Abstract
Background and Objective
The pulpal chamber of each tooth contains the vasculature necessary to maintain a viable tooth. A critical need exists to develop an objective, repeatable method to assess pulpal viability. We hypothesized that the existence of blood perfusion within the pulp can be determined with analysis of laser speckle imaging (LSI) patterns generated by transillumination of the tooth.
Study Design/Materials and Methods
We used nine extracted human cuspids and incisors. A Tygon tube was inserted into a channel created within each tooth and Intralipid pumped through the tube in a controlled manner with a syringe infusion pump. We evaluated the feasibility of LSI for flow assessment using both transillumination and epiillumination imaging configurations. With the transillumination geometry, we also assessed the effect of the angle of incidence of the probe laser light on the speckle flow index (SFI) values extracted from the collected speckle images.
Results
Transillumination LSI, and not epiillumination LSI, enables differentiation between the absence and presence of perfusion in an in vitro tooth model. SFI values are insensitive to the relative angle of incidence of the laser light, over a wide range of angles.
Conclusions
Our preliminary in vitro data suggest that transillumination LSI is a promising method to identify the presence of blood flow in the pulpal chamber. Future in vivo evaluation is warranted.
Keywords: blood flow, dentistry, laser Doppler, laser speckle contrast analysis, laser speckle contrast imaging, perfusion
INTRODUCTION
The pulpal chamber of each tooth contains the vasculature necessary to maintain a viable tooth. Trauma or infection typically elicit an inflammatory response, which could either resolve on its own or cause the pulpal tissue to become necrotic, leading to gangrene and abscess formation. The most common methods used to assess pulpal health, are hot-and-cold thermal testing and electrical stimulation of the Aδ nerve fibers [1]. These methods assess the degree of intact neural innervation in the interrogated tooth. However, recent reports demonstrate that blood flow is a far better indicator of pulpal health [2]. The current inability to accurately diagnose and monitor pulpal perfusion, and its response to noxious stimuli or treatment, provides a strong incentive for clinicians to avoid the risk of attempting measures to maintain pulpal vitality by performing devitalization and root canal therapy as the treatment of choice. Disadvantages include a long, costly and arduous procedure, medical contraindications, unsuitability of some teeth, as well as long-term discoloration and risk of tooth fracture.
Research groups have studied the feasibility of various methods to assess the viability of the pulpal chamber. Gazelius et al. [3] demonstrated that laser Doppler flowmetry (LDF) can monitor blood flow in the pulpal cavity of intact enamel and dentin. Their data suggest that LDF can differentiate between healthy and necrotic pulpal tissue. However, problems with LDF include low signal-to-noise ratio [3,4] and the strong dependence of the signal on the probe position [5]. Fried et al. [6] determined that enamel and dentin are strongly forward scattering and minimally absorb infrared light. This seminal observation led to subsequent investigation of various optical methods such as pulse oximetry [7] and photoplethysmography [8–10], to assess pulpal vitality. Due to the difficulty in obtaining reproducible data, these methods are not accepted in the dental clinic. Additionally, each method requires the use of a dental splint, further limiting its widespread use.
A critical need exists to develop an objective, repeatable method to assess pulpal viability. For widespread clinical acceptance, this method ideally involves rapid data collection and does not require use of a dental splint. Based on our laboratory’s prior experience with laser speckle imaging (LSI) to study blood-flow dynamics in preclinical animal models [11,12] and human subjects [13,14], we investigated its efficacy in assessing fluid flow in an in vitro tooth model. We hypothesized that the existence of blood perfusion within the pulp can be determined with analysis of laser speckle patterns generated by transillumination of the tooth. This hypothesis is based on the high scattering anisotropy of dental tissue reported previously [6,9].
MATERIALS AND METHODS
Ex Vivo Tooth Preparation
We used nine extracted human cuspids and incisors. Upper and lower cuspids and incisors were chosen as they are easy to access in the clinical setting. Samples were stored in water with thymol at a temperature of 0–4°C. We used a diamond-wafering blade (Buehler 10 × 0.3 mm2) to detach the tooth crown from its root approximately 2–5 mm below the cement–enamel junction (CEJ). The root canal was enlarged to a 1-mm diameter channel from the apical end up to the CEJ using a Rite Dent V® hand piece equipped with a White® HP4 carbide dental bur. This work was performed in compliance with a protocol approved by the Institutional Review Board at University of California, Irvine, and with the documented, informed consent of the subjects.
