Abstract
Several mechanisms have evolved to ensure the survival of cells under adverse conditions. The heat shock response is one such evolutionarily conserved survival mechanism. Heat shock factor-1 (HSF1) is a transcriptional regulator of the heat shock response. By the very nature of its prosurvival function, HSF1 may contribute to the pathogenesis of cancer. The current study investigates the role of HSF1 in the pathogenesis of pancreatobiliary tumors. HSF1 was downregulated in pancreatic cancer (MIA PaCa-2 and S2-013) and cholangiocarcinoma (KMBC and KMCH) cell lines by HSF1-specific small interfering RNA (siRNA). Nonsilencing siRNA was used as control. The effect of HSF1 downregulation on viability and apoptosis parameters, i.e., annexin V, terminal deoxynucleotidyl transferase dUTP-mediated nick end labeling (TUNEL), and caspase-3, was measured. To evaluate the cancer-specific effects of HSF1, the effect of HSF1 downregulation on normal human pancreatic ductal cells was also evaluated. HSF1 is abundantly expressed in human pancreatobiliary cancer cell lines, as well as in pancreatic cancer tissue, as demonstrated by Western blot and immunohistochemistry, respectively. Inhibition of HSF1 expression by the HSF1 siRNA sequences leads to time-dependent death in pancreatic and cholangiocarcinoma cell lines. Downregulation of HSF1 expression induces annexin V and TUNEL positivity and caspase-3 activation, suggesting activation of a caspase-dependent apoptotic pathway. Although caspase-3 inhibition protects against cell death induced by HSF1 expression, it does not completely prevent it, suggesting a role for caspase-independent cell death. HSF1 plays a prosurvival role in the pathogenesis of pancreatobiliary tumors. Modulation of HSF1 activity could therefore emerge as a novel therapeutic strategy for cancer treatment.
Keywords: heat shock protein 70, cancer, apoptosis
several survival mechanisms have become evolutionarily ingrained in the genome. The heat shock response is one of the most ancient and evolutionarily conserved protective mechanisms found in nature. It is initiated when the organism is exposed to a variety of harmful conditions, including, but not limited to, heat shock, alcohols, inhibitors of energy metabolism, heavy metals, oxidative stress, fever, and inflammation (12). The heat shock response leads to synthesis of a multitude of proteins, which then protect the cells from injurious stimuli. Dysregulation of the heat shock response has been associated with health and disease (5, 11, 18). Given its prosurvival nature, it is highly plausible that the heat shock response may play a role in carcinogenesis and protect cancer cells by inhibiting cell death in response to a variety of stresses. We, among others, have shown that heat shock proteins (HSPs), which are the prototypical products of the heat shock response, are upregulated in cancer, suggesting activation of the heat shock response in cancer cells (1, 5, 6, 14–17).
Although multiple heat shock factors (HSFs) exist, HSF1 is the dominant regulator of the heat shock response in mammals (11). The heat shock response has been classically associated with increased expression of HSPs. Recent studies in yeast have suggested that HSF1 can regulate as much as 3% of the genome and cellular processes, ranging from energy production to signal transduction, vesicular transport, and carbohydrate metabolism (9). This suggests that the expression of HSPs may just be one of the events regulated by HSF1 and that the effect of HSF1-regulated heat shock response may be far more profound than hitherto believed. In the present study, we explore HSF1 as a novel therapeutic target for the treatment of pancreatobiliary tumors. Even though we have evaluated the role of HSF1 in only two cancers, pancreatic cancer and cholangiocarcinoma, we believe that the findings will be generalizable to cancer biology as a whole.
MATERIALS AND METHODS
HSF1 small interfering RNAs (siRNAs), nonsilencing siRNA, and HSF1 and 18S primers for quantitative PCR analysis of mRNA expression were obtained from Qiagen (Valencia, CA); Lipofectamine 2000 reagent, Opti-MEM I, MitoTracker Red, DMEM, and RPMI 1640 tissue culture media from Invitrogen (Carlsbad, CA); the WST-8 viability assay from Dojindo Molecular Technologies (Gaithersburg, MD); the Guava Nexin apoptosis kit from Guava Technologies (Hayward, CA); the Caspase-Glo 3/7 assay kit from Promega (San Luis Obispo, CA); the terminal deoxynucleotidyl transferase dUTP-mediated nick end labeling (TUNEL) assay from Roche Applied Science (Indianapolis, IN); the bicinchoninic acid protein assay kit from Pierce (Rockford, IL); HSF1 and HSP70 antibodies from Assay Designs (Ann Arbor, MI); antibody to HSF1 phosphorylated at Ser326 (pSer326HSF1) from Abcam (Cambridge, MA); and actin antibody from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were obtained from Sigma Aldrich (St. Louis, MO). Pancreatic cancer cells (MIA PaCa-2 and Panc-1) were obtained from American Type Culture Collection. Cholangiocarcinoma cell lines (KMBC and KMCH) were a kind gift from Dr. Gregory Gores (Mayo Clinic, Rochester, MN), pancreatic cancer cells (S2-013) were a kind gift from Dr. Masato Yamamoto (University of Minnesota, Minneapolis, MN), and human pancreatic ductal cells were a kind gift from Dr. Craig Logsdon (7) (MD Anderson Cancer Center, Houston, TX).
