Abstract
Mesothelioma is an insidious disease with long latency after asbestos exposure. New cases are continually diagnosed, although levels are declining with recognition of the asbestos risk and efforts to remove asbestos from the workplace. Treatment for early stage disease with surgery and radiation is potentially curative, but many patients either are too ill to undergo aggressive surgery or present with advanced disease. Chemotherapy with cisplatin and pemetrexed is considered standard, although relapse is common. Second-line therapy is disappointing. New targeted therapies may pose promise and are being addressed in various clinical trial settings. Palliative care remains an important component of the management of this devastating illness.
Keywords: Asbestos, diagnosis, malignant pleural mesothelioma, targeted therapies, treatment
INTRODUCTION
Mesothelioma has been described as an insidious neoplasm because of its long latency period—up to 40 years in some series—after exposure to asbestos. It arises in the mesothelial surfaces of tissues in the pleura but can also occur in the peritoneum and the tunica vaginalis.1 Peak incidence occurs in the 5th and 6th decades of life. Surveillance Epidemiology and End Results (SEER) registry data report approximately 3,300 new cases annually, compared to nearly 200,000 cases of lung cancer.2 With recognition of asbestos exposure risks in the workplace and better controls, the incidence of mesothelioma in the United States (US) has declined over the past decade; however, there are still areas of endemic clustering, usually around regions of high asbestos-related industry such as shipping. In some parts of the world, the incidence is still on the rise.
In Louisiana, for the period between 2000 and 2008, SEER registry data recorded 182 cases in the greater New Orleans area compared to 309 cases statewide. The impact of Hurricane Katrina in 2005 was taken into account. Louisiana Tumor Registry data for 2009 documented 12 cases in the New Orleans area—including the parishes of Jefferson, Orleans, and St. Bernard—and 57 cases statewide.3 Males were three times more likely to be diagnosed than females, and more than half of the patients presented with stage III or stage IV disease.
PATHOGENESIS
Researchers have examined the association between asbestos and respiratory ailments for decades. A 1980 comprehensive review of asbestos-associated disease estimated that 8% of asbestos workers died of respiratory failure from the chronic morbidity of asbestos-induced pulmonary fibrosis.4 The risk of developing mesothelioma was described as 10% over the lifetime of an asbestos worker, with up to 70% of all mesothelioma cases involving documented asbestos exposure. Concomitant smoking enhances the risk of malignancy in an asbestos worker, with a 60-fold increased risk of developing non–small cell lung cancer. The chance of dying of a malignancy (mesothelioma or lung cancer) versus a nonmalignant cause is 50% in an individual exposed to asbestos compared to 18% in an individual not exposed. Asbestos workers are at highest risk, but family members can also be at risk via exposure to fibers brought home on the clothing of the primary individual.
The majority of asbestos fibers are either amphibole (sharp, rod-like) or serpentine (Figure 1). The serpentine fibers make up 90% of the type seen in the US and are considered less carcinogenic than the amphibole type. These fibers are typically found in brake linings, ship building, cement, and ceiling and pool tiles. The Occupational Safety and Health Administration (OSHA) set acceptable levels of exposure at 0.2 fibers/mL3 for fibers 5 microns or greater and up to 5 fibers/mL3 for smaller fibers.
Figure 1.
Asbestos fiber types.
Inhaled asbestos fibers are trapped in the lower third of the lung, where they initiate an inflammatory response. The fibers are phagocytosed into mesothelial cells and initiate an oncogenic cascade of events that includes activation of c-Myc and c-Jun oncogenes, binding with epidermal growth factor receptors (EGFRs), and promotion of antiapoptotic genes such as Bcl-xl.5
Radiation therapy has also been implicated as a possible cause of mesothelioma. In a study of 77,876 non-Hodgkin lymphoma patients under the age of 25 treated with radiation only, 18 developed mesothelioma.6 In a study of 40,000 testicular cancer patients treated with radiation during the years 1943-2001, 10 developed mesothelioma without any obvious asbestos exposure.7 A review of 22,140 breast cancer patients treated with radiation on 1 of 11 National Surgical Adjuvant Breast and Bowel Project clinical trials showed that 3 developed mesothelioma.8
Carbon nanotubes in some small appliances have similar shapes and chemical characteristics as asbestos fibers. They have been found to induce mesothelioma-like tumors when injected intraperitoneally in mice.9,10
The Simian Virus SV-40 is a polyoma virus that is thought to inactivate tumor suppressor genes of the retinoblastoma family. SV-40 nucleic acids have been identified in mesothelioma cases without obvious asbestos exposure. Research to develop a possible vaccine therapy has been hindered by the low number of cases connected to the virus and problems with laboratory procedures; however, researchers continue to explore this possible avenue of treatment.
CLINICAL PRESENTATION
Signs and symptoms associated with mesothelioma are relatively nonspecific and can be seen with almost any intrathoracic disease process, benign or malignant. Most patients have a cough, usually nonproductive. Dyspnea is also common. Chest wall pain may be a relatively unique symptom, usually described as a focal ache. There may be palpable soft tissue fullness or mass and decreased respiratory sounds with dullness to percussion due to an underlying pleural effusion. Some patients develop splinting and even scoliosis toward the ipsilateral side.