LSI Instrument
The LSI instrument consisted of a laser source, a CCD camera, and a macro lens. It is similar to the instrument described in previous publications [13,14]. Briefly, a 12-bit thermoelectrically cooled CCD camera (1,600 × 1,200 pixel resolution, Model 2000R, QImaging, Surrey, Canada) was used to image the raw speckle image remitted from the tooth. A 633-nm HeNe laser (30 mW power) was used to transilluminate each tooth sample. By controlling the magnification and aperture of the external macro lens, we set the minimum resolvable speckle size to be at least the width of two camera pixels [15].
Data Reduction
Each raw speckle image first was converted to a speckle contrast image, then to a speckle flow index (SFI) image, using the methodology described previously [16]. Each set of SFI images was averaged to a single mean SFI image. A region of interest was extracted from each mean SFI image and a single mean SFI value calculated. The selected region included pixels between the top of the root and the CEJ.
Experimental Design
Flow phantom
A Tygon tube was inserted into the channel created within each tooth. The tube had an outer diameter of 1 mm and inner diameter of 0.25 mm. One end of the tube was connected to a 29-gauge insulin syringe. The syringe was mounted onto an infusion pump (Harvard Instruments). The syringe contained 1 ml of a Liposyn solution. The stock solution (20% intravenous fat emulsion) diluted to a 1:20 Liposyn:water volume ratio. The tooth was fixed in space with an optomechanical mount. The buccal surface of the tooth was imaged on to the camera sensor. We used the following average flow speeds: 0, 0.34, 1.7, and 3.4 mm/second.
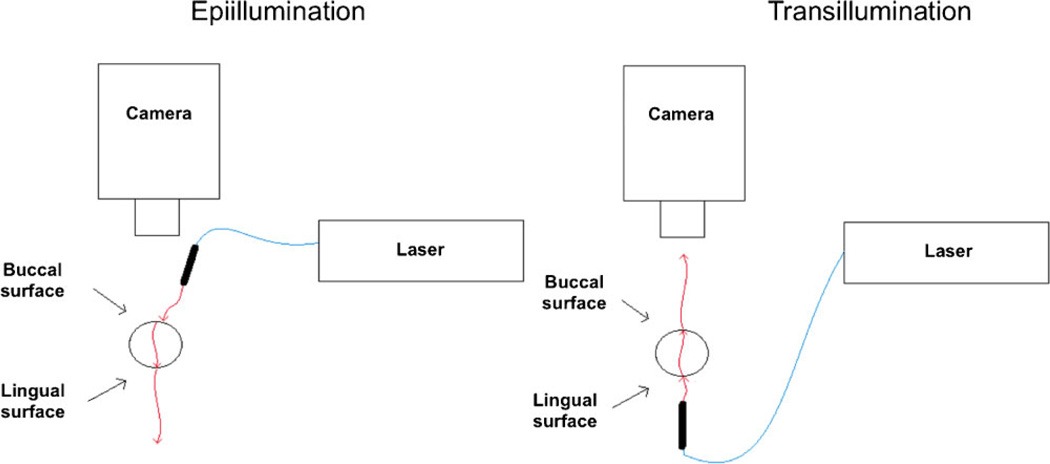
Epiillumination Versus transillumination
We evaluated two imaging configurations. In one set of experiments, we used a transillumination configuration, for which laser light was incident on the lingual side (i.e., the side facing the tongue) and resultant speckle pattern imaged from the buccal side (i.e., the side facing the lips). In a second set of experiments, we used an epiillumination configuration, for which laser light was incident on the buccal side and the pattern imaged from the buccal side. For each flow-speed setting and imaging configuration, a sequence of 10 raw speckle images was collected. The data were reduced as described above, to a single mean SFI value. Three replicates were performed for each flow-speed setting and imaging configuration. For transillumination experiments, data were collected from nine teeth. For epiillumination experiments, data were collected from three teeth. For each imaging configuration, a t-test was used to test the null hypothesis that the SFI values collected at 0 mm/second were similar to those collected at 0.34 mm/second (i.e., the lowest nonzero blood-flow speed used) (Fig. 1).
Fig. 1.
Schematic of experimental setups used to evaluate (left) epiillumination and (right) transillumination LSI. The buccal and lingual sides of the tooth are those that face the cheek and tongue, respectively.
