Abstract
Background
Overactivity and/or dysregulation of the endocannabinoid system (ECS) contribute to development of obesity. In vitro studies indicate a regulatory role for the cannabinoid receptor 1 (CB1) in adipocyte function and CB1-receptor deficient (CB1-/-) mice are resistant to high fat diet-induced obesity. Whether this phenotype of CB1-/- mice is related to altered fat metabolism in adipose tissue is unknown.
Methods
We evaluated adipose tissue differentiation/proliferation markers and quantified lipogenic and lipolytic activities in fat tissues of CB1-/- and CB1+/+ mice fed a high-fat (HF) or a high-fat/fish oil (HF/FO) diet as compared to animals receiving a low-fat chow diet. Comparison between HF diet and HF/FO diet allowed to investigate the influence of dietary fat quality on adipose tissue biology in relation to CB1 functioning.
Results
The adiposity-resistant phenotype of the CB1-/- mice was characterized by reduced fat mass and adipocyte size in HF and HF/FO-fed CB1-/- mice in parallel to a significant increase in energy expenditure as compared to CB1+/+ mice. The expression levels of adipocyte differentiation and proliferation markers were however maintained in these animals. Consistent with unaltered lipogenic gene expression, the fatty acid synthesis rates in adipose tissues from CB1-/- and CB1+/+ mice were unchanged. Whole-body and adipose-specific lipoprotein lipase (LPL) activities were also not altered in CB1-/- mice.
Conclusions
These findings indicate that protection against diet-induced adiposity in CB1-deficient mice is not related to changes in adipocyte function per se, but rather results from increased energy dissipation by oxidative and non-oxidative pathways.
Keywords: CB1-receptor, diet-induced adiposity, fat tissue, lipogenesis, lipolysis
Background
The endocannabinoid system (ECS) comprises the endogenous cannabinoids (endocannabinoids or ECs), the cannabinoid receptors and the enzymes involved in the synthesis and degradation of endocannabinoids [1]. The two most studied ECs anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) are amides and esters, respectively, of long-chain polyunsaturated fatty acids (PUFAs) [2]. To date, two G protein-coupled cannabinoid receptors have been identified. Because of its role in the central regulation of food intake and energy balance, the cannabinoid 1 (CB1)-receptor has emerged as an interesting drug target for treatment of obesity, dyslipidemia and insulin resistance. CB2-receptors, on the other hand, are mainly involved in immune function [3]. Administration of (endo)cannabinoids increases food intake, while CB1-receptor antagonism results in hypophagia [4]. Mice deficient for the cannabinoid 1 receptor gene (Cnr1, further announced as CB1-/- mice) are lean and have less fat stores compared to their wild type littermates [5]. This reduction in adiposity appears to be independent of food intake [5], suggesting that CB1 deficiency alters the balance between energy intake, utilization and storage.
CB1-receptors are localized in the brain, peripheral nerves and several peripheral organs [3]. Adipose tissue represents an important peripheral node of the ECS and several in vitro studies indicate that CB1-receptor activity is critical for adipocyte function [5-7]. CB1 expression has been reported to increase during adipocyte maturation [8] and ECs are required for adipocyte differentiation and growth [6,9,10]. ECS signalling in adipocytes supposedly promotes lipid storage [6,11-13]via the induction of lipogenic genes and by increasing fatty acid uptake [5,6]. Accordingly, CB1-receptor antagonists arrest adipocyte proliferation and reduce lipogenic gene expression [6,13]. Since growth retardation in CB1-/- mice is already apparent during the first weeks of life [5], appropriate CB1-receptor functioning may be critical for adipose tissue development in vivo.
Because EC receptor signalling impacts on energy balance, overactivity and/or dysregulation of the ECS have been proposed contribute to the development of obesity [7,11,14]. High-fat feeding induces obesity in rodents and has been reported to increase the EC tone in their adipose tissue [11,15] while CB1-deficient mice are resistant to high-fat diet-induced obesity [16]. Besides their effects on systemic energy expenditure, adipocyte-specific actions of the cannabinoid receptors potentially impact on adipose tissue physiology and thereby determine the degree of fat storage. Different types of dietary fats exert specific effects on body weight regulation [17] and n-3 PUFAs have been shown to decrease EC levels in adipocytes [18]. In vitro studies indicate that enhanced CB1-receptor signalling directs metabolism towards fat storage [6,11] while reducing fat oxidation [7,11,19]. In addition, it has been shown that peripheral CB1-receptor antagonism reduces the expression of lipogenic genes in mice fed a high-fat diet [20]. The protection against diet-induced obesity in CB1-deficient mice may therefore, at least in part, be related to changes in fat cell metabolism in these animals. Very few studies have however systematically evaluated the consequences of CB1 ablation for adipocyte biology in vivo. Furthermore, it is unknown whether CB1-receptor mediated changes in adipose tissue function contribute to high-fat diet induced obesity in rodents.
We therefore investigated whether resistance to diet-induced adiposity in CB1-deficient mice was related to functional changes in adipocyte biology. In order to evaluate the influence of dietary fat quality in relation to CB1 functioning in adipose tissue, we determined the expression levels of differentiation and proliferation markers, the rate of fatty acid synthesis and lipoprotein lipase (LPL) activity in epididymal fat pads of CB1+/+ and CB1-/- mice receiving a standard, low-fat chow diet, a high-fat (HF) or a HF diet in which part of the fat was replaced by fish oil (HF/FO diet). The results from this study indicate that obesity-resistance in CB1-deficient mice is not related to changes in adipocyte metabolism, but rather results from increased energy expenditure.
Methods
Animals and experimental design
Initial breeding pairs for the generation of cannabinoid 1 receptor gene (Cnr1, further announced as CB1) knockout mice were kindly provided by prof. dr. A. Zimmer, Laboratory of Molecular Neurobiology, Department of Psychiatry, University of Bonn, Germany. Male CB1+/+ and CB1-/- mice on a pure C57BL/6N background were bred within our own facility from heterozygous crossing. Animals were housed under light- and temperature-controlled conditions (lights on 4:00 AM-4:00 PM, 21°C) with free access to food and drinking water. From four weeks of age onwards, they were divided into groups and fed three different diets during six weeks. All diets were obtained from Abdiets, Woerden, The Netherlands. One group received normal laboratory chow (RMH-B), the second group received high-fat (HF; beef tallow) diet and the third group received a diet in which 42% (w/w) of the beef tallow was replaced by fish oil (HF/FO diet; menhaden oil). For diet composition see Additional File 1 Table S1. The HF/FO diet was renewed three times per week to prevent oxidation. Body weight and food intake were registered regularly. Separate cohorts of mice were generated to evaluate the effects on basal parameters, energy expenditure, lipogenic fluxes and lipolytic activities. Prior to all experiments, mice were subjected to a short postprandial fasting period of 3 hours (6-9 AM) with drinking water available to exclude acute postprandial effects without the induction of a fasting response. Experimental procedures were approved by the Ethics Committees for Animal Experiments of the University of Groningen.
Determination of body composition by whole-body carcass analysis
During the 5th week of dietary intervention, feces were collected over a 72-hour period. Fecal energy content was determined as previously described [21]. After six weeks of diet, animals were fasted (6-9 AM) and blood glucose concentrations were measured using a glucometer (Lifescan Benelux, Beerse, Belgium). Then, the mice were sacrificed by cardiac puncture under isoflurane anaesthesia and subcutaneous, epididymal, retroperitoneal and brown adipose fat depots were first collected and weighed to determine their mass. Intestine, organs, skin, and the remaining carcass including the head were separately collected to determine mesenteric, organ, non-removable subcutaneous and muscular fat mass, respectively. Therefore, these samples were dried until all moist was evaporated, after which the total (lean+fat) masses were determined. Lipids were subsequently extracted using Soxleth petroleum ether distillation. Then, the samples were again dried, after which the fat free masses were determined. The fat mass from the different samples was finally calculated by subtracting the lean mass from the total mass.