Pleural effusions are common and are right sided 60% of the time. Five percent may present with bilateral effusions. Pleural plaques are common, and 1 out of 5 patients develop bibasilar fibrosis, characteristic of chronic asbestosis. Computed tomography (CT) may show pleural-based nodularity. Magnetic resonance imaging can define invasion of the diaphragm or mediastinal structures, important in preoperative assessment. Positron emission tomography (PET) is useful because mesothelioma has hypermetabolic characteristics and PET can be used not only for staging but for posttreatment follow-up as well. A pleurodesis procedure should ideally be performed after a PET scan because the inflammatory reaction of the pleurodesis may affect the fludeoxyglucose avidity of the PET scan and lead to false-positive results. Preoperative PET may actually understage the patient, but it is useful for identifying distant metastases.11
Several paraneoplastic syndromes have been described with mesothelioma. These include hypercalcemia, hypoglycemia, autoimmune hemolytic anemia, hypercoagulable states, and disseminated intravascular coagulation. These syndromes are nonspecific and can be seen with a number of malignancies.
PATHOLOGY
Diagnosis of mesothelioma can be difficult. The disease is relatively uncommon, and many pathologists may not have extensive experience with it. The amount of tissue obtained is often minimal and may not be adequate to perform the necessary battery of tests that can distinguish mesothelioma from other pleural-based malignancies. Histologic variability may make diagnosis challenging.
The most common histologic type is epithelioid and is associated with the best prognosis. Sarcomatoid variants with characteristic spindle morphology are associated with a worse prognosis. Often, mixed epithelioid and sarcomatoid histologies can be seen. Tissue obtained by cytologic analysis of pleural fluid or blind pleural biopsy is limited and underclassifies the correct histology up to 25% of the time. If pleural fluid is obtained, large volume collections should be performed and a cytospin analysis conducted to increase diagnostic accuracy. Thoracoscopic biopsies with direct visualization of pleural nodules provide the best yield.
Immunohistochemical staining is important to distinguish mesothelioma from adenocarcinomas of lung origin or metastatic from other sites. Calretinin is commonly positive in mesothelioma, with a reported sensitivity of 95% and specificity of 87%.12 Thrombomodulin has the best specificity at 92% but is less sensitive at 68%. Other useful antibodies directed against mesothelial-associated antigens include mesothelin, cytokeratin 5, Wilms' tumor-1 gene product, and HBME-1 and the nonmesothelial antigens Lewis-Y blood group (antibody BG8), MOC-31, BerEp4, CD15, and the carcinoembryonic antigen family. A consensus statement from an expert panel of 16 pathologists from the International Mesothelioma Interest Group established guidelines for diagnosing mesothelioma and distinguishing it from other tumors such as adenocarcinoma by using a panel of histochemical markers with at least 80% or more sensitivity.13 According to the panel of experts, by utilizing this approach a pathologist can make the diagnosis of mesothelioma 95% of the time. In the remaining 5%, tissue may not be sufficient, not representative of the tumor, or, in some cases, so poorly differentiated that diagnosis is difficult.
DIAGNOSIS
Accurate diagnosis of mesothelioma depends on adequate tissue. Traditional diagnostic procedures have included pleural fluid cytology obtained through thoracentesis, needle biopsy of pleural tissue under CT guidance, video-assisted thoracoscopy surgery with direct visualization and biopsy of pleural nodules, and open thoracotomy. Pleural fluid is usually bloody and exudative with elevated protein, lactase dehydrogenase, and cell counts, but this finding is nonspecific and the sensitivity of pleural fluid cytology is low, ranging from 0% with a single sampling to 64% with serial samplings.14 CT-guided fine needle aspiration (FNA) is limited by small sample size, which decreases the sensitivity and is associated with increased risk of pneumothorax (9.5%) and tumor seeding the needle track (21%).15 Video-assisted thoracoscopy has a diagnostic accuracy of 98% in experienced hands and allows for the possibility of simultaneous pleurodesis. Radiation therapy to the entry port is often recommended 10 to 12 days after the procedure to prevent tumor seeding. Other diagnostic procedures include esophageal ultrasound (EUS), mediastinoscopy, and laparoscopy, which are used more for staging purposes.
STAGING
Several staging systems for mesothelioma have been used over the years, almost exclusively dealing with primary pleural mesothelioma. Peritoneal mesothelioma does not have its own staging system. The oldest staging system used for pleural mesothelioma is the Butchart system; it is still commonly used in some parts of the world. The Butchart system is based on a simple description of the extent of the disease regardless of histologic subtype: pleural contained (Stage I), chest wall or mediastinal invasion (Stage II), peritoneal or diaphragmatic penetration (Stage III), or distant metastases (Stage IV).
Meanwhile, the Brigham system attempts to define surgical resectability and lymph node involvement. Stage I disease is resectable without nodal spread; Stage II is resectable with lymph node involvement; Stage III involves the chest wall, heart, diaphragm, or abdominal cavity, with or without lymph node involvement. Stage III tumors are considered unresectable. Stage IV disease is distant metastases. This system is not utilized at present.
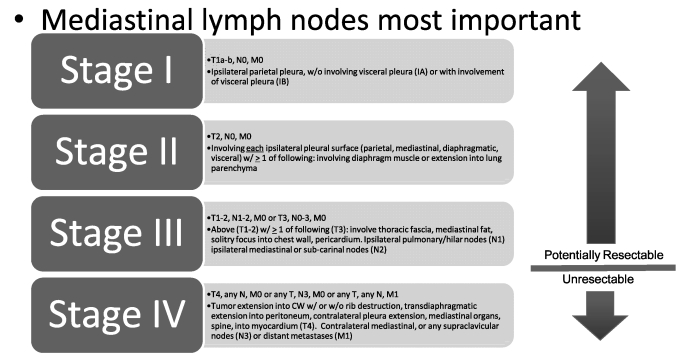
The most practical and most commonly used system is the tumor-node-metastasis system developed by the International Mesothelioma Interest Group (Figure 2). This system is the currently accepted standard adopted by the American Joint Committee on Cancer.16 Most patients present with advanced disease and are considered unresectable. The 2009 Louisiana Tumor Registry reported 57 cases of mesothelioma statewide, with 24 having stage III or IV disease and another 14 with unknown stage. In New Orleans, 75% of the reported cases for 2009 were stage III or IV disease.3
Figure 2.
