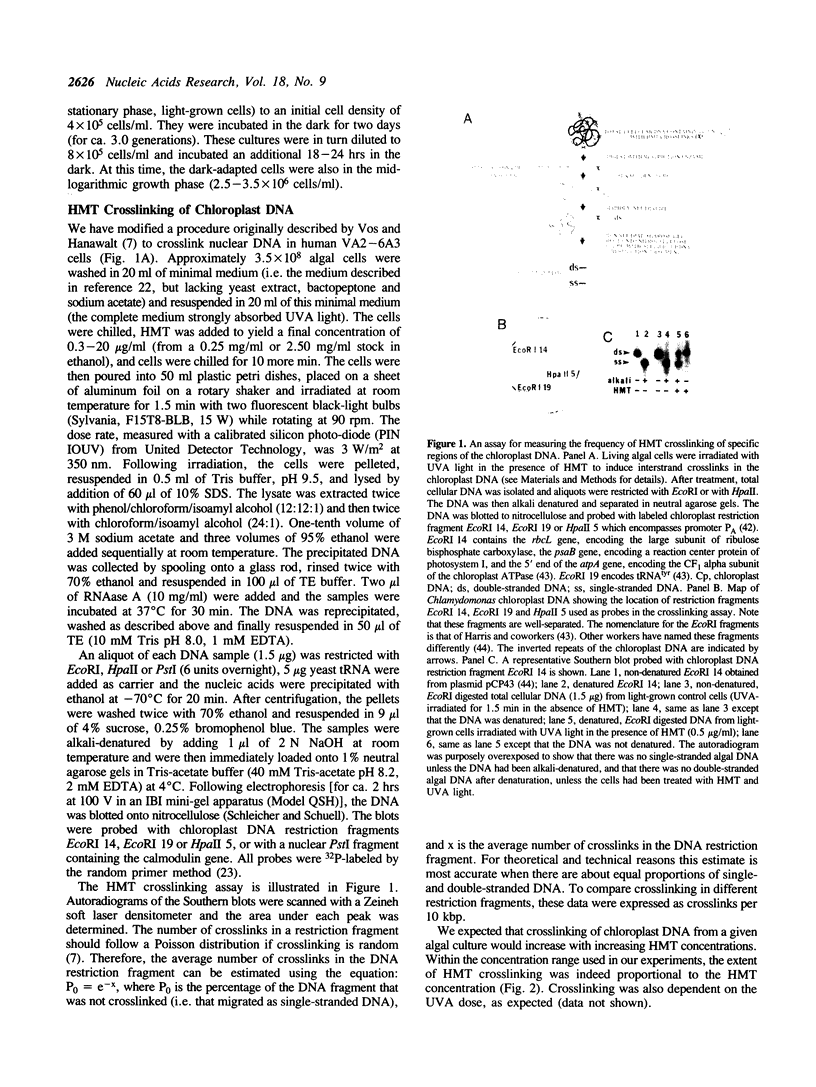
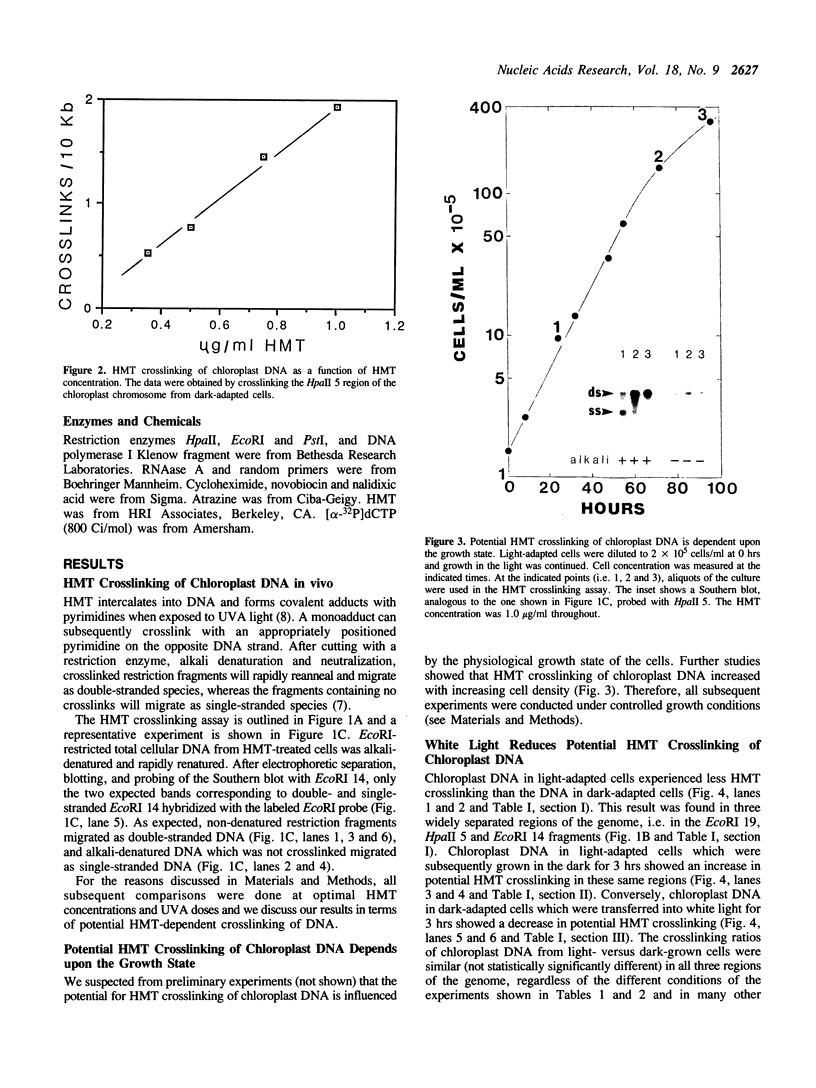
We have analyzed changes in the structure of chloroplast chromosomes in response to light in growing Chlamydomonas cells using a crosslinking assay based on the intercalation of HMT (4'-hydroxymethyl-4,5',8-trimethylpsoralen) into DNA. Our results show that the structure of chloroplast chromosomes in at least three widely separated regions is different in light-grown vs. dark-grown cells. Structural changes in chloroplast chromosomes occur within 3 hrs after exposure to light or darkness, respectively. The response to light is not inhibited by atrazine and can be elicited by dim blue light incapable of evolving O2, indicating that it does not require photosynthesis. Inhibition of cytoplasmic protein synthesis with cycloheximide prevents this response to light, indicating that it depends, at least in part, on proteins imported from the cytoplasm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke V. L., Gralla J. D. Changes in the linking number of supercoiled DNA accompany growth transitions in Escherichia coli. J Bacteriol. 1987 Oct;169(10):4499–4506. doi: 10.1128/jb.169.10.4499-4506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat J. F., Gigot C., Laulhere J. P., Mache R. Visualization of a Spinach Plastid Transcriptionally Active DNA-Protein Complex in a Highly Condensed Structure. Plant Physiol. 1982 May;69(5):1205–1211. doi: 10.1104/pp.69.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang K. S., Sueoka N. Replication of chloroplast DNA in Chlamydomonas reinhardi during vegetative cell cycle: its mode and regulation. Proc Natl Acad Sci U S A. 1967 May;57(5):1506–1513. doi: 10.1073/pnas.57.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Henderson N. C., Austin S. DNA supercoiling and aerobic regulation of transcription from the Klebsiella pneumoniae nifLA promoter. Nucleic Acids Res. 1988 Nov 11;16(21):9933–9946. doi: 10.1093/nar/16.21.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Barr G. C., Ni Bhriain N., Higgins C. F. DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol. 1988 Jun;170(6):2816–2826. doi: 10.1128/jb.170.6.2816-2826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Snyder M. Superhelical Escherichia coli DNA: relaxation by coumermycin. J Mol Biol. 1978 Apr 5;120(2):145–154. doi: 10.1016/0022-2836(78)90061-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Kochel T. J., Sinden R. R. Hyperreactivity of B-Z junctions to 4,5',8-trimethylpsoralen photobinding assayed by an exonuclease III/photoreversal mapping procedure. J Mol Biol. 1989 Jan 5;205(1):91–102. doi: 10.1016/0022-2836(89)90367-7. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. The molecular size and conformation of the chloroplast DNA from higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):372–390. doi: 10.1016/0005-2787(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983 Aug;34(1):105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Nagy F., Kay S. A., Chua N. H. Gene regulation by phytochrome. Trends Genet. 1988 Feb;4(2):37–42. doi: 10.1016/0168-9525(88)90064-9. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. DNA supercoiling and prokaryotic transcription. Cell. 1989 Feb 24;56(4):521–523. doi: 10.1016/0092-8674(89)90574-6. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Kuchka M., Mayfield S., Schirmer-Rahire M., Girard-Bascou J., Bennoun P. Nuclear and chloroplast mutations affect the synthesis or stability of the chloroplast psbC gene product in Chlamydomonas reinhardtii. EMBO J. 1989 Apr;8(4):1013–1021. doi: 10.1002/j.1460-2075.1989.tb03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction endonuclease map of the chloroplast DNA of Chlamydomonas reinhardii. J Mol Biol. 1978 Dec 25;126(4):597–617. doi: 10.1016/0022-2836(78)90011-6. [DOI] [PubMed] [Google Scholar]
- SAGER R., PALADE G. E. Structure and development of the chloroplast in Chlamydomonas. I. The normal green cell. J Biophys Biochem Cytol. 1957 May 25;3(3):463–488. doi: 10.1083/jcb.3.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. DNA binding by proteins. Science. 1988 Sep 2;241(4870):1182–1187. doi: 10.1126/science.2842864. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Thompson R. J., Davies J. P., Mosig G. ;Dark-Lethality' of Certain Chlamydomonas reinhardtii Strains Is Prevented by Dim Blue Light. Plant Physiol. 1985 Nov;79(3):903–907. doi: 10.1104/pp.79.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. J., Mosig G. An ATP-dependent supercoiling topoisomerase of Chlamydomonas reinhardtii affects accumulation of specific chloroplast transcripts. Nucleic Acids Res. 1985 Feb 11;13(3):873–891. doi: 10.1093/nar/13.