Fat cell histology, basal plasma metabolite and gene expression analysis
Epididymal adipose tissue was quickly removed, snap-frozen in liquid nitrogen and stored at -80°C. Part of the fat tissue was fixed in 4% paraformaldehyde in PBS and embedded in paraffin. For adipocyte histology, 3 μm paraffin sections were stained with hematoxylin and eosin and analyzed at 10× magnification. The area of 240-420 fat cells per group was quantified using image analysis software (Qwin, Leica, Wetzlar, Germany). Fat cell area data were analyzed using the percent relative cumulative frequency (PCRF) approach and EC50 values were calculated according to Riachi et al. [22]. Adipose tissue-derived LPL activity was determined as described under 'lipolytic activity'. Blood was centrifuged (4000 × g for 10 minutes at 4°C) and plasma was stored at -20°C. Plasma insulin, triglyceride, cholesterol and free fatty acids were quantified using commercially available kits (Mercodia, Uppsala, Sweden for insulin ELISA; Roche diagnostics, Mannheim, Germany for triglyceride and cholesterol; Wako Chemicals, Neuss, Germany for free fatty acids). Plasma leptin, resistin and adiponectin concentrations were determined by Luminex® Multiplex technology (Luminex Corporation, Austin, TX) using Multiplex Immunoassays (Millipore, Amsterdam, The Netherlands) [23]. RNA was extracted from adipose tissue using Tri reagent (Sigma-Aldrich, St. Louis, MO) and subsequently converted into cDNA by a reverse transcription procedure using M-MLV and random primers according to the manufacturer's protocol (Sigma-Aldrich). For quantitative PCR (qPCR), cDNA was amplified using the appropriate primers and probes. The sequences for all primers and probes are given in Additional File 1 Table S2, and those that have been published are deposited at RTprimerdB http://www.rtprimerdb.org/. All mRNA levels were calculated relative to the expression of cyclophilin and normalized for expression levels of chow-fed CB1+/+ mice.
Indirect calorimetry
Homecage-housed animals were placed in an indirect calorimeter chamber with free access to food and drinking water. During 24 hours, gas exchange measurements were performed using an eight-channel open flow system. Flow rates were measured and controlled by a mass flow controller. O2 and CO2 concentrations of dried inlet and outlet air from each chamber were measured at 10-minute intervals using a paramagnetic O2 detector and an infrared CO2 detector. Estimated energy expenditure in kcal/24 hours was calculated using the following equation, which was derived from [24,25]: (24 * (16.18*VO2*0.001) + (5.02*VCO2*0.001))/4.184, with VO2 and VCO2 expressed in mL/h.
Fat and carbohydrate oxidation in mg/h were calculated using the following equation, which was derived from [26]:
Fat oxidation: 38.461*VO2 - VCO2, with VO2 and VCO2 expressed in mol/h.
Carbohydrate oxidation: 94.017*VCO2 - 66.239*VO2, with VO2 and VCO2 expressed in mol/h.
Data were calculated per animal or normalized for total lean body mass, which was determined by carcass analysis. Total lean mass represents the actual dry lean mass, and was calculated by adding the lean masses from intestine, organs, skin and carcass. Raw calorimetry- and detailed substrate utilization data are presented in Additional File 1 Figure S1A-S1C and Additional File 1 Table S3, respectively.
Determination of de novo lipogenesis and chain elongation in adipose tissue
The assessment of lipogenic fluxes and chain elongation is derived from the enrichment of the adipose tissue acetyl-CoA precursor pool used for fatty acid synthesis using non-radioactive steady state isotope labelling. Lipogenic fluxes were calculated from the incorporation of 13C-labelled acetate into palmitate, stearate and oleate molecules within the adipose tissue [27,28]. In order to determine these parameters, animals received sodium [1-13C]-acetate (99 atom %, Isotec/Sigma-Aldrich) via the drinking water (2%) during the final 72 hours of the dietary period. After a postprandial fast, mice were sacrificed by cardiac puncture under isoflurane anaesthesia. Epididymal adipose tissue was quickly removed, snap-frozen in liquid nitrogen and stored at -80°C. Lipids were hydrolyzed in HCl/acetonitrile (1:22 v/v) for 45 minutes at 100°C. Fatty acids were extracted in hexane and derivatized for 15 minutes at room temperature using α-Br-2,3,4,5,6-pentafluorobenzyl (PFB)/acetonitrile/triethanolamine (1:6:2 v/v). Derivatization was stopped by adding HCl and fatty acid-PFB derivatives were extracted in hexane. The fatty acid-PFB mass isotopomer distributions were measured using an Agilent 5975 series GC/MSD (Agilent Technologies, Santa Clara, CA). Gas chromatography was performed using a ZB-1 column (Phenomenex, Torrance, CA). Mass spectrometry analysis was performed by electron capture negative ionization using methane as moderating gas.
The normalized mass isotopomer distributions measured by GC-MS (m0-mx) were corrected for natural abundance of 13C by multiple linear regression [29] to obtain the excess fractional distribution of mass isotopomers (M0-Mx) due to incorporation of [1-13C]-acetate. This distribution was used in mass isotopomer distribution analysis (MIDA) algorithms to calculate the acetyl-CoA precursor pool enrichment (pacetate), fractional palmitate synthesis rates (fC16:0) and the fraction of stearate and oleate generated by elongation of de novo synthesized palmitate (fC18:0/1(C16DNL)), or by elongation of pre-existing palmitate (fC18:0/1(C16PE)) as described [28].
Lipolytic activity
Following the postprandial fast, a baseline blood sample was drawn by retro-orbital bleeding under isoflurane anaesthesia into heparinized capillaries. Mice subsequently received an intra-orbital injection of heparin in saline (0.1 U/g body weight). After 10 minutes, a post-heparin blood sample was drawn by retro-orbital bleeding. Mice were sacrificed by cardiac puncture and blood was centrifuged (4000 × g for 10 minutes at 4°C). Total lipase activities were determined by incubating 10 μL of plasma with 200 μL of ultrasonified substrate containing 1 mL Triton X-100 (1%), 1 mL Tris.HCl (1 M), 2 mL of heat-inactivated human serum, 2 mL of fat-free BSA (10%), 42 mg triolein and 5 μL glycerol-tri-9,10(n)-[3H]- oleate (5 mCi/mL), with or without addition of 50 μL NaCl (5 M) to block LPL activity. After 30-min incubation at 37°C, lipolysis was stopped by adding 3.25 mL of heptane/methanol/chloroform (100:128:137, vol/vol/vol) and 1 mL of 0.1 M K2CO3. After centrifugation for 15 min at 3,600 rpm at room temperature, extracted hydrolyzed fatty acids were quantified by scintillation counting. Lipase activities were calculated according to the formula: [disintegrations per second (dps) sample - dps blank]/dps 200 μL LPL-substrate * factor, in which the factor = {2.45 (volume aqueous phase) * 2.85 (total added free fatty acids in micromoles)/[0.76 (extraction efficiency) * 0.5 (reaction time in hours) * 0.01 (plasma volume in mL)]}. Postheparin LPL activity was calculated by subtracting postheparin hepatic lipase activity (i.e., lipase activity inhibited by 1 M NaCl) from the total postheparin lipase activity.
Adipose tissue specific LPL activity was determined in biopsies collected after a postprandial fast. Tissue was first homogenized in 1 mL of a buffer containing sucrose (0.25 M), EDTA (1 mM), Tris.HCl (10 mM) and Deoxycholate (12 mM), pH 7.4 [30]. Tissue homogenates were centrifuged (20 min at 12000 × g for at 4°C) and the fraction between the upper fat layer and the bottom sediment was collected. Lipase activities were determined as described using 50 μl of tissue fraction. LPL activity was calculated by subtracting lipolytic activity determined in a final NaCl concentration of 0.83 M (non-LPL) from total lipolytic activity measured without NaCl. Data obtained in adipose tissue were normalized for protein concentration [30].