Tumor-node-metastasis staging for mesothelioma.
(Adapted from Edge S, Byrd DR, Compton CC, Fritz AG, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer Publications; 2010.16)
STANDARD MANAGEMENT
Once a diagnosis of pleural mesothelioma is confirmed, a thorough staging work-up should be undertaken to determine if a patient is amenable to surgical resection. This work-up includes not only imaging and surgical staging as mentioned above, but a complete assessment of comorbidities, cardiac status, and pulmonary function testing.
Surgery is recommended for patients with clinical stage I disease who are considered medically fit and can tolerate the surgery. Patients who are not operable because of comorbidity or impaired cardiopulmonary function can be observed (an appropriate option in the very elderly, those with poor performance status and significant comorbidity, etc) or treated with chemotherapy. Patients with stage II-III disease should be offered trimodality therapy with surgery, chemotherapy, and radiotherapy, while chemotherapy alone is recommended for patients who are not medically fit for surgery, have stage IV disease, and/or show sarcomatoid histology.
Pleural effusions, considered stage IV disease and therefore unresectable in non–small cell lung cancer, are not absolute contraindications to surgery and to aggressive trimodality therapy in mesothelioma patients who are otherwise fit for such therapy. Effusions can be managed with either talc pleurodesis (thoracoscopic drainage of the fluid followed by instillation of a sterile talc slurry) or by placement of a pleural catheter for continuous drainage.11,17-19
Surgery
For patients considered fit for surgery, the standard procedure for many years has been an extrapleural pneumonectomy (EPP): a radical excision of the entire lung, both visceral and parietal pleura, pericardium, and diaphragm with synthetic reconstruction. Methodical dissection of intra- and extrapleural lymph nodes is important. Sugarbaker et al20 described the outcome in a large series of 183 patients treated with EPP: Perioperative deaths were 3.8% with a median and 5-year survival of 19 months and 15%, respectively. Patients with epitheloid histology, lack of extrapleural nodal involvement, and negative resection margins fared better, with median and 5-year survival of 51 months and 46%, respectively. The poor outcome in patients with extrapleural nodal involvement underscores the importance of accurate preoperative staging with PET, EUS-guided FNA, and/or mediastinoscopy.
Other studies addressing EPP have shown median survival rates ranging from 10-24 months.21-24 Rusch and Venkatraman21 compared EPP in a nonrandomized manner with pleurectomy. The median survival for pleurectomy patients was 18 months compared to 10 months for EPP patients; however, the patients treated with pleurectomy tended to be in an earlier stage. Sites of relapse were more often local after a pleurectomy, whereas patients treated with EPP were more likely to have distant relapse in the contralateral lung or the abdominal cavity.
In some centers, the use of intracavitary chemotherapy, usually cisplatin, is favored. Concentrations 3 to 5 times those of systemic administration can be achieved. Most experience with this approach has been with intraperitoneal (IP) administration. Response rates with IP have been around 23% compared to 12% for intrapleural administration.25 Nephrotoxicity is still a concern with intracavitary administration, which requires hydration and close monitoring of renal function.
Standards of care recommend that patients diagnosed with mesothelioma undergo a multidisciplinary evaluation and work-up with careful preoperative evaluation to determine nodal and distant metastatic disease. Patients who are ultimately considered for surgery should have a good performance status, minimal comorbidities, epithelioid histology, and stage I or perhaps stage II (without nodal involvement) disease.26 Patients with sarcomatoid histology, biphasic histologies, or extrapleural nodal involvement (stage III-IV) have poor outcomes and should probably not be offered radical surgery. No randomized trial has yet shown a survival advantage with EPP. This question is being addressed in the Mesothelioma and Radical Surgery feasibility trial that randomized patients to EPP or observation after they received 3 cycles of platinum-based chemotherapy.27 The study closed after 50 patients were accrued over a 3-year period and results are pending, but criticism is already mounting that the study will have too small a power to detect any significant difference.
Lung-sparing cytoreductive surgery offers a more conservative approach than EPP and is advocated by some, especially when combined with chemotherapy and radiation (trimodality therapy). Lung-sparing approaches typically include a pleurectomy, removal of the parietal pleural layer, and decortication. In a large surgical series, Flores et al28 reported a median survival of 15.8 months in 176 patients treated with pleurectomy and decortication. Teh et al29 conducted a systematic review of lung-sparing extirpative surgery in mesothelioma patients, analyzing results on 1,270 patients from 26 studies. The average survival at 1, 2, 3, 4, and 5 years was 51%, 26%, 16%, 11%, and 9%, respectively. Variables included the use of adjuvant chemotherapy or radiation therapy and even surgical approaches within specific trials. The authors acknowledged that lacking any controlled trials, no firm conclusions could be drawn regarding lung-sparing surgery versus more radical approaches. Nonetheless, in the modern era of more common use of adjuvant and neoadjuvant chemotherapy, a more conservative approach to surgery warrants consideration.