Relative angle of incidence of laser light
For in vivo application of LSI, we postulated that the relative angle of incidence of the laser light on the tooth surface, may differ for each tooth. To determine the effect of a varying angle of incidence, we performed experiments with the transillumination configuration described above. We used four angles of incidence, relative to normal incidence: 0°, 15°, 30°, and 45°. A 360° manual rotational mount was used to control precisely the angle. For each flow speed setting and relative angle of incidence, a sequence of 10 raw speckle images was collected. The data were reduced as described above, to a single mean SFI value. Three replicates were performed for each flow-speed setting and angle of incidence. For each flow speed, a single-factor analysis of variance test was used to test the null hypothesis that the SFI values collected at each angle of incidence were similar.
RESULTS
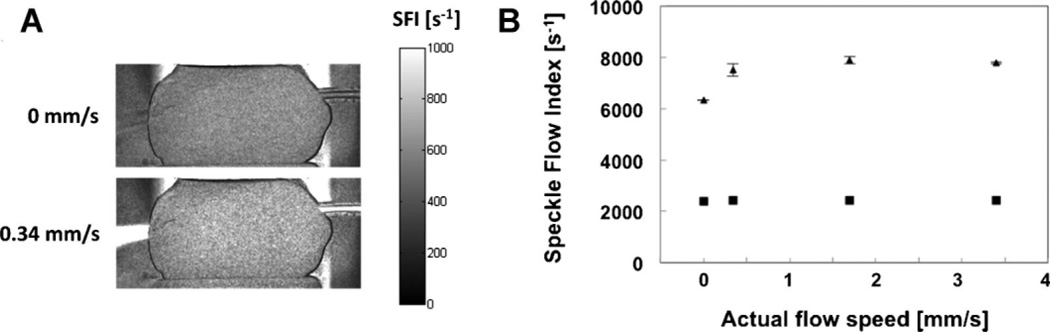
Transillumination LSI, and not epiillumination LSI, enables differentiation between the absence and presence of perfusion in an in vitro tooth model (Fig. 2). With transillumination, an ~70% increase in SFI was observed (Fig. 2A). In general, the mean SFI values were at least three times higher for transillumination LSI than for epiillumination LSI, for all flow speeds. For transillumination LSI experiments, the mean SFI value was significantly lower at 0 mm/second than at 0.34 mm/second (P < 0.05). For epiillumination LSI experiments, the mean SFI value was insensitive to flow speed (P > 0.30).
Fig. 2.
A: With a transillumination LSI configuration, SFI images enabled visualization of a small increase in flow speed. The mean SFI value increased by 70% as the flow speed increased from 0 to 0.34 mm/second. B: With transillumination LSI (triangles), an increase in SFI values was observed as the fluid flow changed from Brownian fluid motion (0 mm/second) to forced fluid flow (≥0.34 mm/second) within the tooth. In contrast, epiillumination LSI (squares) was insensitive to any change in flow speed. For (A), the error bars represent the standard deviation of the mean. For (B), the error bars are not visible due to the low coefficient of variation (< 3.2%) in measured SFI values.
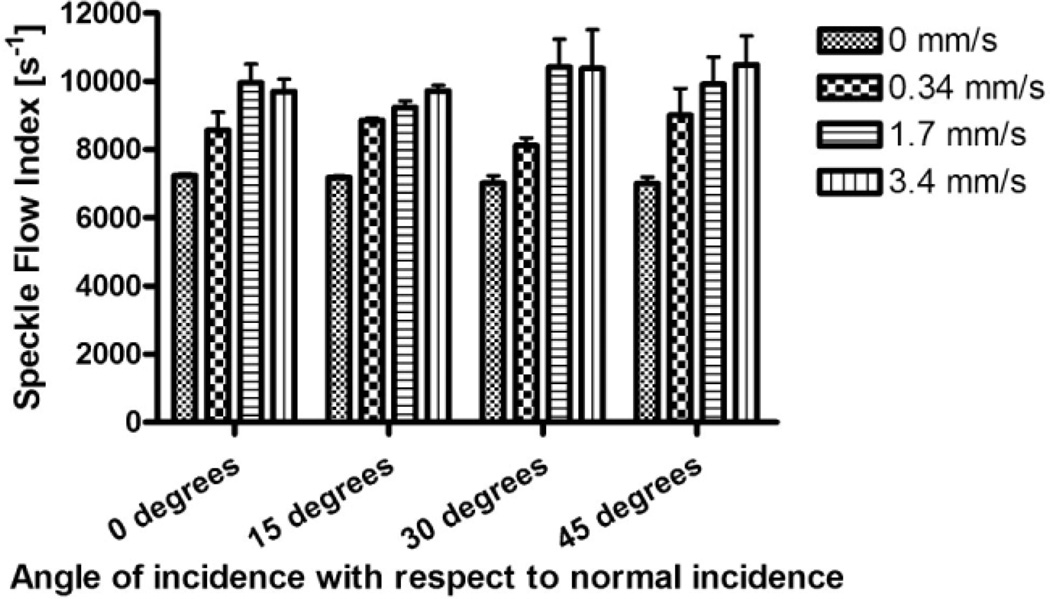
SFI values are insensitive to the relative angle of incidence of the laser light, over a wide range of angles (Fig. 3). Based on a single-factor analysis of variance test, SFI values for a given flow speed, are identical over a large range of incidence angle settings (P > 0.62).