Statistics
All data are presented as mean values ± SEM. Statistical analysis was performed using SPSS for Windows software (SPSS 16.0, Chicago, IL, USA). All parameters were first tested for equality of variances by Levene's test. Then, full-factorial General Linear Model analysis was performed to test for overall changes caused by diet or genotype, or for interactions between diet and genotype. Data were therefore initially analysed with a large statistical power to analyse overall effects, using groups of n > 10. For the overall dietary effects, Bonferoni post-hoc analysis was applied to correct for multiple comparisons. Outcome of general linear model analysis is given in the legends. Independent Student t-tests were run to analyse differences between genotypes within diets, and between dietary groups within genotypes. In the few cases where data transformation did not result in equality of variance, non-parametric analysis was performed. The null hypothesis was rejected at the 0.05 level of probability. Fat cell areas were considered significantly different between groups if the 95%-confidence intervals of the EC50 values obtained by PCRF analysis did not overlap.
Results
The resistance against high-fat diet-induced adiposity in CB1-/- mice is associated with increased energy expenditure and reduced adipocyte size
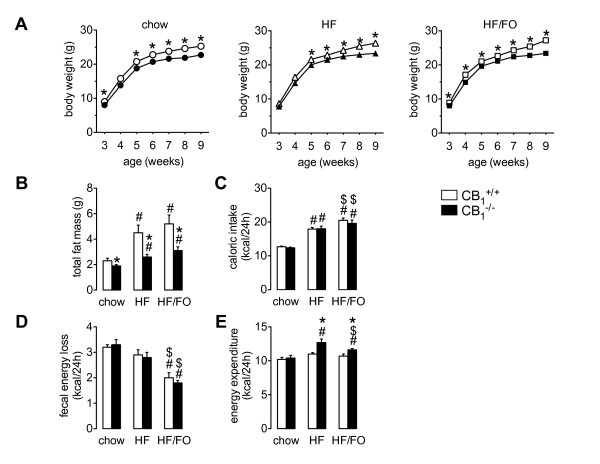
We first evaluated the adiposity-inducing effects of two high-fat diets in young CB1+/+ and CB1-/- mice. Immediately after weaning, a 6-week dietary intervention was started during which animals were fed chow, HF or HF/FO diets. Body weight development during this intervention is presented in Figure 1A. We observed a steep increase in body weight during the first weeks of the dietary intervention in all groups, which likely indicates growth. Body weight development in this study therefore reflects both growth and increasing adiposity. Body mass was however always lower in CB1-deficient mice as compared to wild-type littermates, irrespective of the diet they received (Figure 1A). During the final weeks of the intervention, body weight curves of CB1+/+ and CB1-/- mice diverged, particularly in the HF and HF/FO group (Figure 1A). To assess to what extent the difference in body weight reflected changes in adiposity, we quantified the fat mass after finalisation of the dietary intervention. As expected, HF and HF/FO feeding resulted in a comparable increase in fat mass compared to chow in CB1+/+ mice, while CB1-/- mice remained relatively lean on the HF and HF/FO diets (Figure 1B). Whole-body carcass analysis revealed that, with the exception of brown fat, all adipose tissue depots contributed to the diet-induced adiposity (Table 1). Caloric intake was significantly higher in HF and HF/FO fed CB1+/+ and CB1-/- mice as compared to chow-fed controls (Figure 1C). The resistance to adiposity in CB1-/- mice was however independent of caloric intake since we did not observe differences in energy consumption between CB1+/+ and CB1-/- mice during the dietary interventions (Figure 1C). Fecal energy loss was also similar in CB1+/+ and CB1-/- mice, indicating that intestinal energy absorption was comparable (Figure 1D). Fish oil replacement, however, increased nutrient absorption in HF-fed mice, since fecal energy loss was reduced in CB1+/+ and CB1-/- mice that were fed the HF/FO diet. A linear relationship between body weight and energy expenditure was observed for chow-fed CB1+/+ and CB1-/- mice only (data not shown), indicating that the increase in body weight upon HF and HF/FO feeding was not accompanied by elevated energy expenditure in this study. Nevertheless, we found that estimated energy expenditure was clearly and significantly increased in HF and HF/FO-fed CB1-deficient mice as compared to wild-type controls (Figure 1E and Additional File 1 Table S3). Altogether, analysis of the different components of energy balance (Figures 1C-E) reveals equal energy intake and -expenditure in chow-fed CB1+/+ and CB1-/- mice. On the other hand, HF and HF/FO feeding induced a positive energy balance, which is less marked in CB1-/- versus CB1+/+ mice, as a result of increased energy expenditure. Interestingly, chow-fed CB1-/- mice exhibited higher fat oxidation rates in the dark phase (Additional File 1 Table S3), which likely explains the lower fat mass as compared to CB1+/+ mice (Figure 1B and Table 1).
Figure 1.
Body weight, fat mass and energy balance in CB1+/+ and CB1-/- mice fed chow, a HF or a HF/FO diet during 6 weeks. A, Body weight development during the dietary intervention. B, Total fat mass at the end of the dietary intervention. C, Caloric intake, determined by manual food weighing, and averaged over the third to fifth week of dietary intervention. D, Fecal energy loss and E, Energy expenditure, derived from indirect calorimetry. Open symbols/bars, CB1+/+ mice; closed symbols/bars, CB1-/- mice. Values are given as means ± SEM for n = 5-13; # p < 0.05 compared to chow group of the same genotype; $ p < 0.05 compared to HF group of the same genotype, * p < 0.05 CB1-/- vs. CB1+/+ (Student t-test). General linear model analysis revealed overall effects for the following parameters (p < 0.05): Genotype: body weight at every timepoint, total fat mass, 24 h-energy expenditure. Chow versus HF: total fat mass, caloric intake, 24 h-energy expenditure. Chow versus HF/FO: total fat mass, caloric intake, fecal energy loss. HF versus HF/FO: caloric intake, fecal energy loss.
Table 1.
Body composition and plasma metabolite concentrations in CB1+/+ and CB1-/- mice fed chow, a HF or a HF/FO diet during 6 weeks
| chow | HF | HF/FO | ||||
|---|---|---|---|---|---|---|
| CB1+/+ | CB1-/- | CB1+/+ | CB1-/- | CB1+/+ | CB1-/- | |
| Subcutaneous fat (g) | 0.9 ± 0.1 | 0.7 ± 0.0 | 2.1 ± 0.3# | 1.1 ± 0.1#* | 2.5 ± 0.4# | 1.4 ± 0.1#* |
| Epididymal fat (mg) | 319 ± 40 | 227 ± 19 | 716 ± 113# | 361 ± 34#* | 644 ± 85# | 406 ± 64#* |
| Retroperitoneal fat (mg) | 116 ± 17 | 66 ± 6* | 217 ± 33# | 108 ± 7#* | 300 ± 54# | 145 ± 19#* |
| Brown fat (mg) | 45 ± 6 | 41 ± 5 | 47 ± 6 | 36 ± 3 | 52 ± 5 | 36 ± 5 |
| Mesenteric fat (mg) | 194 ± 18 | 148 ± 12 | 303 ± 36# | 181 ± 11* | 336 ± 47# | 190 ± 14* |
| Muscular fat (g) | 0.6 ± 0.0 | 0.5 ± 0.0* | 1.0 ± 0.1# | 0.7 ± 0.0#* | 1.1 ± 0.1# | 0.8 ± 0.0#* |
| Organ fat (mg) | 113 ± 15 | 103 ± 14 | 176 ± 28 | 161 ± 15# | 238 ± 30# | 133 ± 14* |
| Lean mass (g) | 5.2 ± 0.6 | 4.6 ± 0.1* | 5.0 ± 0.1 | 4.7 ± 0.1 | 5.0 ± 0.1 | 4.7 ± 0.1 |
| Plasma metabolites and adipokines | ||||||
| Triglycerides (μM) | 333 ± 31 | 245 ± 31 | 277 ± 29 | 275 ± 33 | 240 ± 63 | 183 ± 19 |
| Cholesterol (mM) | 1.3 ± 0.2 | 0.9 ± 0.1 | 1.9 ± 0.1# | 1.8 ± 0.1# | 1.4 ± 0.1$ | 1.3 ± 0.1#$ |
| Non-esterified fatty acids (μM) | 607 ± 32 | 391 ± 34* | 586 ± 40 | 588 ± 51# | 464 ± 38# | 372 ± 26$ |
| Blood glucose (mM) | 9.3 ± 0.5 | 7.7 ± 0.8 | 9.1 ± 0.3 | 7.4 ± 0.4* | 9.0 ± 0.4 | 8.1 ± 0.3 |
| Insulin (pg/mL) | 334 ± 53 | 356 ± 86 | 325 ± 39 | 270 ± 64 | 371 ± 82 | 300 ± 84 |
| Leptin (ng/mL) | 0.7 ± 0.1 | 0.6 ± 0.1$ | 2.1 ± 0.5#* | 1.0 ± 0.2#* | 2.2 ± 0.5# | 1.7 ± 0.3# |
| Adiponectin (μg/mL) | 14.3 ± 1.3 | 18.5 ± 2.4 | 15.4 ± 1.5 | 17.0 ± 2.9 | 18.1 ± 2.2 | 19.1 ± 2.2 |
| Resistin (ng/mL) | 2.3 ± 0.2 | 2.5 ± 0.4 | 3.0 ± 0.5 | 2.9 ± 0.3 | 2.1 ± 0.2 | 2.6 ± 0.4 |
Values are given as means ± SEM for n = 6-8; # p < 0.05 compared to chow group of the same genotype; $ p < 0.05 compared to HF group of the same genotype, * p < 0.05 CB1-/- vs. CB1+/+ (Student t-test).