Cell culture.
MIA PaCa-2, KMBC, and KMCH cells were cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin. S2-013 cells were cultured in RPMI 1640 with 10% FBS and 1% penicillin-streptomycin. All cells were maintained at 37°C in a humidified air atmosphere with 5% CO2.
Transfection with siRNA.
HSF1 expression was inhibited using HSF1 siRNA. Two different siRNA sequences, HSF1 siRNA #1 (catalog no. SI03246348, Qiagen) and HSF1 siRNA #2 (catalog no. SI02656052, Qiagen), were used to rule out any off-target effects. A nonsilencing siRNA sequence (catalog no. 1027280, Qiagen) bearing no homology to any human gene was used as an appropriate control. Cells treated with the transfection reagent Lipofectamine 2000 without siRNA were used as an additional control. Caspase-3 siRNA was also obtained from Qiagen (catalog no. SI02654603). For transfection, 6 × 104 cells/well in a six-well plate or 1 × 103 cells/well in a 96-well plate were treated with siRNA complexed to Lipofectamine 2000 at a final concentration of 25–100 nM (based on cell line) in Opti-MEM I medium for 5 h. The final concentration of Lipofectamine 2000 was 2–4.5 μg/ml (based on cell line). After 5 h, the medium was replaced with DMEM with 10% FCS but no penicillin-streptomycin, and this time point was used as the start time for duration-of-treatment calculations.
Western blotting and quantitative PCR.
HSF1 and HSP70 proteins and mRNA levels were evaluated in cancer cell lysates by Western blotting and quantitative PCR, as described by us previously (17).
Determination of cell viability.
Cell viability was determined with the Dojindo Cell Counting Kit-8. After treatment with siRNA for the appropriate duration (24–120 h), cell viability was measured by incubation with 10 μl of the tetrazolium substrate for 1 h at 37°C; absorbance at 450 nm was measured.
TUNEL.
TUNEL assay for measurement of the late stage of apoptosis was carried out as described by us previously (17).
Measurement of annexin V-positive cells.
Phosphatidylserine externalization (a marker of early apoptosis) was analyzed using the Guava Nexin kit. After the appropriate duration of treatment with HSF1 siRNA, cancer cells were stained with the reagents according to the manufacturer's protocol and then analyzed (2,000 events) on a flow cytometer (Guava PCA).
Immunohistochemistry.
For immunohistochemistry, paraffin tissue sections were received mounted on charged slides. The slides were deparaffinized in xylene and hydrated through graded ethanols. Slides were steamed with a Reveal Decloaker (Biocare Medical, Concord, CA) to minimize background staining. Sniper Universal Blocking Sera (Biocare Medical) were used throughout the protocol. The slides were stained using an antibody against HSF1 (rabbit polyclonal, Stressgen, Ann Arbor, MI). A Vectastain biotinylated horse universal secondary antibody (Vector Laboratories, Burlingame, CA) was used after the primary antibody, followed by a Vectastain avidin-biotin protocol (ABC kit, Vector Laboratories), which was prepared according to the manufacturer's instructions. A diaminobenzidine peroxidase substrate kit (Vector Laboratories) was used to reveal staining for HSF1. The tissue sections were counterstained with Gill's hematoxylin (Vector Laboratories). The primary antibody was omitted for the negative controls.
Caspase-3 activity assays.
Caspase-3 activity was measured by the Caspase-Glo 3/7 assay according to the manufacturer's protocol.
Statistical analysis.
Values are means ± SE. All experiments with cells were repeated at least three times. The significance of the difference between the control and each experimental test condition was analyzed by unpaired Student's t-test. P < 0.05 was considered statistically significant.
Institutional review board approval.
The pancreatic cancer specimens used for immunohistochemistry were devoid of patient identifier, and appropriate permission for their use was obtained from the Institutional Review Board at the University of Minnesota.