Photodynamic therapy is a light-based treatment that utilizes a porphyrin-based compound that reacts in the presence of visible light to cause direct cellular destruction and to initiate a series of apoptotic events. Photodynamic therapy has been approved in some malignancies but remains experimental for malignant pleural mesothelioma. Trials have assessed its use in eradicating microscopic residual disease after macroscopic complete resection by delivering an intrathoracic cavity treatment.30
Palliative surgery remains a viable option for patients who are not fit for a more aggressive radical EPP or who have advanced disease. Talc pleurodesis via an indwelling chest tube provides symptomatic relief of dyspnea associated with a malignant pleural effusion. A thoracoscopically applied talc poudrage is usually more effective and durable and, with the use of a video-assisted thoracoscope, can also allow the surgeon to perform a cytoreductive procedure. As a comfort measure, a permanent tunneled chest drain can be placed, allowing the patient or caregivers the ability to periodically drain fluid.26
Radiotherapy
Radiotherapy is potentially helpful for reducing chest wall masses or alleviating pain, but these responses are transient; radiotherapy has seldom demonstrated significant response as the primary modality for intrathoracic disease and has not been shown to improve survival.31 Intensity-modulated radiotherapy (IMRT) is a sophisticated modality that uses small radiation beams at various angles in a 3-dimensional conformal pattern, allowing for more intense radiation at the target with greater precision. It is often used after EPP because the ipsilateral hemithorax is a common site of relapse. An M.D. Anderson study of 28 patients treated with EPP followed by IMRT found that local control was 100% at 9 months' follow-up.32
Recommending radiotherapy, usually IMRT, after an EPP has been standard since 2003, as has been suggesting radiotherapy within 2 weeks of any pleural biopsy or tube drainage procedure to prevent possible seeding. Whether such treatment is truly effective or has any impact on survival is controversial.
Chemotherapy
For many years, chemotherapy treatment of mesothelioma was disappointing, partly because of the relative chemoresistance of mesothelioma and the lack of active agents with acceptable toxicity. Older phase I and II trials were fraught with small numbers. Studies of single agents such as anthracyclines, antimetabolites, and platinum analogs showed response rates around 10%.33 Researchers and practitioners were even concerned that chemotherapy did not impart any better outcome than best supportive care (BSC) alone. The United Kingdom's Medical Research Council attempted to establish a benefit for chemotherapy over supportive care in a large phase III trial in previously untreated patients.34 The study used a 3-arm design and randomized patients to BSC with or without 1 of 2 chemotherapy arms: single-agent vinorelbine or the combination of mitomycin, vinblastine, and cisplatin (MVP). The study intended to accrue 840 patients but, because of slow accrual, was closed after only 409 patients enrolled. For statistical analysis, the results of the 2 chemotherapy arms were combined and compared against BSC alone. The median survival for patients in the chemotherapy arms was 8.5 months compared to 7.6 months for BSC, which was not statistically different. A subsequent exploratory analysis of the 2 chemotherapy arms separately showed that those treated with vinorelbine had a median survival of 9.4 months, but patients in the MVP arm had no significant survival advantage.
Vinorelbine was further tested as monotherapy in the salvage or second-line setting. Stebbing et al35 gave weekly vinorelbine to 63 patients with relapsed or refractory mesothelioma and obtained a response rate of 16% and median survival of 9.6 months. With the combination of vinorelbine and cisplatin, the response rate was 30%, median time to progression was 7.2 months, and median survival was 16.8 months.36
Gemcitabine as a single agent demonstrated a response rate of 31% and symptom improvement in 40% in a trial of 23 patients with untreated disease.37 However, this trial was criticized because of its small size and because most of the patients had early-stage disease and favorable epithelial histology. Other trials of gemcitabine monotherapy had response rates of 0%-7% and median survivals of 4.7-8 months.38,39 Trials combining gemcitabine with cisplatin or carboplatin resulted in response rates ranging from 12%-48% with median time to progression ranging from 6-9 months.40
In a meta-analysis of phase II trials conducted between 1965 and 2001, cisplatin was the most active single agent for the treatment of unresectable malignant pleural mesothelioma.41 Cisplatin has served as the backbone of most doublet regimens. In 2003, a phase III randomized trial compared cisplatin alone versus cisplatin plus pemetrexed in untreated malignant pleural mesothelioma.42 With the combination, the response rate was 41.3% compared to 16.7% for cisplatin alone (P < .0001). Median time to progression was 5.7 vs 3.9 months, (P = .001), and median overall survival was 12.1 vs 9.3 months (P = .02), both in favor of the combination arm. As a result, the combination of cisplatin and pemetrexed is considered standard first-line therapy for unresectable malignant pleural mesothelioma. It has also become a standard recommendation in the adjuvant combined modality approach to resectable disease.
Methotrexate, an antifolate agent, was one of the earliest such agents to demonstrate activity in mesothelioma. High-dose therapy with 3 gm/m2 utilizing leucovorin rescue resulted in a 37% response rate and a median survival of 11 months in a small phase II trial of 60 patients.43 However, pemetrexed in combination with cisplatin has largely replaced this regimen.
Despite the improvement shown with the combination of cisplatin and pemetrexed, nearly two-thirds of patients still fail to show a response to this regimen and most patients will progress after first-line therapy and often die within a year of diagnosis. Efforts have been undertaken to find better markers of response to cisplatin and pemetrexed to identify not only patients who would benefit from therapy but, just as important, to exclude those who would not. Also, better second-line agents need to be developed.
The excision repair cross-complementing 1 (ERCC-1) gene, located on chromosome 19, is an essential gene for physiologic repair of damaged DNA adducts. ERCC-1 also repairs DNA strand damage caused by cisplatin and correlates with a favorable prognosis in non–small cell lung cancer.44,45 Thymidylate synthase (TS) is an enzyme targeted by pemetrexed; studies have attempted to correlate TS-mRNA and protein expression levels with response and/or survival to pemetrexed-based therapy in mesothelioma. Zucali et al46 showed a positive correlation between low TS protein expression and disease control, longer progression-free survival, and overall survival in mesothelioma patients treated with carboplatin-pemetrexed; however, the researchers did not find any associations with ERCC-1 protein expression. Righi et al47 showed that TS-mRNA and protein expression are inversely correlated with pemetrexed sensitivity and outcome in non–small cell lung cancer but failed to find a correlation between TS-RNA and patient outcome in mesothelioma, although the mesothelioma specimens used had small numbers of tumor cells. Using these tests is difficult because of the lack of uniform standards for both immunohistochemical and polymerase-chain reaction methods.