General linear model analysis revealed overall effects for the following parameters (p < 0.05):
Genotype: subcutaneous/epidydimal/retroperitoneal/brown/mesenteric/muscular/organ fat mass, lean mass, non-esterified fatty acid levels, glucose levels.
Chow versus HF: subcutaneous/epididymal/retroperitoneal/mesenteric/muscular/organ fat mass, leptin levels.
Chow versus HF/FO: subcutaneous/epididymal/retroperitoneal/mesenteric/muscular/organ fat mass, cholesterol levels, leptin levels.
HF versus HF/FO: non-esterified fatty acid levels.
Interaction between genotype and diet: non-esterified fatty acid levels.
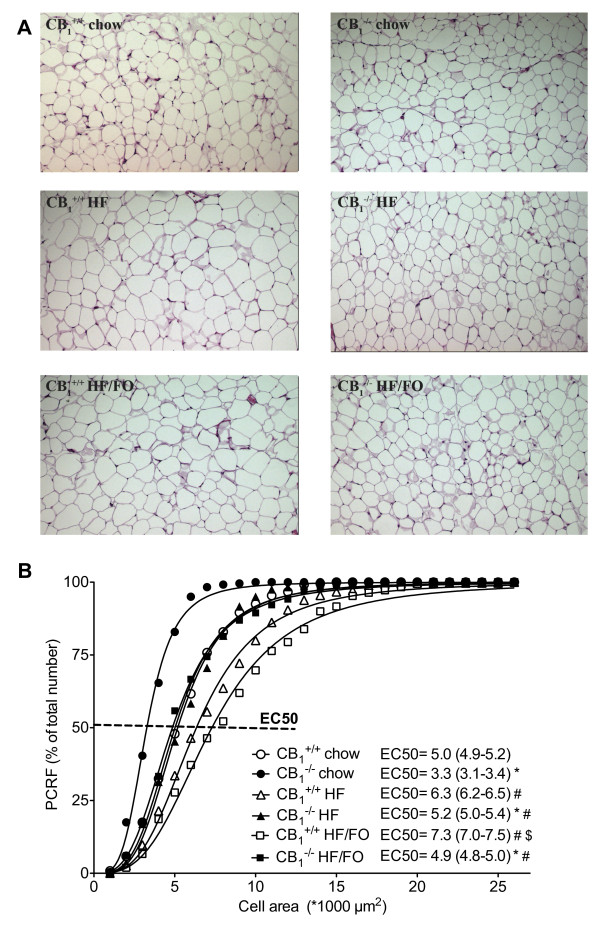
HF and HF/FO feeding led to an increase in adipocyte size in both CB1+/+ and CB1-/- mice (Figure 2A and 2B). Similar to body and fat mass, CB1-/- mice always had smaller adipocytes (Figure 2A), and we observed a significant difference between the 95%-confidence intervals of the fat cell area EC50s in CB1-/- versus CB1+/+ mice fed either of the three diets (Figure 2B). Adipose tissue expression of Faah, Pparγ2, C/ebpα, Ap2 and Adiponectin were not affected by CB1 deficiency or HF and HF/FO feeding, while Napepld expression was increased in HF-fed mice of both genotypes (Table 2). Plasma triglyceride, insulin, adiponectin and resistin levels were comparable in all mice (Table 1). Cholesterol concentrations were increased in HF-fed CB1+/+ and CB1-/- mice as compared to those receiving chow, and normalized in HF/FO-fed mice (Table 1). Non-esterified fatty acid levels were significantly lower in chow- and HF/FO-fed CB1-deficient mice (Table 1). Glucose levels tended to be lower in CB1-/- versus CB1+/+ mice, however the difference reached statistical significance for HF-fed animals only (Table 1). Plasma leptin concentrations (Table 1) correlated to the degree of HF and HF/FO-induced adiposity (Figure 1B) in CB1+/+ and CB1-/- mice.
Figure 2.
Fat cell area in CB1+/+ and CB1-/- mice fed chow, a HF or a HF/FO diet during 6 weeks. A, Representative pictures of 3 μm paraffin hematoxylin and eosin-stained sections (1 cm = 100 μm) and B, percent relative cumulative frequency (PCRF) curves of 240-420 fat cell areas from adipose tissue sections. Inset: EC50 values of the PCRF curves and their 95%-confidence intervals. Open symbols, CB1+/+ mice; closed symbols, CB1-/- mice. # p < 0.05 compared to chow group of the same genotype; $ p < 0.05 compared to HF group of the same genotype, * p < 0.05 CB1-/- vs. CB1+/+ (p < 0.05 in case of no overlap between EC50 95%-confidence intervals).
Table 2.