RESULTS
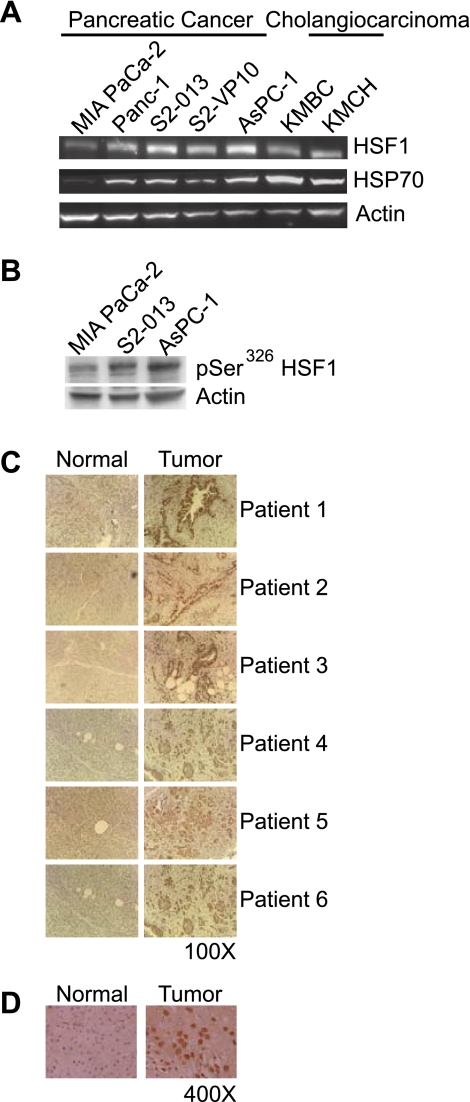
To evaluate the role of HSF1 in the pathogenesis of pancreatic cancer and cholangiocarcinoma, we compared the level of HSF1 in various pancreatic cancer and bile duct cancer cell lines. MIA PaCa-2 and Panc-1 are primary pancreatic cancer cell lines, i.e., originally derived from patients with localized primary tumor, and, thus, believed to be less aggressive than AsPC-1, S2-013, and S2-VP10 cells, which are derived from metastatic tumors and malignant ascites. As seen in Fig. 1A, the level of HSF1 is markedly higher in the more aggressive cell lines, AsPC-1, S2-013, and S2-VP10, than in the less aggressive cell lines, MIA PaCa-2 and Panc-1. Actin was used as the loading control. Next, we wanted to evaluate whether HSF1 in these cancer cell lines is in the active form. Since HSP70 is one of the products of HSF1 activation, we used HSP70 levels in the pancreatic cancer and bile duct cancer cell lines as a marker of HSF1 activation. As seen in Fig. 1A, HSP70 is expressed in all the cancer cell lines, suggesting that HSF1 is present in the active form. We further evaluated HSF1 activation in pancreatic cancer cells by comparing the level of HSF1 phosphorylated at serine 326 (pSer326HSF1). As seen in Fig. 1B, all the pancreatic cancer cell lines tested express pSer326HSF1, suggesting that HSF1 is present in the active form. Furthermore, pSer326HSF1 levels are markedly higher in the aggressive cell lines, such as S2-013 and AsPC-1, than in the less aggressive cell line, MIA PaCa-2.
Fig. 1.
Heat shock factor 1 (HSF1) is expressed and activated in pancreatobiliary tumor cell lines and human pancreatic cancer tissue. A: representative Western blot demonstrating levels of HSF1 and heat shock protein (HSP) 70 (a transcriptional product of HSF1) in pancreatobiliary tumor cell lines. Actin was used as loading control. B: levels of HSF1 phosphorylated at serine 326 (pSer326HSF1) are higher in the more aggressive pancreatic cancer cell lines S2-013 and AsPC-1 than the less aggressive cell line MIA PaCa-2. C: HSF1 expression in pancreatic cancer tissue of 6 pancreatic cancer patients was measured by immunohistochemistry and compared with normal pancreatic tissue at the margin. Expression of HSF1 was observed to be markedly higher in pancreatic cancer tissue than normal pancreatic tissue from the margin. Negative control (no primary antibody) did not demonstrate staining (data not shown), underscoring specificity of immunostaining. D: higher-magnification images of normal and pancreatic cancer tissue from a representative patient. HSF1 staining is most prominent in the nucleus.
To correlate the data obtained from cell lines with human pancreatic cancer tissue data, we evaluated HSF1 expression in pancreatic cancer tissue obtained from 12 patients by immunohistochemistry and compared it with HSF1 expression in normal pancreas tissue at the margin. Figure 1C shows immunohistochemistry data from six representative patients; all the human pancreatic cancer tissue specimens demonstrate marked expression of HSF1 compared with normal pancreatic tissue from the margin. HSF1 in the cancer cells appears to be localized primarily in the nucleus of the cancer cells (Fig. 1D). These findings from human pancreatic tissue samples, which confirm data from cell lines, emphasize the relevance of information obtained from cell lines.