Fig. 3.
With transillumination LSI, the measured SFI values are independent of angle of incidence, for each flow-speed setting. The coefficient of variation of the measured SFI values was < 5% for each of the four flow speeds.
DISCUSSION
Our preliminary in vitro data support our hypothesis that use of the transillumination LSI method enables determination of the presence of pulpal perfusion. A significant increase in SFI values was observed with a change from stagnant flow to an average flow speed of 0.34 mm/ second (Fig. 2). In contrast, epiillumination LSI was insensitive to flow speeds ranging between 0 and 3.4 mm/ second (Fig. 2).
We propose that transillumination LSI has several advantages over other diagnostic methods which have been described in the peer-reviewed literature. It has the capability to serve as an objective method to study blood flow in the pulpal chamber, as opposed to thermal testing. Furthermore, SFI values obtained with transillumination LSI, were insensitive to the relative angle of incidence of the laser light (Fig. 3). Multiple optical scattering of the light is expected to occur, resulting in a homogenization of both the spatial intensity distribution and the degree to which the speckle pattern is modulated by the moving scatterers in the tube. This result suggests that precise positioning of an eventual probe design in the mouth, is unnecessary to enable accurate interrogation of the pulpal chamber for the presence of blood flow. Published studies using LDF probes, required the use of stents to affix the probe in place, and the measurements were easily corrupted by relative differences in probe placement or even angulation [5].
To the best of our knowledge, in vivo measurements of blood flow in the pulp, remain unknown. Arterioles, venules, and capillaries are present in the pulp. If we assume that the blood flow in these vessels is similar to that found in other arterioles, venules, and capillaries in other organs, then the maximum blood flow speed is several mm/second [17]. Hence, the range of flow speeds used in our experimental design, is appropriate to represent in vivo pulpal blood flow.
Our data in Figure 2B suggest that transillumination LSI potentially enables identification of the presence or absence of viable perfusion in the interrogated tooth, but it has limited utility to enable reliable quantitation of the actual perfusion value over potential blood-flow speeds in the pulpal vasculature. A possible explanation for this result is the presence of static optical scatterers in the hard dental tissue. Static scattering is known to modulate the measured speckle pattern and diminish the sensitivity of LSI to blood flow [18]. Additional studies are planned to determine the effects of tooth thickness on measured SFI values and the dynamic range and flow-speed resolution of LSI.
Our current experimental setup is bulky and hence not well suited for imaging of teeth other than the incisors. With the rapid advances being made in both laser diode and digital-camera technology, we plan to reduce considerably the footprint of the LSI instrument, to enable development of a probe that could be used with other teeth.
A potential issue is that the intra- and interpatient difference in SFI values in healthy teeth may be greater than the difference observed within our data set. The thickness of the tooth is expected to affect the SFI measurements. To address this issue, various methods can be used to assess tooth thickness, such as the use of calipers. A more difficult issue to address, is that the ratio of dentin to enamel thicknesses is expected to differ for each type of tooth.
One approach that we plan to explore in vivo, is to determine the degree of similarity between SFI values associated with contralateral teeth (i.e., the left and right central incisors, the left and right lateral incisors, etc.) from each subject’s mouth. If matched teeth are associated with similar SFI values, then transillumination LSI may be used to identify relative differences in blood flow between matched teeth, which may be used as an objective indicator that pulpal blood flow is compromised.
In conclusion, our preliminary in vitro data suggest that transillumination LSI is a promising method to identify the presence of blood flow in the pulpal chamber. Future work involves development of a robust in vivo transillumination LSI instrument and pilot clinical data collection to assess intra- and interpatient variability in SFI values.
ACKNOWLEDGMENTS
We thank Joe Youssef and Elaine Nguyen for their assistance on this project. This study was funded in part by the Arnold and Mabel Beckman Foundation and the National Institutes of Health Laser Microbeam and Medical Program (LAMMP, a P41 Technology Research Resource, award RR001192).
Contract grant sponsor: Arnold and Mabel Beckman Foundation; Contract grant sponsor: National Institutes of Health Laser Microbeam and Medical Program; Contract grant number: RR001192.