Adipose tissue gene expression levels in CB1+/+ and CB1-/- mice fed chow, a HF or a HF/FO diet during 6 weeks
| chow | HF | HF/FO | ||||
|---|---|---|---|---|---|---|
| CB1+/+ | CB1-/- | CB1+/+ | CB1-/- | CB1+/+ | CB1-/- | |
| Endocannabinoid system | ||||||
| CB1 (Cnr1) | 1.0 ± 0.1 | ND | 1.0 ± 0.1 | ND | 1.1 ± 0.2 | ND |
| Napepld | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.4 ± 0.1# | 1.3 ± 0.2# | 1.2 ± 0.1 | 1.1 ± 0.1 |
| Faah | 1.0 ± 0.3 | 1.8 ± 0.5 | 1.1 ± 0.6 | 1.1 ± 0.4 | 1.0 ± 0.5 | 1.3 ± 0.6 |
| Adipocyte proliferation and differentiation | ||||||
| Pparγ2 (Pparg) | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.3 | 1.0 ± 0.1$ | 0.9 ± 0.1 |
| C/ebpα (Cebpa) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.0 | 1.3 ± 0.3 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| Ap2 (Fabp4) | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.5 ± 0.0# | 1.2 ± 0.2 | 1.4 ± 0.1 | 1.2 ± 0.1 |
| Adiponectin (Adipoq) | 1.0 ± 0.2 | 0.9 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.4 | 1.3 ± 0.1 | 1.0 ± 0.1 |
| Trigyceride synthesis | ||||||
| Srebp-1c (Srebf1) | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1# | 1.8 ± 0.4 | 1.1 ± 0.0$ | 1.1 ± 0.0 |
| Acc1 (Acaca) | 1.0 ± 0.2 | 1.1 ± 0.3 | 0.9 ± 0.1 | 0.9 ± 0.3 | 0.6 ± 0.1$ | 0.5 ± 0.1# |
| Fas (Fasn) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.3 | 0.7 ± 0.1$ | 0.4 ± 0.0# |
| Scd1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 2.8 ± 0.1# | 3.1 ± 0.7# | 0.9 ± 0.1$ | 1.1 ± 0.1$ |
| Pepck (Pck1) | 1.0 ± 0.2 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.4 | 1.1 ± 0.1 | 0.9 ± 0.1 |
| Fatty acid uptake and transport | ||||||
| Cd36 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.1 ± 0.1 |
| Fatp4 (Slc27a4) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.0 | 1.4 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| Fatty acid hydrolysis | ||||||
| Lpl | 1.0 ± 0.3 | 0.9 ± 0.2 | 1.2 ± 0.1 | 0.9 ± 0.2 | 1.4 ± 0.2 | 1.0 ± 0.1 |
| Gpihbp1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.9 ± 0.0 |
| Angptl3 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.0 |
| Angptl4 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1 | 1.1 ± 0.1 |
| Apoc1 | 1.0 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.0 | 1.1 ± 0.0 | 0.7 ± 0.1$ | 0.9 ± 0.1 |
| Apoc3 | 1.0 ± 0.2 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.0 | 0.2 ± 0.0$ | 0.2 ± 0.0# |
| Macrophage infiltration | ||||||
| Cd68 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.0 | 1.0 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 |
| Adipocyte lipolysis | ||||||
| Hsl (Lipe) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.1 | 1.0 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| Atgl (Pnpla2) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.4 | 1.2 ± 0.1 | 1.2 ± 0.2 |
| Fatty acid oxidation | ||||||
| Cpt1a | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.6 ± 0.2# | 1.2 ± 0.2 | 1.3 ± 0.1# | 1.1 ± 0.1 |
Expression levels were normalized to cyclophilin expression and values are given as means ± SEM for n = 4-8; # p < 0.05 compared to chow group of the same genotype, $ p < 0.05 compared to HF group of the same genotype (Student t-test). ND, non-detectable.
General linear model analysis revealed overall effects for the following parameters (p < 0.05):
Chow versus HF: aP2, Scd1, Srebp-1c.
Chow versus HF/FO: Fas.
HF versus HF/FO: Fas, Scd1, Srebp-1c.
Fatty acid synthesis in adipose tissue is not affected in CB1-/- mice
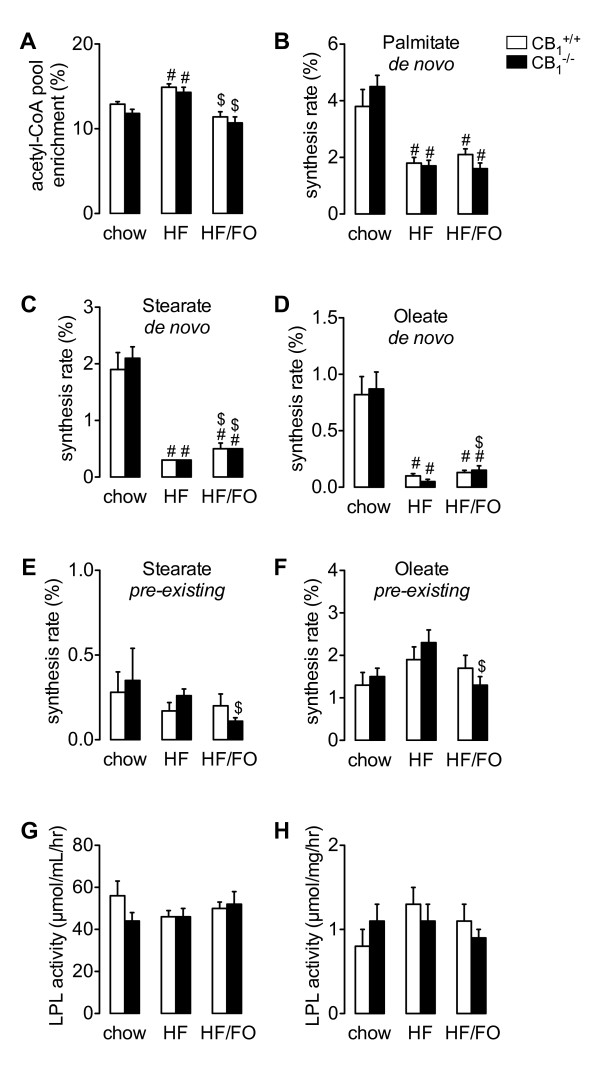
To assess whether lipogenesis was reduced in CB1-/- mice, we determined fatty acid synthesis in adipose tissue using 13C-acetate incorporation. Acetyl-CoA pool enrichments were increased in HF-fed CB1+/+ and CB1-/- mice as compared to chow-fed animals (Figure 3A), and normalized by FO replacement in both genotypes. No differences between CB1+/+ and CB1-/- mice were observed. De novo palmitate synthesis was similar in CB1+/+ and CB1-/- mice, but was reduced in mice fed the HF and HF/FO diets as compared to chow-fed animals (Figure 3B). Similarly, fractional synthesis of stearate and oleate via de novo lipogenesis was reduced in CB1+/+ and CB1-/- mice fed the HF and HF/FO diets as compared to chow-fed animals (Figure 3C and 3D) with no differences between CB1+/+ and CB1-/- mice. Compared to HF-fed mice, de novo stearate synthesis was slightly increased in HF/FO-fed mice of both genotypes (Figure 3C). The unaltered fatty acid synthesis rates in CB1-/- mice were supported by gene expression analysis in adipose tissue: we did not observe any changes in lipogenic gene expression between CB1+/+ and CB1-/- mice under the different dietary conditions (Table 2). HF feeding did not affect Acc1, Fas and Pepck (Table 2) expression. HF feeding induced Scd1 expression in both CB1+/+ and CB1-/- mice while we observed a tendency for an increased Srebp-1c expression. These inductions were normalized in HF/FO-fed CB1+/+ and CB1-/- mice (Table 2). HF/FO feeding also reduced Acc and Fas expression levels as compared to HF (Table 2). Fatty acids are toxic molecules, which are immediately processed after they enter cells to prevent apoptosis [31]. Chain-elongation and desaturation of pre-existing palmitate may therefore be used as a measure for fatty acid entry [31]. In contrast to the reduced lipogenic fluxes from elongation of de novo synthesized palmitate (Figure 3B-3D), we did not observe any consistent differences in synthesis of stearate (Figure 3E) and oleate (Figure 3F) from elongation of pre-existing palmitate between CB1+/+ and CB1-/- mice. Reduced fatty acid influx therefore does not explain the protection against HF and HF/FO-induced adiposity in CB1-deficient mice. This is further supported to the unchanged mRNA levels of the fatty acid transporters Cd36 and Fatp4 in these animals (Table 2).
Figure 3.
Fractional fatty acid synthesis rates and lipolytic activities in CB1+/+ and CB1-/- mice fed chow, a HF or a HF/FO diet during 6 weeks. A, Acetyl-CoA precursor pool enrichment. B, De novo palmitate synthesis. C De novo stearate synthesis. D, De novo oleate synthesis. E, Stearate synthesis from pre-existing palmitate. F, Oleate synthesis from pre-existing palmitate. G, Post-heparin plasma LPL activity and H, Adipose tissue-derived LPL activity normalized for protein content. Open bars, CB1+/+ mice; closed bars, CB1-/- mice. Values are given as means ± SEM for n = 4-7; # p < 0.05 compared to chow group of the same genotype; $ p < 0.05 compared to HF group of the same genotype (Student t-test). General linear model analysis revealed overall effects for the following parameters (p < 0.05): Chow versus HF: acetyl-CoA pool enrichment, de novo palmitate/stearate/oleate synthesis. Chow versus HF/FO: de novo palmitate/stearate synthesis. HF versus HF/FO: acetyl-CoA pool enrichment, de novo stearate/oleate synthesis.