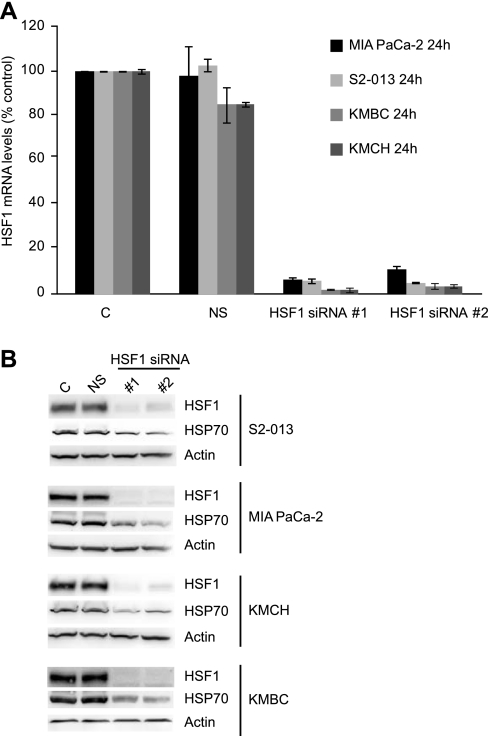
We next evaluated whether HSF1 expression is important for survival of pancreatobiliary cancer cells by evaluating the effects of HSF1 downregulation in cancer cell lines. We used HSF1-specific siRNA to decrease HSF1 expression in pancreatic cancer and cholangiocarcinoma cell lines. Two different sequences of HSF1 siRNA were used to rule out any off-target effects. Cells treated with nonsilencing siRNA (with no homology to any human gene) were used as controls. As seen in Fig. 2, both HSF1 siRNA sequences lead to specific downregulation of HSF1 mRNA (Fig. 2A) and HSF1 protein levels (Fig. 2B) in pancreatic and cholangiocarcinoma cells, whereas nonsilencing siRNA does not influence HSF1 levels. Furthermore, a decrease of HSF1 protein levels is associated with a decrease of HSP70 levels (Fig. 2B), suggesting downregulation of processes downstream of HSF1.
Fig. 2.
Downregulation of HSF1 by HSF1-specific small interfering RNA (siRNA) sequences. Transfection of pancreatobiliary cancer cells lines with 2 different specific siRNA sequences for HSF1 (HSF siRNA #1 and #2) led to a marked decrease in HSF1 mRNA levels at 24 h (A) and HSF1 protein levels at 48 h (B). Nonsilencing siRNA did not influence HSF1 levels, suggesting specificity of the effect. Also, inhibition of HSF1 expression by siRNA is associated with reduced levels of HSP70 protein (B), one of the many downstream proteins regulated by HSF1, suggesting that inhibition of HSF1 expression is influencing downstream pathways. C, control; NS, nonsilencing.
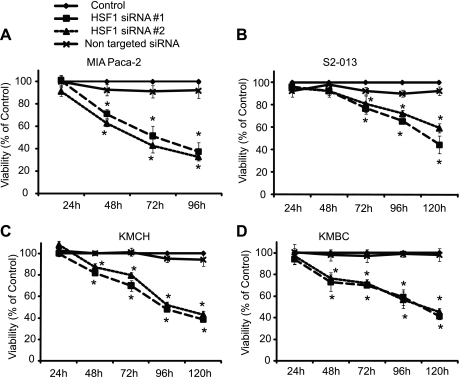
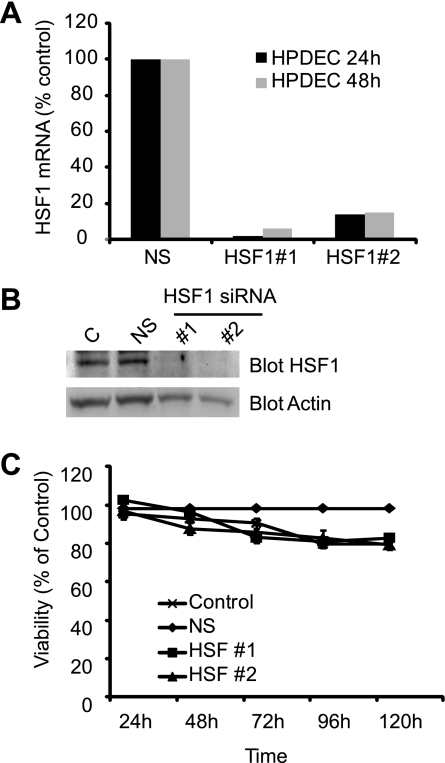
We next evaluated the effect of inhibition of HSF1 expression on the viability of pancreatobiliary cancer cell lines and compared it with cells treated with transfection reagent or nonsilencing siRNA only. As seen in Fig. 3, inhibition of HSF1 expression by both siRNA sequences leads to a decrease in viability in pancreatic cancer and cholangiocarcinoma cells, whereas nonsilencing siRNA does not influence cancer cell viability significantly. Cell death was observed at 48–72 h and continued in a time-dependent fashion. To evaluate whether the effect of HSF1 downregulation on decreasing viability is specific to cancer cells or whether it is observed in normal cells also, we evaluated the effect of HSF1 downregulation on the viability of immortalized pancreatic ductal cells. As seen in Fig. 4, HSF1-specific siRNAs lead to a marked reduction in HSF1 mRNA (Fig. 4A) and protein (Fig. 4B) levels at 48 h in human immortalized pancreatic ductal cells, whereas nonsilencing siRNA does not affect HSF1 levels. Interestingly, inhibition of HSF1 expression by HSF1-specific siRNA does not significantly decrease the viability of immortalized pancreatic ductal cells (Fig. 4C), suggesting that downregulation of HSF1 can emerge as cancer-specific therapy and may have minimal, if any, effect on normal cells.