Pemetrexed, a useful second-line agent, was shown to be better than BSC in a phase III trial of 243 patients with mesothelioma previously treated with one prior chemotherapy regimen that excluded pemetrexed.48 However, with the combination of pemetrexed and cisplatin as the more common standard first-line regimen, the use of pemetrexed as a second-line treatment is less likely.
NEW APPROACHES—TARGETED THERAPIES
The identification of various growth factors, glycoproteins, genetic mutations, and enzymatic catalases has led to the development of new agents to specifically target these oncogenic abnormalities. Bevacizumab is a humanized monoclonal antibody directed at the vascular endothelial growth factor receptor (VEGFR). It has demonstrated increased survival when combined with chemotherapy in non–small cell lung cancer and metastatic colon cancer and has become a Food and Drug Administration–approved standard of care for those diagnoses.49,50 VEGFR1 and VEGFR2 receptors have been detected in the majority of mesothelioma cases.51-53 In vitro studies showed that VEGF stimulates the growth of mesothelioma cells and that anti-VEGF rabbit polyclonal antibodies inhibit the growth.53 Thus, it seemed reasonable to utilize the anti-VEGF agent bevacizumab in the treatment of malignant pleural mesothelioma in humans.
A randomized phase II trial of untreated mesothelioma patients compared cisplatin-gemcitabine alone or with bevacizumab.54 The addition of bevacizumab did not improve the response rate (25% vs 22%), progression-free survival (6.9 vs 6.0 months), or overall survival (15.6 vs 14.7 months) compared to chemotherapy alone. A number of other agents, mainly small molecule tyrosine kinase inhibitors that target multiple sites including VEGFR, have been tested, with similarly disappointing results.55-57 Most of these trials have been small phase II designs with limited numbers of patients. Agents tested have included vatalanib (targets VEGFR1, VEGFR2, c-kit, platelet-derived growth factor receptor [PDGFR], and c-Fms); sorafenib (VEGFR2, VEGFR3, Raf, PDGFR, and c-kit); and sunitinib (VEGFR1, VEGFR2, VEGFR3, PDGFR, and c-kit). When the latter agent was used as second-line therapy, 3 out of 22 patients responded. Despite limited success with these early phase II trials, ongoing study with antiangiogenic-targeted therapies continues with bevacizumab, vatalabin, cediranib, pazopanib, and others.
Erlotinib and gefitinib are tyrosine kinase inhibitors that target EGFR and have demonstrated activity in non–small cell lung cancers. Erlotinib also has use in pancreatic carcinomas. EGFR expression has been demonstrated by immunohistochemistry in 68%-96% of mesothelioma specimens, especially the epithelioid type.58 Gefitinib has been shown to inhibit the growth of mesothelioma cells in vitro.59 Despite these encouraging in vitro data, phase II trials in patients with untreated mesothelioma using gefitinib or erlotinib have failed to demonstrate significant activity.60-62
Histone deacetylase (HDAC) inhibitors block the enzyme HDAC, which regulates the wrapping and unwrapping of DNA around protein spools called histones. These inhibitors can alter the access of transcription factors and thereby either increase or decrease the expression of various genes. Vorinostat, an oral HDAC inhibitor, has shown activity against mesothelioma in phase I trials.63 A phase III, multicenter trial of vorinostat versus placebo in relapsed or refractory mesothelioma is ongoing, although accrual has been difficult.
RNA exists in tightly wound cohesive molecules. Ribonucleases catalyze these bonds and unravel the RNA, resulting in impaired protein synthesis and cell cycle arrest. Ranpirnase is an amphibian ribonuclease that targets tRNA. A phase II trial with this agent in untreated mesothelioma patients demonstrated a response in 6 of 105 patients.64 A randomized trial of ranpirnase versus single-agent doxorubicin showed no significant difference, although subset analysis of patients with favorable prognoses revealed an improved median survival for ranpirnase: 11.3 vs 9.1 months.65 Ribonucleases are somewhat problematic because they involve multiple subtypes with no one dominant pathway and indiscriminately target any RNA, leading to potentially increased toxicity.
Arginine, a semiessential amino acid in humans, is essential for some cancers, including mesothelioma. Argininosuccinate synthetase catalyzes a rate-limiting step in the synthesis of arginine. Downregulation of this enzyme results in the cell becoming dependent on extracellular sources of arginine, a process known as arginine auxotrophy. Several tumors have demonstrated this dependence, including mesothelioma. In these arginine-dependent cells, the depletion of arginine by pegylated forms of the enzyme arginine deiminase may prove to be a novel anticancer approach.66,67 Phase I/II trials of this process have shown activity in hepatocellular carcinomas and melanomas, 2 tumor types that are known arginine auxotrophs. Similar investigation in malignant pleural mesothelioma is warranted and under consideration (verbal communication with Paolo Rodriguez, Louisiana State University Stanley S. Scott Cancer Center, New Orleans, LA, May 2011).
Tumor necrosis factor-alpha (TNF-α) has been studied for decades for its antitumor activity in a variety of malignancies; however, early enthusiasm has been dampened by severe toxicities associated with its administration. In an effort to create a more targeted approach, investigators have coupled TNF-α with ligand-directed agents. A phase II trial of human TNF-α fused with a cyclic tumor-homing peptide, asparagine-glycine-arginine, was administered to 57 previously treated patients with mesothelioma on an every 3-week schedule in 43 patients and a weekly schedule in 14 patients.68 Toxicity was primarily chills during administration and was no greater in the dose-dense weekly schedule than in the triweekly schedule. The overall disease control rate was 46%, and median progression-free survival was 2.8 months with a median overall survival of 12.1 months, thus warranting the investigators to recommend further investigation of the weekly regimen.