Lipolytic activity is maintained in CB1-/- mice
CB1-receptor agonist treatment enhances lipolytic activity in vitro [5]. As a consequence, CB1 deficiency may result in a reduction of lipoprotein triglyceride lipolysis, thereby limiting fat storage in adipose tissue and as such protecting against adiposity. Yet, the expression of Lpl and its stimulatory factor Gpihbp1 [32] in adipose tissue were not affected by CB1 deficiency or HF and HF/FO feeding (Table 2). In parallel, we determined LPL activities in CB1+/+ and CB1-/- mice fed either of the three diets. In accordance with unaltered Lpl and Gpihbp1 mRNA levels, we observed that both post-heparin plasma LPL activity and adipose tissue-derived LPL-activity were similar in all groups (Figure 3G and 3H). The expression levels of the LPL inhibitory proteins Angptl3, Angplt4, ApoC1 and ApoC3 [32,33] were also unchanged between genotypes (Table 2). Because macrophage products have been shown to inhibit adipocyte differentiation [34], we determined CD68 macrophage antigen expression. Consistent with the unaltered mRNA levels of adipocyte differentiation/proliferation markers, we found no significant differences in Cd68 expression between CB1-deficient and wild-type mice (Table 2). Finally, we did not observe significant differences in adipose tissue expression of Hsl, Atgl and Cpt1a between CB1+/+ and CB1-/- mice (Table 2), suggesting that fatty acid release and -oxidation were also not affected by CB1 deficiency.
Discussion
Overactivity and/or dysregulation of the EC system potentially contribute to the development of obesity [7,11,14]. Several in vitro studies point to the involvement of the CB1-receptor in controlling adipocyte development and metabolism [5-13]. Here, we report that the protection against high-fat diet induced adiposity and a concomitant reduced fat cell size in CB1-deficient mice are not related to alterations in adipocyte differentiation and proliferation markers, or to changes lipogenic fluxes and lipoprotein lipolysis in fat tissue.
Diet-induced adiposity was prevented in CB1-/- mice as compared to wild-type littermates. Analysis of the energy balance in chow, HF and HF/FO-fed animals revealed that CB1 deficiency did not impact on energy intake. Consistent with previous findings [35], no major effects on food intake were observed. Although both HF and HF/FO feeding increased intestinal fatty acid uptake, this occurred to a similar extent in CB1-/- and CB1+/+ mice. Interestingly, isocaloric replacement of the saturated fat in the HF diet for PUFA provoked an additional increase in intestinal fatty acid uptake. This most likely resulted from an upregulation of intestinal fatty acid transport proteins and a subsequent increase in lipid uptake [36]. On the other hand, we observed that energy expenditure was increased in HF and HF/FO-fed CB1-/- mice as compared to wild-type littermates. Reduced diet-induced adiposity upon CB1 ablation was therefore associated with a less positive energy balance in the current study.
Impaired CB1-signalling has been shown to protect against high-fat diet-induced obesity in adult mice [16,35], however, the adipocyte-specific consequences in this context had not been evaluated. The ECS has been implicated in the regulation of adipocyte proliferation and differentiation in vitro [5-13] and CB1-deficient mice are retarded in growth from an early age on [5]. We therefore decided to study the metabolic consequences of high-fat feeding in young CB1-/- mice. Six weeks of HF and HF/FO feeding induced adiposity in CB1+/+ mice, which was characterized by an increased fat mass and adipocyte size. This effect was clearly blunted in CB1-/- mice, although these mice did become more obese on the HF and HF/FO diets as compared to chow-fed CB1-/- mice. Gene expression analysis indicated that this was not due to a lower expression of adipocyte differentiation and proliferation markers in CB1-/- mice, since we did not observe differences in Pparγ2, Cebp/α and Ap2 mRNA levels. The expression of these genes was also not different prior to the start of the dietary interventions (Additional File 1 Figure S1D).
PPARγ and C/EBPα are two master transcription factors which play key roles in controlling adipocytes differentiation and proliferation, as well as adiponectin gene transcription [37,38]. We did not observe differences in adiponectin mRNA levels, indicating that, in parallel to the unaltered expression levels of PPARγ and C/EBPα, the transcriptional activity of these regulators was not impaired by CB1 deficiency. In accordance with these findings, we observed that plasma adiponectin concentrations remained unchanged among the different groups studied. Because of the relatively short timeframe of the dietary intervention, HF and HF/FO feeding did not yet alter adiponectin levels as compared to chow-fed mice [39]. Although CB1-receptor activity has been proposed to modulate adiponectin expression and release [7,11], pharmacological CB1-receptor activation and blockade do not directly affect adiponectin expression in cultured adipocytes [12,40]. The reported effects on adiponectin expression and -secretion are therefore rather related to acute changes in food intake [41]. Consistent with the unchanged adiponectin levels and similar to what has been found by others [35], voluntary caloric intake was not different between CB1+/+ and CB1-/- mice.
Pharmacological CB1-receptor activation promotes lipid storage in adipocytes [6,11-13], in parallel to elevated expression levels of lipogenic genes in fat tissue [6]. We therefore quantified the lipogenic flux in vivo in adipose tissue of CB1+/+ and CB1-/- mice. Fatty acid synthesis rates were not affected by CB1 deficiency, in line with unaltered expression levels of lipogenic genes. In both genotypes, HF and HF/FO feeding reduced de novo lipogenesis (indicated by lower fractional palmitate synthesis as well as by reduced stearate and oleate synthesis from de novo synthesized palmitate). This is in agreement with a report that shows reduced fatty acid synthesis from glucose in the fat tissue of high-fat fed mice compared to animals receiving a low-fat diet [42]. Thus, high-fat diet-induced adiposity is caused by increased storage and elongation of dietary fatty acids. The latter process was however not affected by CB1 deficiency in the current study, since we found that fatty acid elongation of pre-existing palmitate was maintained in CB1-/- mice. The obesity-resistant phenotype of these animals can therefore not be ascribed to a defect in fatty acid synthesis or -elongation.
Lipid supply to organs and tissues requires the activity of lipases. Adipocyte-specific LPL activity directs fatty acids to the adipose tissue, thereby enabling their storage. To assess whether the protection against high-fat diet-induced adiposity in CB1-/- mice was secondary to a reduced lipolytic activity, we determined whole-body and adipose tissue-specific LPL activity. CB1+/+ and CB1-/- mice fed either of the three diets exhibited comparable LPL activities. Elevated plasma EC concentrations have been associated with increased LPL activity [43]. In vitro studies have furthermore shown that CB1-receptor antagonism inhibits LPL activity in adipocytes, however, only when these cells are co-treated with a CB1-receptor agonist [5]. Together, these findings indicate that CB1-receptor blockade only impacts on LPL activity under conditions of active ECS signalling. Consistent with previous work [35], we did not observe an increase in LPL activity upon high-fat feeding. LPL activity was furthermore not different between CB1+/+ and CB1-/- mice, suggesting that the HF diet did not increase CB1-mediated LPL activity, which rendered this process insensitive to CB1 ablation. In further support of this were the unchanged expression levels of LPL and its regulators among the different groups. Thus, the reduced fat stores in CB1-deficient mice were not related to impaired LPL-mediated lipolysis. The unaltered expression levels of Hsl and Atgl and the lack of an increase in plasma non-esterified fatty acid levels in CB1-deficient mice furthermore argues against increased fatty acid release from adipose tissue in these animals.