Fig. 3.
Inhibition of HSF1 expression induces cell death in pancreatobiliary tumor cell lines. HSF1 expression was inhibited by HSF1 siRNA #1 and #2. Cells treated with transfection medium alone (Lipofectamine 2000) were used as controls. Cells transfected with nonsilencing siRNA were used as additional controls. Inhibition of HSF1 expression by both siRNA sequences leads to a time-dependent decrease in viability of the pancreatic cancer cell lines MIA PaCa-2 (A) and S2-013 (B). Similar results were observed in the cholangiocarcinoma cell lines KMCH (C) and KMBC (D). Values are means ± SE; n ≥ 4. *P < 0.05 vs. transfection medium-only (control).
Fig. 4.
HSF1 downregulation does not influence viability of immortalized human pancreatic ductal cells. Transfection with HSF1 siRNA #1 and #2 leads to a decrease in HSF1 mRNA (A) and protein (B) expression in immortalized human pancreatic ductal cells, whereas nonsilencing siRNA does not have an effect. C: HSF1 downregulation does not significantly influence viability of immortalized human pancreatic ductal cells, thus supporting the cancer-specific prosurvival effects of HSF1.
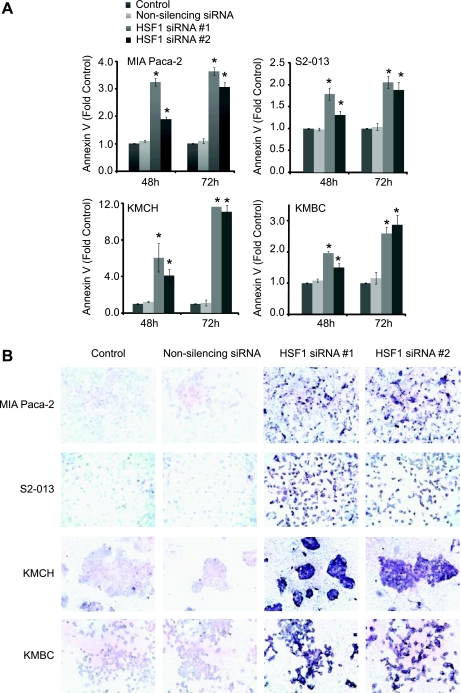
Cell death can occur by multiple mechanisms, such as apoptosis and necrosis. Thus our next step was to determine whether inhibition of HSF1 expression leads to apoptotic cell death in pancreatobiliary tumors. Annexin V (a marker of early apoptosis) and TUNEL (a marker of late apoptosis) were used to evaluate apoptotic cell death. As shown in Fig. 5A, inhibition of HSF1 expression in pancreatobiliary cancer cell lines by both sequences of HSF1 siRNA leads to time-dependent annexin positivity, suggesting apoptotic cell death. Nonsilencing siRNA did not significantly influence apoptosis at any time, thus pointing toward the specificity of the effect observed. We further evaluated the effect of inhibition of HSF1 expression on apoptosis by evaluating TUNEL positivity. As mentioned previously, TUNEL is a marker of late apoptosis. As seen in Fig. 5B, inhibition of HSF1 expression induces TUNEL positivity at 72 h in all pancreatic cancer and cholangiocarcinoma cell lines tested. Similar results were observed with both HSF1 siRNA sequences. Nonsilencing siRNA did not induce apoptosis as measured by TUNEL positivity.