REFERENCES
- 1.Petersson K, Soderstrom C, Kiani-Anaraki M, Levy G. Evaluation of the ability of thermal and electrical tests to register pulp vitality. Endod Dent Traumatol. 1999;15(3):127–131. doi: 10.1111/j.1600-9657.1999.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 2.Chambers IG. The role and methods of pulp testing in oral diagnosis: A review. Int Endod J. 1982;15(1):1–15. doi: 10.1111/j.1365-2591.1982.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 3.Gazelius B, Olgart L, Edwall B, Edwall L. Non-invasive recording of blood flow in human dental pulp. Dent Traumatol. 1986;2(5):219–221. doi: 10.1111/j.1600-9657.1986.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 4.Vongsavan N, Matthews B. Experiments on extracted teeth into the validity of using laser Doppler techniques for recording pulpal blood flow. Arch Oral Biol. 1993;38(5):431–439. doi: 10.1016/0003-9969(93)90215-8. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay DS, Artun J, Martinen SS. Reliability of pulpal blood-flow measurements utilizing laser Doppler flowmetry. J Dent Res. 1991;70(11):1427–1430. doi: 10.1177/00220345910700110601. [DOI] [PubMed] [Google Scholar]
- 6.Fried D, Glena RE, Featherstone JDB, Seka W. Nature of light-scattering in dental enamel and dentin at visible and near-infrared wavelengths. Appl Opt. 1995;34(7):1278–1285. doi: 10.1364/AO.34.001278. [DOI] [PubMed] [Google Scholar]
- 7.Schnettler JM, Wallace JA. Pulse oximetry as a diagnostictool of pulpal vitality. J Endod. 1991;17(10):488–490. doi: 10.1016/S0099-2399(06)81795-4. [DOI] [PubMed] [Google Scholar]
- 8.Miwa Z, Ikawa M, Iijima H, Saito M, Takagi Y. Pulpal blood flow in vital and nonvital young permanent teeth measured by transmitted-light photoplethysmography: A pilot study. Pediatr Dent. 2002;24(6):594–598. [PubMed] [Google Scholar]
- 9.Kakino S, Takagi Y, Takatani S. Absolute transmitted light plethysmography for assessment of dental pulp vitality through quantification of pulp chamber hematocrit by a three-layer model. J Biomed Opt. 2008;13(5):054023. doi: 10.1117/1.2976112. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt JM, Webber RL, Walker EC. Optical determination of dental pulp vitality. IEEE Trans Biomed Eng. 1991;38(4):346–352. doi: 10.1109/10.133229. [DOI] [PubMed] [Google Scholar]
- 11.Bui AK, Teves KM, Indrawan E, Jia WC, Choi B. Longitudinal, multimodal functional imaging of microvascular response to photothermal therapy. Opt Lett. 2010;35:3216–3218. doi: 10.1364/OL.35.003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi B, Jia WC, Channual J, Kelly KM, Lotfi J. The importance of long-term monitoring to evaluate the microvascular response to light-based therapies. J Invest Dermatol. 2008;128(2):485–488. doi: 10.1038/sj.jid.5700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YC, Ringold TL, Nelson JS, Choi B. Noninvasive blood flow imaging for real-time feedback during laser therapy of port wine stain birthmarks. Lasers Surg Med. 2008;40:167–173. doi: 10.1002/lsm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YC, Tran N, Shumaker PR, Kelly K, Ross EV, Nelson JS, Choi B. Blood flow dynamics after laser therapy of port wine stain birthmarks. Lasers Surg Med. 2009;41(8):563–571. doi: 10.1002/lsm.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkpatrick SJ, Duncan DD, Wells-Gray EM. Detrimental effects of speckle-pixel size matching in laser speckle contrast imaging. Opt Lett. 2008;33(24):2886–2888. doi: 10.1364/ol.33.002886. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez-San-Juan JC, Ramos-Garcia R, Guizar-Iturbide I, Martinez-Niconoff G, Choi B. Impact of velocity distribution assumption on simplified laser speckle imaging equation. Opt Expr. 2008;16(5):3197–3203. doi: 10.1364/oe.16.003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer. 2002;2(4):266–276. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 18.Parthasarathy AB, Tom WJ, Gopal A, Zhang XJ, Dunn AK. Robust flow measurement with multi-exposure speckle imaging. Opt Expr. 2008;16(3):1975–1989. doi: 10.1364/oe.16.001975. [DOI] [PubMed] [Google Scholar]