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. J., Mosig G. Light and Genetic Determinants in the Control of Specific Chloroplast Transcripts in Chlamydomonas reinhardtii. Plant Physiol. 1984 Sep;76(1):1–6. doi: 10.1104/pp.76.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. J., Mosig G. Stimulation of a Chlamydomonas chloroplast promoter by novobiocin in situ and in E. coli implies regulation by torsional stress in the chloroplast DNA. Cell. 1987 Jan 30;48(2):281–287. doi: 10.1016/0092-8674(87)90431-4. [DOI] [PubMed] [Google Scholar]
- Tse-Dinh Y. C. Regulation of the Escherichia coli DNA topoisomerase I gene by DNA supercoiling. Nucleic Acids Res. 1985 Jul 11;13(13):4751–4763. doi: 10.1093/nar/13.13.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987 Aug 28;50(5):789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- Widmer R. M., Koller T., Sogo J. M. Analysis of the psoralen-crosslinking pattern in chromatin DNA by exonuclease digestion. Nucleic Acids Res. 1988 Jul 25;16(14B):7013–7024. doi: 10.1093/nar/16.14.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Droffner M. L. Mechanisms determining aerobic or anaerobic growth in the facultative anaerobe Salmonella typhimurium. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2077–2081. doi: 10.1073/pnas.82.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G. H., Cole R. S. Cross-linking and relaxation of supercoiled DNA by psoralen and light. Biochim Biophys Acta. 1978 Dec 21;521(2):529–546. doi: 10.1016/0005-2787(78)90295-2. [DOI] [PubMed] [Google Scholar]
- Zhen W. P., Jeppesen C., Nielsen P. E. Psoralen photofootprinting of protein-binding sites on DNA. FEBS Lett. 1988 Feb 29;229(1):73–76. doi: 10.1016/0014-5793(88)80800-7. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Hearst J. E. Transcription of oxygen-regulated photosynthetic genes requires DNA gyrase in Rhodobacter capsulatus. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4209–4213. doi: 10.1073/pnas.85.12.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer W. E., Schloss J. A., Silflow C. D., Youngblom J., Watterson D. M. Structural organization, DNA sequence, and expression of the calmodulin gene. J Biol Chem. 1988 Dec 25;263(36):19370–19383. [PubMed] [Google Scholar]