Fig. 5.
Inhibition of HSF1 expression induces apoptosis in pancreatobiliary tumor cell lines. HSF1 expression was inhibited by HSF1 siRNA #1 and #2. A: early-stage apoptosis was measured as annexin V positivity by flow cytometry. Cells treated with transfection medium (Lipofectamine 2000) alone were used as controls. Cells transfected with nonsilencing siRNA were used as additional controls. Inhibition of HSF1 expression by both siRNA sequences leads to a time-dependent increase in annexin V positivity, suggesting apoptotic cell death in the pancreatic cancer cell lines MIA PaCa-2 and S2-013. Similar results were observed in the cholangiocarcinoma cell lines KMCH and KMBC. Values are means ± SE; n ≥ 4. *P < 0.05 vs. transfection medium-only (control). B: HSF1 downregulation leads to marked terminal deoxynucleotidyl transferase dUTP-mediated nick end labeling (TUNEL) positivity, suggesting apoptotic cell death. Data are from a representative experiment. TUNEL was measured by immunohistochemistry. Sites of TUNEL positivity stain purple.
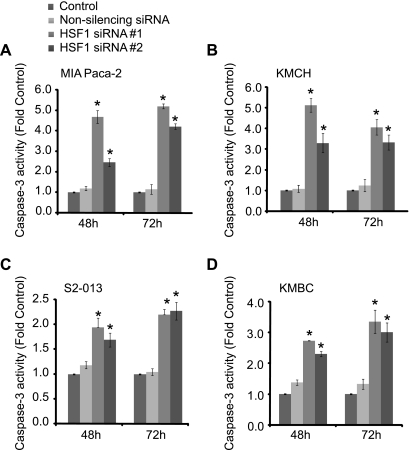
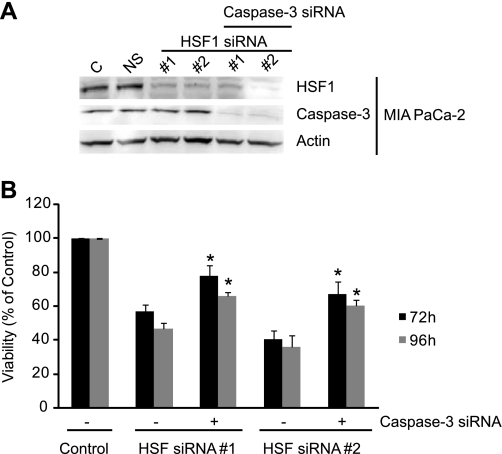
Apoptosis can occur via a caspase-dependent or -independent mechanism. To determine whether caspases are activated during apoptosis induced by HSF1, we measured caspase-3 activation by a luminescence-based assay. As seen in Fig. 6, inhibition of HSF1 expression by both siRNA sequences leads to caspase-3 activation. Caspase-3 activation is observed at 48 h and increases in a time-dependent fashion. Nonsilencing siRNA did not significantly affect caspase-3 activation. Similar results were observed in cholangiocarcinoma cell lines, where inhibition of HSF1 expression leads to time-dependent activation of caspase-3. To further evaluate the role of caspase-3 in cell death induced by HSF1 downregulation, we silenced caspase-3 and then evaluated the effect of HSF1 downregulation on MIA PaCa-2 viability. As seen in Fig. 7, although silencing caspase-3 protects against HSF1 downregulation-induced cell death, it does not completely prevent cell death, suggesting the involvement of caspase-independent cell death.
Fig. 6.
Inhibition of HSF1 expression induces caspase-3 activation in pancreatobiliary tumor cell lines. HSF1 expression was inhibited by HSF1 siRNA #1 and #2. Caspase-3 activation was measured by a luminescence-based method. Cells treated with transfection medium (Lipofectamine 2000) alone were used as controls. Cells transfected with nonsilencing siRNA were used as additional controls. Inhibition of HSF1 expression by both siRNA sequences leads to a time-dependent increase in caspase-3 activation in the pancreatic cancer cell lines MIA PaCa-2 (A) and S2-013 (C). Similar results were observed in the cholangiocarcinoma cell lines KMCH (B) and KMBC (D). Values are means ± SE; n ≥ 4. *P < 0.05 vs. transfection medium-only (control).
Fig. 7.