CONCLUSIONS
Mesothelioma remains a relatively uncommon but potentially very lethal disease. Recognition of the disease's association with asbestos has improved risk exposure in the workplace and other environmental areas, but the long latency period means physicians will continue to see cases for years to come. Diagnosis, especially in the early stages, is difficult, and medicine has no satisfactory screening modalities for high-risk populations. Avoidance of tobacco is extremely important to decrease the risk of lung cancer with asbestos exposure.
Treatment—especially with trimodality approaches combining IMRT, cisplatin-pemetrexed chemotherapy, and surgery—has improved and patients are living longer. Unfortunately, relapses and progression are common, and second-line therapies are far from satisfactory. The exact type of surgery that is most effective remains controversial, with more interest in volume-sparing approaches. Intracavitary chemotherapy with surgery is favored by some and disputed by others.
Targeted therapies offer the potential advantages of disease-specific treatment with reduced toxicity but have yet to prove sufficient activity. Continued research for novel agents and ongoing clinical trials is critical.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
Footnotes
The author has no financial or proprietary interest in the subject matter of this article.
REFERENCES
- 1.Teta MJ, Mink PJ, Lau E, Sceurman BK, Foster ED. US mesothelioma patterns 1973-2002: indicators of change and insights into background rates. Eur J Cancer Prev. 2008 Nov;17(6):525–534. doi: 10.1097/CEJ.0b013e3282f0c0a2. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Cancer Statistics Review—Mesothelioma fast stats. http://seer.cancer.gov/faststats/selections.php?series=cancer. Accessed May 27, 2011. [Google Scholar]
- 3.Louisiana Tumor Registry Data, 2009. http://publichealth.lsuhsc.edu/tumorregistry. Accessed March 27, 2011. [Google Scholar]
- 4.Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer. 1980 Dec 15;46(12):2736–2740. doi: 10.1002/1097-0142(19801215)46:12<2736::aid-cncr2820461233>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010 Feb;42(2):133–139. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006 Jul 1;107(1):108–115. doi: 10.1002/cncr.21971. [DOI] [PubMed] [Google Scholar]
- 7.Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005 Sep 21;97(18):1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch M, Land SR, Begovic M, Cecchini R, Wolmark N. An association between postoperative radiotherapy for primary breast cancer in 11 National Surgical Adjuvant Breast and Bowel Project (NSABP) studies and the subsequent appearance of pleural mesothelioma. Am J Clin Oncol. 2007 Jun;30(3):294–296. doi: 10.1097/01.coc.0000256102.40842.78. [DOI] [PubMed] [Google Scholar]
- 9.Takagi A, Hirose A, Nishimura T, et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008 Feb;33(1):105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 10.Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008 Jul;3(7):423–428. doi: 10.1038/nnano.2008.111. Epub 2008 May 20. [DOI] [PubMed] [Google Scholar]
- 11.Flores RM, Akhurst T, Gonen M, Larson SM, Rusch VW. Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2003 Jul;126(1):11–16. doi: 10.1016/s0022-5223(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 12.Yaziji H, Battifora H, Barry TS, et al. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol. 2006 Apr;19(4):514–523. doi: 10.1038/modpathol.3800534. [DOI] [PubMed] [Google Scholar]
- 13.Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009 Aug;133(8):1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 14.Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008 Feb;83(2):235–250. doi: 10.4065/83.2.235. Erratum in: Mayo Clin Proc. 2009 Sep;84(9):847. [DOI] [PubMed] [Google Scholar]
- 15.Boutin C, Rey F, Gouvernet J, et al. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer. 1993 Jul 15;72(2):394–404. doi: 10.1002/1097-0142(19930715)72:2<394::aid-cncr2820720214>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Edge S, Byrd DR, Compton CC, Fritz AG, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer Publications; 2010. [Google Scholar]
- 17.Arapis K, Caliandro R, Stern JB, Girard P, Debrosse D, Gossot D. Thoracoscopic palliative treatment of malignant pleural effusions: results in 273 patients. Surg Endosc. 2006 Jun;20(6):919–923. doi: 10.1007/s00464-005-0534-6. Epub 2006 May 2. [DOI] [PubMed] [Google Scholar]
- 18.Aelony Y, Yao JF. Prolonged survival after talc poudrage for malignant pleural mesothelioma: case series. Respirology. 2005 Nov;10(5):649–655. doi: 10.1111/j.1440-1843.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- 19.Schulze M, Boehle AS, Kurdow R, Dohrmann P, Henne-Bruns D. Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg. 2001 Jun;71(6):1809–1812. doi: 10.1016/s0003-4975(01)02586-3. [DOI] [PubMed] [Google Scholar]
- 20.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999 Jan;117(1):54–63; discussion 63-65. doi: 10.1016/s0022-5223(99)70469-1. [DOI] [PubMed] [Google Scholar]
- 21.Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1996 Apr;111(4):815–825; discussion 825-826. doi: 10.1016/s0022-5223(96)70342-2. [DOI] [PubMed] [Google Scholar]
