Abstract
Growth factor signaling plays an essential role in regulating processes such as tissue development, maintenance, and repair. Gene expression levels, diffusion, degradation, and sequestration by extracellular matrix components all play a role in regulating the concentration of growth factors within the cellular microenvironment. Herein, we describe the synthesis and characterization of hydrogel microspheres that mimic the ability of the native extracellular matrix to reversibly bind vascular endothelial growth factor (VEGF) out of solution. A peptide ligand derived from the VEGF receptor 2 (VEGFR2) was covalently incorporated into the hydrogel microspheres in order to achieve binding affinity and specificity. In addition to being able to both bind and release VEGF in a controllable manner, the microspheres were also shown to affect human umbilical vein endothelial cell (HUVEC) proliferation. The resulting microspheres may enable new strategies to specifically upregulate or downregulate growth factor signaling in the cellular microenvironment.
Keywords: Biomimetic, Bioinspired, Microparticle, ECM, Extracellular Matrix, Growth Factors
Introduction
Regulation of growth factor signaling is essential to tissue development[1], maintenance[2], and repair[3]. For example, vascular endothelial growth factor (VEGF) is a critical regulatory molecule in both physiological and pathological angiogenesis[4–5], and temporarily increasing VEGF signaling is an attractive approach for creating vascular networks in engineered tissue constructs. In healthy tissues, soluble molecules (e.g. sFlt-1, a soluble splice variant of VEGF receptor 1 (Flt-1)) and extracellular matrix components (e.g. collagen) help to regulate VEGF signaling by competitively binding the molecule out of solution, thereby preventing binding to growth factor receptors on the cell surface[6]. During inflammation, the bound growth factor can be rapidly released back into the cellular microenvironment to initiate a cellular response[7]. Conversely, reducing growth factor signaling has therapeutic value in pathological diseases where dysregulation leads to excess growth factor signaling. For example, dysregulation of VEGF is associated with disease states such as tumor growth and age-related macular degeneration[8–10]. Anti-VEGF antibodies and other soluble antagonists represent one therapeutic approach that can be used to reduce excess levels of growth factor in these diseased states. One limitation of soluble antagonist treatments is that they can readily diffuse away from the injection site, which can lead to side effects in both proximal [11] and distant locations [12]. Further, antibody treatments can present unique safety risks in clinical treatments [13]. The ability to both bind and release molecules in the cellular microenviroment allows the native ECM to effectively buffer the activity of specific growth factors. Given the fact that VEGF plays a prominent role in both healthy tissue and pathological states, it would be beneficial to have a therapeutic means of both upregulating and downregulating the molecule.
A number of biomaterial-based approaches have been pursued in an attempt to mimic the ability of native ECM to locally regulate growth factors. Sustained release of basic fibroblast growth factor (bFGF) was achieved from a fibrin gel by conjugating heparin into the network using a heparin binding peptide ligand [14]. Similarly, vinyl-functionalized heparin was covalently incorporated into a polyethylene glycol (PEG) hydrogel network using photoinitiated radical crosslinking to achieve sustained bFGF release[15]. Cells cultured on the heparin functionalized PEG hydrogels exhibited a decrease in mitogen-activated protein kinase (MAPK) activation, a critical protein activated in the bFGF signaling pathway, as compared to cells cultured on PEG hydrogels crosslinked in the absence of photo-crosslinkable heparin [16]. The results implied that the heparin-functionalized PEG hydrogels were capable of binding bFGF, which subsequently modulated downstream biological effects of the growth factor. Similarly, maleimide-functionalized heparin was covalently incorporated into a thiol-terminated PEG hydrogel network using Michael-type addition [17]. The work demonstrated that the release rate of bFGF from the hydrogel could be controlled by varying the amount of heparin that was incorporated into the network. Finally, two recent studies have shown that sequestering of serum-borne heparin can in turn result in local amplification of endogenous or exogenous growth factor activity in the cellular microenvironment[18–19]. Taken together, these studies demonstrate that biomaterials can mimic the ability of the ECM to locally modulate growth factor concentrations and affect downstream cellular responses. One potential limitation of these materials is that heparin, along with many other molecules, readily binds a variety of growth factors and would likely impact many different growth factor signaling pathways simultaneously.
In this work, we present synthetic hydrogel microspheres that mimic the native ECM’s ability to locally sequester and release VEGF. However, instead of using a naturally occurring ligand that may show binding affinity for a variety of growth factors, the PEG microspheres utilize a synthetic VEGF binding peptide (VBP) derived from VEGF receptor 2 [20] in order to confer affinity and specificity. The use of a VEGF-specific binding peptide instead of a broader growth factor-binding molecule such as heparin results in targeted VEGF binding and release, and represents a distinction from many current growth factor binding biomaterials. The use of hydrogel microspheres with VBP conjugated throughout the porous network is also distinct and potentially advantageous when compared to systems that use solid particles or bulk hydrogels, as the functional area for VBP-VEGF interaction in porous microspheres is potentially greater than in a bulk hydrogel or solid particles with VBP conjugated to the surface. The VBP concentration was optimized to maximize VEGF binding while minimizing non-specific interactions. The binding and release of VEGF from the functionalized microspheres was characterized and a human umbilical vein endothelial cell (HUVEC) proliferation assay was used to evaluate the ability of the microspheres to modulate VEGF activity in vitro. Results demonstrate specific and reversible VEGF binding, which was used to downregulate (via binding) and upregulate (via release) VEGF signaling.