HSF1 protects pancreatic cancer cells against caspase-dependent and -independent cell death. A: Western blot showing efficacy and specificity of siRNA against HSF1 and caspase-3 at 48 h postsilencing. HSF1 siRNA #1 and #2 silence HSF1 effectively. Caspase-3 siRNA also silences caspase-3 levels very effectively. Nonsilencing siRNA (NS) does not affect HSF1 or caspase-3 levels. B: HSF1 siRNA #1 and #2 induce cell death in the pancreatic cancer cell line MIA PaCa-2 at 72 or 96 h postsilencing. Downregulation of caspase-3 protects against cell death induced by HSF1 silencing but does not completely prevent it, suggesting that caspase-independent cell death plays a role. Values are means ± SE; n ≥ 4. *P < 0.05 vs. cells treated with HSF1 siRNA only.
DISCUSSION
The heat shock response is an essential, highly conserved and exquisitely regulated cellular response to suboptimal physiological conditions. HSF1, the primary regulator of the heat shock response, is thus inseparably linked to survival (13). We have demonstrated that HSF1 contributes to the survival of pancreatobiliary cancer cells. Thus, in contrast to its beneficial effect in ensuring the continued existence of organisms in the face of adversity, HSF1, by virtue of its prosurvival functions, may contribute to the pathogenesis of cancer. In congruence with its role in the pathogenesis of pancreatobiliary tumors, HSF1 protein is highly expressed in pancreatobiliary cell lines. Furthermore, the levels of pSer326HSF1, the transcriptionally active form of HSF1, as well as HSP70, a protein with HSF1-regulated transcription, are high in pancreatobiliary tumor cell lines, suggesting that HSF1 is not only expressed in cancer cell lines but also that it is transcriptionally active. Our studies in human pancreatic tumor specimens corroborate our cell line data. We have shown that HSF1 is highly expressed in human pancreatic cancer tissue. We previously showed that the level of HSP70 is high in human pancreatic cancer tissue (1). This finding confirms the conclusion from our cell line data that not only is HSF1 highly expressed but also that it is active in human pancreatic cancer tissue (1). We have further observed that the level and activity of HSF1 are higher in more aggressive metastatic cell lines than in less aggressive primary cell lines, suggesting that activation of the heat shock response may contribute to the aggressive phenotype of pancreatic cancer, which arguably is one of the most deadly malignancies known. Previous studies have also suggested that the levels of proteins that are typically considered to be the result of the heat shock response are upregulated in cancer cells compared with their nonmalignant counterparts (1, 5, 8), suggesting that the heat shock response is upregulated in many cancer cell types.
We have demonstrated that silencing HSF1 expression induces cell death in pancreatobiliary cancer cell lines. Furthermore, using multiple markers of apoptosis, i.e., annexin V, TUNEL, and caspase-3 activation, we have shown that inhibition of HSF1 induces cell death by activating apoptosis. Since inhibition of HSF1 expression induces apoptosis in two cancer cell lines of different origin (pancreatic and bile duct), these effects may be applicable to other cancers as well. These data have important therapeutic implications. Pancreatic cancer and cholangiocarcinoma are relatively difficult cancers to treat and are believed to have marked resistance to apoptosis. Given our data, it may be possible to modulate the heat shock response in pancreatobiliary as well as other cancer cells by chemical inhibitors of HSF1 activity and, thus, induce cell death. Additionally, it may be possible to make these otherwise chemoresistant tumor cells susceptible to cell death by conventional chemotherapy. Given the poor effectiveness of the current therapies for these tumors, silencing HSF1 expression or negative modulation of HSF1 activity may open new avenues for treatment of these cancers. Activation of the heat shock response is a multistep process. Heat shock response activation requires the binding of HSF1 to the heat shock element, which is present in the promoter region of the genes activated by HSF1. Upon activation, HSF1 trimerizes, translocates to the nucleus, and undergoes inducible serine phosphorylation, leading to transcriptional activation (2). Once transcriptionally active, HSF1 induces a rapid and coordinated increase in molecular chaperone levels. This multistep process therefore offers multiple sites at which potential inhibitors can intervene.