- 22.Buduhan G, Menon S, Aye R, Louie B, Mehta V, Vallières E. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2009 Sep;88(3):870–875; discussion 876. doi: 10.1016/j.athoracsur.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Trousse DS, Avaro JP, D'Journo XB, et al. Is malignant pleural mesothelioma a surgical disease? A review of 83 consecutive extra-pleural pneumonectomies. Eur J Cardiothorac Surg. 2009 Oct;36(4):759–763. doi: 10.1016/j.ejcts.2009.04.044. Epub 2009 Jun 11. [DOI] [PubMed] [Google Scholar]
- 24.Yan TD, Boyer M, Tin MM, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg. 2009 Sep;138(3):619–624. doi: 10.1016/j.jtcvs.2008.12.045. Epub 2009 Mar 9. [DOI] [PubMed] [Google Scholar]
- 25.Taub RN, Antman KH. Chemotherapy for malignant mesothelioma. Semin Thorac Cardiovasc Surg. 1997 Oct;9(4):361–366. [PubMed] [Google Scholar]
- 26.Rudd RM. Malignant mesothelioma. Br Med Bull. 2010;93:105–123. doi: 10.1093/bmb/ldp047. Epub 2010 Jan 4. [DOI] [PubMed] [Google Scholar]
- 27.Treasure T, Waller D, Tan C, et al. The Mesothelioma and Radical Surgery randomized controlled trial: the MARS feasibility study. J Thorac Oncol. 2009 Oct;4(10):1254–1258. doi: 10.1097/JTO.0b013e3181ae26ae. [DOI] [PubMed] [Google Scholar]
- 28.Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol. 2007 Oct;2(10):957–965. doi: 10.1097/JTO.0b013e31815608d9. [DOI] [PubMed] [Google Scholar]
- 29.Teh E, Fiorentino F, Tan C, Treasure T. A systematic review of lung-sparing extirpative surgery for pleural mesothelioma. J R Soc Med. 2011 Feb;104(2):69–80. doi: 10.1258/jrsm.2010.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedberg JS. Photodynamic therapy as an innovative treatment for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009 Summer;21(2):177–187. doi: 10.1053/j.semtcvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Bissett D, Macbeth FR, Cram I. The role of palliative radiotherapy in malignant mesothelioma. Clin Oncol (R Coll Radiol). 1991 Nov;3(6):315–317. doi: 10.1016/s0936-6555(05)80582-5. [DOI] [PubMed] [Google Scholar]
- 32.Ahamad A, Stevens CW, Smythe WR, et al. Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2003 Mar 1;55(3):768–775. doi: 10.1016/s0360-3016(02)04151-2. [DOI] [PubMed] [Google Scholar]
- 33.Su S. Mesothelioma: path to multimodality treatment. Semin Thorac Cardiovasc Surg. 2009 Summer;21(2):125–131. doi: 10.1053/j.semtcvs.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Muers MF, Stephens RJ, Fisher P, et al. MS01 Trial Management Group. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet. 2008 May 17;371(9625):1685–1694. doi: 10.1016/S0140-6736(08)60727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stebbing J, Powles T, McPherson K, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer. 2009 Jan;63(1):94–97. doi: 10.1016/j.lungcan.2008.04.001. Epub 2008 May 16. [DOI] [PubMed] [Google Scholar]
- 36.Sørensen JB, Frank H, Palshof T. Cisplatin and vinorelbine first-line chemotherapy in non-resectable malignant pleural mesothelioma. Br J Cancer. 2008 Jul 8;99(1):44–50. doi: 10.1038/sj.bjc.6604421. Epub 2008 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bischoff HG, Manegold C, Knopp M, Blatter J, Drings P. Gemcitabine (Gemzar) may reduce tumor load and tumor associated symptoms in malignant pleural mesothelioma. Proc Am Soc Clin Oncol. 1998;17(Abstract 1784):A464. [Google Scholar]
- 38.Kindler HL, Millard F, Herndon JE, 2nd, Vogelzang NJ, Suzuki Y, Green MR. Gemcitabine for malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B. Lung Cancer. 2001 Feb-Mar;31(2-3):311–317. doi: 10.1016/s0169-5002(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 39.van Meerbeeck JP, Baas P, Debruyne C, et al. A Phase II study of gemcitabine in patients with malignant pleural mesothelioma. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. Cancer. 1999 Jun 15;85(12):2577–2582. doi: 10.1002/(sici)1097-0142(19990615)85:12<2577::aid-cncr13>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 40.Jackman DM. Current options for systemic therapy in mesothelioma. Semin Thorac Cardiovasc Surg. 2009 Summer;21(2):154–158. doi: 10.1053/j.semtcvs.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Berghmans T, Paesmans M, Lalami Y, et al. Activity of chemotherapy and immunotherapy on malignant mesothelioma: a systematic review of the literature with meta-analysis. Lung Cancer. 2002 Nov;38(2):111–121. doi: 10.1016/s0169-5002(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 42.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003 Jul 15;21(14):2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 43.Solheim OP, Saeter G, Finnanger AM, Stenwig AE. High-dose methotrexate in the treatment of malignant mesothelioma of the pleura. A phase II study. Br J Cancer. 1992 Jun;65(6):956–960. doi: 10.1038/bjc.1992.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Ding L, Yu JJ, et al. Cisplatin and phorbol ester independently induce ERCC-1 protein in human ovarian carcinoma cells. Int J Oncol. 1998 Nov;13(5):987–992. doi: 10.3892/ijo.13.5.987. [DOI] [PubMed] [Google Scholar]
- 45.Simon GR, Sharma S, Cantor A, Smith P, Bepler G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest. 2005 Mar;127(3):978–983. doi: 10.1378/chest.127.3.978. [DOI] [PubMed] [Google Scholar]