Although several studies have implicated the ECS in control of adipocyte differentiation/proliferation, lipogenesis and lipolysis [5-13], the current work excludes the possibility that these processes contribute to the development of obesity through CB1. One of the explanations for these discrepancies presumably lies in the acute and transient nature of responses to pharmacological treatments [44], whereas our knockout mice rather provide a model of sustained impaired CB1 activity. The adaptations of adipocyte biology in response to altered EC composition, on the other hand, are most likely mediated by CB1-independent mechanisms. Overall, our data indicate that the reported physiological changes in adipocyte biology that occur in response to altered ECS activity cannot explain the phenotype of CB1-/- mice in the long term.
Reduced diet-induced fat storage in CB1-/- mice was primarily related to a less positive energy balance as compared to their wild type littermates in this study. Recent reports show that decreased ECS signalling promotes energy dissipation as heat. CB1-receptor blockade induces a mitochondria-rich, thermogenic phenotype in adipocytes, characterized by increased mitochondrial uncoupling [45-48]. CB1-agonists, on the other hand, impair mitochondrial biogenesis and -uncoupling [6,49]. Therefore, both oxidative and non-oxidative energy expenditure presumably contribute to the protection against diet-induced adiposity CB1-deficient mice.
Conclusions
This study shows that the obesity-resistant phenotype of CB1-deficient mice is not due to changes in adipose tissue differentiation and -proliferation, lipogenesis or LPL-activity in vivo. Wild-type and CB1-deficient mice exhibited similar energy intakes. Energy expenditure, on the other hand, was increased in CB1-/- mice upon high-fat feeding, resulting in lower adiposity and decreased fat cell size. We therefore propose that the protection against diet-induced adiposity in CB1-/- mice is not related to functional alterations in fat metabolism per se, but rather results from increased energy loss by oxidative and potentially non-oxidative pathways.
List of abbreviations
Acc1: acetyl-CoA carboxylase 1; AEA: anandamide; 2-AG, 2-arachidonoyl glycerol; Angptl: Angiopoietin-like protein; Ap2: fatty acid-binding protein 4; ApoC: apolipoprotein C; Atgl: adipose triglyceride lipase; CB1: cannabinoid receptor 1 (Cnr1); Cd36: fatty acid transporter; Cd68: macrophage antigen 68; C/ebpα: CCAAT enhancer binding protein α; Cpt1a: carnitine palmitoyl transferase 1a; EC: endocannabinoid; ECS: endocannabinoid system; Faah: fatty acid amide hydrolase; Fas: fatty acid synthase; Fatp4: fatty acid transport protein 4; Gpihbp1: glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1; HF: high-fat; HF/FO: high-fat/fish oil; Hsl: hormone sensitive lipase; Lpl: lipoprotein lipase; MIDA: mass isotopomer distribution analysis; Napepld: N-acyl phosphatidylethanolamine phospholipase D; PCRF: percent relative cumulative frequency; Pepck: phosphoenolpyruvate carboxykinase; PFB: pentafluorobenzyl; Pparγ2: peroxisome proliferator-activated receptor gamma isoform 2; PUFA: polyunsaturated fatty acid; qPCR: quantitative PCR; Scd1: stearoyl-CoA desaturase 1; Srebp-1c: sterol regulatory element binding protein 1c.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MHO and AHK were involved in the acquisition and analysis of the data, participated in the design of the study and drafted the manuscript. PTB, TB, AB and THD were responsible for data acquisition. VWB and PJJS and contributed to interpretation of the data and critical revision of the manuscript. FK and GJD conceived of the study, participated in its design and coordination, and critically revised the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Table S1 Composition of experimental diets. Table S2 Primer and probe sequences used for qPCR. Sequence and accessions numbers of qPCR primers and probes used in these study. Figure S1 (A) VO2, (B) VCO2 and (C) RER values during light and dark phases. Open symbols, CB1+/+ mice; closed symbols, CB1-/- mice. Values are given as means ± SEM for n = 5-7. (D) Gene expression levels in epididymal fat tissue of 3-week old CB1-/- and CB1+/+ mice receiving regular chow. Open bars, CB1+/+ mice; closed bars, CB1-/- mice. Values are given as means ± SEM for n = 4-8. Table S3 Detailed indirect calorimetry data in CB1+/+ and CB1-/- mice fed chow, a HF or a HF/FO diet during 6 weeks. Energy expenditure and substrate utilization during dark and light phases, expressed per mouse (upper part) or normalized for lean body mass (lower part).
Contributor Information
Maaike H Oosterveer, Email: M.H.Oosterveer@med.umcg.nl.
Anniek H Koolman, Email: a.koolman@umcg.nl.
Pieter T de Boer, Email: ptdeboer@hotmail.com.
Trijnie Bos, Email: t.bos02@umcg.nl.
Aycha Bleeker, Email: a.bleeker@med.umcg.nl.
Theo H van Dijk, Email: t.van.dijk@umcg.nl.
Vincent W Bloks, Email: v.w.bloks@umcg.nl.
Folkert Kuipers, Email: f.kuipers@umcg.nl.
Pieter JJ Sauer, Email: p.j.j.sauer@umcg.nl.
Gertjan van Dijk, Email: gertjan.van.dijk@rug.nl.
Acknowledgements
This work was supported by the Groningen Expert Centre on Childhood Obesity and the Dutch Diabetes Research Foundation.
The authors thank Nanda Gruben, Rick Havinga, Gerard Overkamp, Theo S. Boer, Jan Keijzer, Jan Bruggink and Juul F.W. Baller for excellent technical assistance.
References
- Vettor R, Pagotto U, Pagano C, Pasquali R. Here, there and everywhere: the endocannabinoid system. JNeuroendocrinol. 2008;20(Suppl 1):iv–vi. doi: 10.1111/j.1365-2826.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- Bisogno T. Endogenous cannabinoids: structure and metabolism. JNeuroendocrinol. 2008;20(Suppl 1):1–9. doi: 10.1111/j.1365-2826.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. JNeuroendocrinol. 2008;20(Suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. IntJObesRelat Metab Disord. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S. et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. JClinInvest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Cervino C, Vicennati V, Pasquali R, Pagotto U. Cannabinoid type 1 receptor: another arrow in the adipocytes' bow. JNeuroendocrinol. 2008;20(Suppl 1):130–138. doi: 10.1111/j.1365-2826.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le FG, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. MolPharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Hilairet S, Marchand J, Fajas L, Le FG, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. EurJPharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Gasperi V, Fezza F, Pasquariello N, Bari M, Oddi S, Agro AF, Maccarrone M. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell MolLife Sci. 2007;64:219–229. doi: 10.1007/s00018-006-6445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De PL, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R. et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. JClinEndocrinolMetab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Pagano C, Pilon C, Calcagno A, Urbanet R, Rossato M, Milan G, Bianchi K, Rizzuto R, Bernante P, Federspil G, Vettor R. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab. 2007;92:4810–4819. doi: 10.1210/jc.2007-0768. [DOI] [PubMed] [Google Scholar]
- Pagano C, Rossato M, Vettor R. Endocannabinoids, adipose tissue and lipid metabolism. JNeuroendocrinol. 2008;20(Suppl 1):124–129. doi: 10.1111/j.1365-2826.2008.01690.x. [DOI] [PubMed] [Google Scholar]
- Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, Pacher P, Schon MR, Jordan J, Stumvoll M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR. The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes. 2008;57:1262–1268. doi: 10.2337/db07-1186. [DOI] [PubMed] [Google Scholar]
- Ravinet TC, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. IntJObesRelat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Pellizzon M, Buison A, Ordiz F Jr, Santa AL, Jen KL. Effects of dietary fatty acids and exercise on body-weight regulation and metabolism in rats. ObesRes. 2002;10:947–955. doi: 10.1038/oby.2002.129. [DOI] [PubMed] [Google Scholar]