The anti-HSF1 therapy may be selective to cancer and may not have the deleterious side effects that are common to many other anticancer chemotherapeutic agents. Although our data suggest that HSF1 is important for the survival of pancreatobiliary tumor cells, we have shown that HSF1 downregulation does not influence the viability of normal human pancreatic ductal cells. The literature also suggests that HSF1 may not be important for the survival of nonmalignant cells in an unstressed condition. For example, even though HSF1 is required for thermotolerance, oogenesis, and early larval development, it is dispensable for normal growth and viability in Drosophila (10). HSF1-deficient mouse embryos suffer from defects in placental development and are recovered from crosses in lower numbers than expected by Mendelian segregation (19). However, other than being ∼20% smaller than wild-type mice, HSF1-deficient mice display no overt organ system abnormalities and, in the absence of acute stress, live to late adulthood (19). We have also shown that inhibition of HSP70 expression, one of the products of the heat shock response, selectively kills pancreatic cancer cells without any deleterious effects in normal cells (1, 5, 17). Thus anti-HSF1 therapy may be an opportunity for cancer-targeted therapy. It is worth noting that although downregulation of HSF1 leads to a time-dependent decrease in the viability of pancreatic cancer cells, some cells (40% in MIA PaCa-2 and 50% in S2-013) are still alive 5 days after transfection. We believe that, given time and with continued suppression of HSF1, all cells should eventually die. However, the possibility that these cells are resistant to anti-HSF1 therapy could not be entirely ruled out. Although therapies targeted against HSF1 could emerge as a novel and multifaceted strategy against cancer, given the role of HSF1 in regulating an organism's response to stress, anti-HSF1 therapy might impair an organism's ability to respond to other acute disease processes, such as infection and inflammation. Careful assessment of the risk-to-benefit ratio in preclinical and clinical trials is therefore imperative before broader application of these therapies.
What leads to activation of the heat shock response with increased synthesis of downstream proteins in cancer cells is less clear. The tumor environment is considered to be suboptimal for the growth of normal cells due to hypoxia, acidosis, nutrient deprivation, and host immune reaction, requiring the tumor cells to be equipped with mechanisms to survive such harsh conditions. Activation of the heat shock response with synthesis of prosurvival proteins may be an adaptive response, and HSF1 may be a facilitator. The other possibility is that HSF1 may be an oncogene. Dai et al. (3) showed that enforced overexpression of HSF1 does not alter the proliferation characteristics of cells, disputing the possibility that HSF1 is a oncogene. However, the possibility that HSF1 is a multifaceted oncogene cannot be entirely ruled out.
Given the role of HSF1 in protecting cancer cells from apoptosis, it is of utmost importance to elucidate how HSF1 protects cancer cells from cell death. As discussed previously, HSF1 is a transcription factor traditionally considered to regulate the transcription of HSP70, HSP27, HSP90, and multiple other proteins of the heat shock response (11). HSPs have been shown to protect cancer cells from cell death in the same way they protect normal cells from injurious stimuli. For example, HSP70 has been shown to protect pancreatic cancer cells from apoptotic cell death by stabilization of lysosomes and attenuation of cytosolic calcium levels (4). Thus one mechanism by which HSF1 may contribute to the survival of cancer cells is synthesis of HSPs. However, recent studies have suggested that the effects of HSF1 may be much more profound than merely the regulation of HSP synthesis. In yeast, HSF1 is shown to impact several pathways, including energy production, signal transduction, carbohydrate metabolism, cytoskeletal organization, and vesicular transport, and is proposed to regulate up to 3% of the genome (9). Dai et al. (3) showed that HSF1 may be important for the carcinogenesis induced by loss of p53 or gain of ras. In this study, HSF1 was shown to influence a multitude of processes, including proliferation, survival, protein synthesis, and glucose metabolism. Thus it may be premature to ascribe all the actions of HSF1 to the synthesis of HSPs.
In summary, our data suggest that HSF1 is important for the survival of pancreatobiliary tumors, making the modulation of HSF1 activity an attractive target for anticancer drug development.
GRANTS
This study was supported in part from NIH grants CA-124723 and DK-058694 (A.K. Saluja).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, Lerch MM, Saluja A. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res 67: 616–625, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. Evidence for a multistep pathway of regulation. J Biol Chem 271: 3355–3358, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130: 1005–1018, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, Vickers SM, Saluja AK. Heat shock protein70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology 136: 1772–1782, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dudeja V, Vickers SM, Saluja AK. The role of heat shock proteins in gastrointestinal diseases. Gut 58: 1000–1009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer 51: 613–619, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol 148: 1763–1770, 1996 [PMC free article] [PubMed] [Google Scholar]
- 8. Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle 5: 2592–2601, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol 24: 5249–5256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jedlicka P, Mortin MA, Wu C. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J 16: 2452–2462, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 92: 1564–1572, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science 259: 1409–1410, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem 267: 21987–21990, 1992 [PubMed] [Google Scholar]
- 14. Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann NY Acad Sci 926: 122–125, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jaattela M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci USA 97: 7871–7876, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M, Weller M, Jaattela M. Eradication of glioblastoma and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res 62: 7139–7142, 2002 [PubMed] [Google Scholar]
- 17. Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res 67: 9407–9416, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Saluja A, Dudeja V. Heat shock proteins in pancreatic diseases. J Gastroenterol Hepatol 23 Suppl 1: S42–S45, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J 18: 5943–5952, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]