- 46.Zucali PA, Giovannetti E, Destro A, et al. Thymidylate synthase and excision repair cross-complementing group-1 as predictors of responsiveness in mesothelioma patients treated with pemetrexed/carboplatin. Clin Cancer Res. 2011 Apr 15;17(8):2581–2590. doi: 10.1158/1078-0432.CCR-10-2873. Epub 2011 Jan 24. [DOI] [PubMed] [Google Scholar]
- 47.Righi L, Papotti MG, Ceppi P, et al. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol. 2010 Mar 20;28(9):1534–1539. doi: 10.1200/JCO.2009.25.9275. Epub 2010 Feb 22. [DOI] [PubMed] [Google Scholar]
- 48.Jassem J, Ramlau R, Santoro A, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol. 2008 Apr 1;26(10):1698–1704. doi: 10.1200/JCO.2006.09.9887. [DOI] [PubMed] [Google Scholar]
- 49.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 50.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355(24):2542–2550. doi: 10.1056/NEJMoa061884. Erratum in: N Engl J Med. 2007 Jan 18;356(3):318. [DOI] [PubMed] [Google Scholar]
- 51.König J, Tolnay E, Wiethege T, Müller K. Co-expression of vascular endothelial growth factor and its receptor flt-1 in malignant pleural mesothelioma. Respiration. 2000;67(1):36–40. doi: 10.1159/000029460. [DOI] [PubMed] [Google Scholar]
- 52.König JE, Tolnay E, Wiethege T, Müller KM. Expression of vascular endothelial growth factor in diffuse malignant pleural mesothelioma. Virchows Arch. 1999 Jul;435(1):8–12. doi: 10.1007/s004280050388. [DOI] [PubMed] [Google Scholar]
- 53.Strizzi L, Catalano A, Vianale G, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001 Apr;193(4):468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 54.Karrison T, Kindler HL, Gadara R, et al. Final analysis of a multi-center, double-blind, placebo-controlled randomized phase II trial of gemcitabine/cisplatin (GC) plus bevacizumab (B) or placebo (P) in patients (pts) with malignant mesothelioma (MM) J Clin Oncol. 2007 Jun 20;25(Suppl 18S Abstract 7526):391s. doi: 10.1200/JCO.2011.41.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jahan TM, Gu L, Wang X, et al. Vatalanib (V) for patients with previously untreated advanced malignant mesothelioma (MM): a phase II study by the Cancer and Leukemia Group B (CALGB 30107) J Clin Oncol. 2006 Jun 20;(Suppl 18S Abstract 7081):364s. [Google Scholar]
- 56.Janne PA, Wang XF, Krug LM, Hodgson L, Vokes EE, Kindler HL. Sorafenib in malignant mesothelioma (MM): a phase II trial of the Cancer and Leukemia Group B (CALGB 30307) J Clin Oncol. 2007 Jun 20;(Suppl 18S Abstract 7707):45s. [Google Scholar]
- 57.Nowak AK, Millward MJ, Francis R, van der Schaaf A, Musk AW, Byrne MJ. Phase II study of Sunitinib as second-line therapy in malignant pleural mesothelioma (MPM) J Clin Oncol. 2008 May 20;26(Suppl–Abstract 8063):439s. doi: 10.1097/JTO.0b013e31825f22ee. [DOI] [PubMed] [Google Scholar]
- 58.Govindan R, Riter J, Suppiah R. EGFR and HER-2 overexpression in malignant mesothelioma. Proc Am Soc Clin Oncol. 2001;20;(Abstract 3106) [Google Scholar]
- 59.Jänne PA, Taffaro ML, Salgia R, Johnson BE. Inhibition of epidermal growth factor receptor signaling in malignant pleural mesothelioma. Cancer Res. 2002 Sep 15;62(18):5242–5247. [PubMed] [Google Scholar]
- 60.Govindan R, Kratzke RA, Herndon JE, 2nd, et al. Cancer and Leukemia Group B (CALGB 30101) Gefitinib in patients with malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res. 2005 Mar 15;11(6):2300–2304. doi: 10.1158/1078-0432.CCR-04-1940. [DOI] [PubMed] [Google Scholar]
- 61.Garland LL, Rankin C, Gandara DR, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol. 2007 Jun 10;25(17):2406–2413. doi: 10.1200/JCO.2006.09.7634. [DOI] [PubMed] [Google Scholar]
- 62.Jackman DM, Kindler HL, Yeap BY, et al. Erlotinib plus bevacizumab in previously treated patients with malignant pleural mesothelioma. Cancer. 2008 Aug 15;113(4):808–814. doi: 10.1002/cncr.23617. [DOI] [PubMed] [Google Scholar]
- 63.Krug LM, Curley T, Schwartz L, et al. Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer. 2006 Jan;7(4):257–261. doi: 10.3816/CLC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 64.Mikulski SM, Costanzi JJ, Vogelzang NJ, et al. Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J Clin Oncol. 2002 Jan 1;20(1):274–281. doi: 10.1200/JCO.2002.20.1.274. [DOI] [PubMed] [Google Scholar]
- 65.Vogelzang N, Taub R, Shin D, et al. Phase III randomized trial of onconase (ONC) vs. doxorubicin (DOX) in patients (Pts) with unresectable malignant mesothelioma (UMM): Analysis of survival. Proc Am Soc Clin Oncol. 2000;19(Abstract 2274):A577. [Google Scholar]
- 66.Delage B, Fennell DA, Nicholson L, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010 Jun 15;126(12):2762–2772. doi: 10.1002/ijc.25202. [DOI] [PubMed] [Google Scholar]
- 67.Feun L, Savaraj N. Pegylated arginine deiminase: a novel anticancer enzyme agent. Expert Opin Investig Drugs. 2006 Jul;15(7):815–822. doi: 10.1517/13543784.15.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gregorc V, Zucali PA, Santoro A, et al. Phase II study of asparagine-glycine-arginine-human tumor necrosis factor alpha, a selective vascular targeting agent, in previously treated patients with malignant pleural mesothelioma. J Clin Oncol. 2010 May 20;28(15):2604–2611. doi: 10.1200/JCO.2009.27.3649. Epub 2010 Apr 20. [DOI] [PubMed] [Google Scholar]