- Matias I, Carta G, Murru E, Petrosino S, Banni S, Di MV. Effect of polyunsaturated fatty acids on endocannabinoid and N-acyl-ethanolamine levels in mouse adipocytes. BiochimBiophysActa. 2008;1781:52–60. doi: 10.1016/j.bbalip.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z. et al. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J. et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolman AH, Bloks VW, Oosterveer MH, Jonas I, Kuipers F, Sauer PJ, van Dijk G. Metabolic responses to long-term pharmacological inhibition of CB1-receptor activity in mice in relation to dietary fat composition. Int J Obes (Lond) 2010;34:374–384. doi: 10.1038/ijo.2009.219. [DOI] [PubMed] [Google Scholar]
- Riachi M, Himms-Hagen J, Harper ME. Percent relative cumulative frequency analysis in indirect calorimetry: application to studies of transgenic mice. Can J Physiol Pharmacol. 2004;82:1075–1083. doi: 10.1139/y04-117. [DOI] [PubMed] [Google Scholar]
- Liu MY, Xydakis AM, Hoogeveen RC, Jones PH, Smith EO, Nelson KW, Ballantyne CM. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin Chem. 2005;51:1102–1109. doi: 10.1373/clinchem.2004.047084. [DOI] [PubMed] [Google Scholar]
- Chwalibog A, Jakobsen K, Tauson AH, Thorbek G. Heat production and substrate oxidation in rats fed at maintenance level and during fasting. Comp Biochem Physiol A Mol Integr Physiol. 1998;121:423–429. doi: 10.1016/S1095-6433(98)10153-8. [DOI] [PubMed] [Google Scholar]
- Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- Lusk G. The elements of the science of nutrition. 4. New York: Johnson Reprint Corp; 1924. [Google Scholar]
- Wiegman CH, Bandsma RH, Ouwens M, van der Sluijs FH, Havinga R, Boer T, Reijngoud DJ, Romijn JA, Kuipers F. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes. 2003;52:1081–1089. doi: 10.2337/diabetes.52.5.1081. [DOI] [PubMed] [Google Scholar]
- Oosterveer MH, Van Dijk TH, Tietge UJF, Boer T, Havinga R, Stellaard F, Groen AK, Kuipers F, Reijngoud DJ. High fat feeding induces hepatic fatty acid elongation in mice. PLoSOne. 2009;4:e6066. doi: 10.1371/journal.pone.0006066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WN, Byerley LO, Bergner EA, Edmond J. Mass isotopomer analysis: theoretical and practical considerations. BiolMass Spectrom. 1991;20:451–458. doi: 10.1002/bms.1200200804. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sell H, MacNaul KL, Richard D, Berger JP, Deshaies Y. PPAR-gamma activation mediates adipose depot-specific effects on gene expression and lipoprotein lipase activity: mechanisms for modulation of postprandial lipemia and differential adipose accretion. Diabetes. 2003;52:291–299. doi: 10.2337/diabetes.52.2.291. [DOI] [PubMed] [Google Scholar]
- Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. JBiolChem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L, Kersten S. Modulation of plasma TG lipolysis by Angiopoietin-like proteins and GPIHBP1. Biochimica et biophysica acta. 2010;1801:415–420. doi: 10.1016/j.bbalip.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Voshol PJ, Rensen PC, van Dijk KW, Romijn JA, Havekes LM. Effect of plasma triglyceride metabolism on lipid storage in adipose tissue: studies using genetically engineered mouse models. Biochimica et biophysica acta. 2009;1791:479–485. doi: 10.1016/j.bbalip.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Constant VA, Gagnon A, Landry A, Sorisky A. Macrophage-conditioned medium inhibits the differentiation of 3T3-L1 and human abdominal preadipocytes. Diabetologia. 2006;49:1402–1411. doi: 10.1007/s00125-006-0253-0. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. JClinInvest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schothorst EM, Flachs P, Franssen-van Hal NL, Kuda O, Bunschoten A, Molthoff J, Vink C, Hooiveld GJ, Kopecky J, Keijer J. Induction of lipid oxidation by polyunsaturated fatty acids of marine origin in small intestine of mice fed a high-fat diet. BMCGenomics. 2009;10:110. doi: 10.1186/1471-2164-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Choi DS, Baik SH, Choi KM. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. BiochemBiophysResCommun. 2005;336:747–753. doi: 10.1016/j.bbrc.2005.08.203. [DOI] [PubMed] [Google Scholar]
- Qiao L, Maclean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M, Friedman JE, Shao J. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes. 2005;54:1744–1754. doi: 10.2337/diabetes.54.6.1744. [DOI] [PubMed] [Google Scholar]
- Bullen JW Jr, Bluher S, Kelesidis T, Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. American journal of physiology Endocrinology and metabolism. 2007;292:E1079–1086. doi: 10.1152/ajpendo.00245.2006. [DOI] [PubMed] [Google Scholar]
- Lofgren P, Sjolin E, Wahlen K, Hoffstedt J. Human adipose tissue cannabinoid receptor 1 gene expression is not related to fat cell function or adiponectin level. J Clin Endocrinol Metab. 2007;92:1555–1559. doi: 10.1210/jc.2006-2240. [DOI] [PubMed] [Google Scholar]
- Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. IntJObes(Lond) 2005;29:183–187. doi: 10.1038/sj.ijo.0802847. [DOI] [PubMed] [Google Scholar]
- Bederman IR, Foy S, Chandramouli V, Alexander JC, Previs SF. Triglyceride synthesis in epididymal adipose tissue: contribution of glucose and non-glucose carbon sources. JBiolChem. 2009;284:6101–6108. doi: 10.1074/jbc.M808668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby MA, Nomura DK, Hudak CS, Mangravite LM, Chiu S, Casida JE, Krauss RM. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. ProcNatlAcadSciUSA. 2008;105:14561–14566. doi: 10.1073/pnas.0807232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molhoj S, Hansen HS, Schweiger M, Zimmermann R, Johansen T, Malmlof K. Effect of the cannabinoid receptor-1 antagonist rimonabant on lipolysis in rats. Eur J Pharmacol. 2010;646:38–45. doi: 10.1016/j.ejphar.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Flamment M, Gueguen N, Wetterwald C, Simard G, Malthiery Y, Ducluzeau PH. Effects of the cannabinoid CB1 antagonist, rimonabant, on hepatic mitochondrial function in rats fed a high fat diet. Am J Physiol Endocrinol Metab. 2009. [DOI] [PubMed]
- Perwitz N, Wenzel J, Wagner I, Buning J, Drenckhan M, Zarse K, Ristow M, Lilienthal W, Lehnert H, Klein J. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes Metab. 2010;12:158–166. doi: 10.1111/j.1463-1326.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- Tedesco L, Valerio A, Cervino C, Cardile A, Pagano C, Vettor R, Pasquali R, Carruba MO, Marsicano G, Lutz B. et al. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes. 2008;57:2028–2036. doi: 10.2337/db07-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty AN, Allen AM, Oldfield BJ. The effects of rimonabant on brown adipose tissue in rat: implications for energy expenditure. Obesity(SilverSpring) 2009;17:254–261. doi: 10.1038/oby.2008.509. [DOI] [PubMed] [Google Scholar]
- Tedesco L, Valerio A, Dossena M, Cardile A, Ragni M, Pagano C, Pagotto U, Carruba MO, Vettor R, Nisoli E. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes. 2010;59:2826–2836. doi: 10.2337/db09-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Composition of experimental diets. Table S2 Primer and probe sequences used for qPCR. Sequence and accessions numbers of qPCR primers and probes used in these study. Figure S1 (A) VO2, (B) VCO2 and (C) RER values during light and dark phases. Open symbols, CB1+/+ mice; closed symbols, CB1-/- mice. Values are given as means ± SEM for n = 5-7. (D) Gene expression levels in epididymal fat tissue of 3-week old CB1-/- and CB1+/+ mice receiving regular chow. Open bars, CB1+/+ mice; closed bars, CB1-/- mice. Values are given as means ± SEM for n = 4-8. Table S3 Detailed indirect calorimetry data in CB1+/+ and CB1-/- mice fed chow, a HF or a HF/FO diet during 6 weeks. Energy expenditure and substrate utilization during dark and light phases, expressed per mouse (upper part) or normalized for lean body mass (lower part).