Materials and methods
2.1 Peptide and polymer synthesis
All reagents were purchased from Sigma Aldrich unless otherwise stated. Peptides were synthesized on rink amide resin (AnaSpec) using standard Fmoc protection chemistry. A cysteine group was added to the c-terminus of the peptide so that the ligand could be coupled into the hydrogel network using thiol-ene chemistry. The peptides were cleaved using 95% trifluoroacetic acid, 2.5% triisopropylsilane, and 2.5% water. The peptide was precipitated and washed four times with cold diethyl ether (Fisher Scientific), vacuum dried, redissolved in DI water, frozen, and lyophilized. Peptides were verified using MALDI-TOF MS and purities were > 85% as determined by HPLC.
2.2 4-arm PEG norbornene synthesis
N,N′-Dicyclohexylcarbodiimide (2.1 g) and 5-norbornene-2-carboxylic acid (2.5 ml) were added to a round bottom flask and dissolved in 30 ml of anhydrous dichloromethane (DCM). The head space was then purged with dry argon and the solution was allowed to stir for 20 minutes.
In a second flask, 10 g of 4-arm PEG (20k MW from JenKem Technology) and 120 mg of 4-(dimethylamino)pyridine were dissolved in 40 ml of anhydrous DCM containing 0.8 ml of pyridine. Next, the PEG solution was transferred into the first flask using a syringe. The reaction was allowed to proceed at room temperature for two hours.
The reaction mixture was passed through a fritted Buchner funnel (medium) to remove suspended urea salts that formed during the reaction. The filtrate was then precipitated using 900 ml of cold diethyl ether and the solids were collected on qualitative grade filter paper. Vacuum was pulled on the sample until a dense cake was formed. Following drying, the reaction product was dissolved in 90 ml of chloroform and extracted twice using 50 mM glycine buffer solution (pH 10) in order to remove residual norbornene acid. Residual water in the chloroform phase was then extracted using a saturated sodium chloride solution. Finally, the chloroform fraction was added to 900 ml of cold diethyl ether in order to precipitate the purified PEG norbornene, which was then collected and dried on filter paper using vacuum filtration.
2.3 Microsphere synthesis
Microspheres were formed in a water-in-water emulsion by adding a 18.6% w/w PEG dithiol (Laysan Bio)/6.4% w/w 4-arm PEG norbornene solution to a 40% w/w dextran 40K, resulting in a PEG rich discontinuous phase. Dextran was weighed to make a 40% w/w solution in buffer (0.22 M KCl/10mM sodium phosphate/pH 8) that had been deoxygenated by purging with nitrogen through a diffusion stone for 10 minutes while stirring. PEG dithiol (3,400 MW) was weighed in a 1:1 functional group ratio with 4-arm PEG norbornene (20,000 MW), resulting in a 25 % w/w PEGDT/PEGNB solution in 0.5% w/w Irgacure 2959 (Ciba) photoinitiator solution. Peptide solutions were made using either VBP or a scramble version of the peptide in concentrations corresponding to various ratios of peptide to norbornene functional groups in the prepolymer solution. DI water was used to make the VBP solution and the scramble peptide solution was prepared in 0.1 M pH 8.5 HEPES in order to fully dissolve the peptides. To make the prepolymer solution, 2.89 ml of 40% w/w dextran, 311 μl 25% w/w PEGDT/PEGNB solution, and 222 μl of peptide solution containing varying amounts of peptide (between 1:128 to 1:8 peptide:norbornene ratio) were pipetted into a 15 ml conical tube using a positive displacement pipette. The tube was then vented with argon, vortexed for 60 seconds, and the resulting emulsion was allowed to stabilized for 30 minutes.
To form microspheres approximately 1–2 orders of magnitude smaller in diameter than those formed by vortexing for 60 seconds and stabilizing for 30 minutes, a sonic probe was placed in the prepolymer solution for 30 seconds and the emulsion was allowed to stabilize for one minute. These smaller spheres are referred to as nanospheres, and were synthesized primarily to determine the relationship between increased sphere surface area and VEGF binding.
Following emulsion stabilization, the tubes were then exposed to a 4 mW/cm2 mercury UV light for 8 minutes to form crosslinked microspheres. Following crosslinking, the microspheres were washed 4 times with DI water, frozen and lyophilized. Size distributions were determined using ImageJ to measure microspheres in phase contrast images at 20X magnification (n>500). Note that all future references made to a peptide:norbornene ratio of a microsphere refer to the ratio used during the synthesis of the microspheres.
2.4 Microsphere Micro BCA
A modified Micro BCA technique (Pierce) was used to determine the mass of peptide ligand incorporated per mass of microspheres for each peptide:norbornene ratio used to synthesize microspheres. A standard curve was constructed by adding 125 μl dH2O and 25 μl of 0, 125, 250, 500, 1000, and 2000 μg/ml bovine serum albumin (BSA) (Fisher Scientific) directly into wells of a 96-well plate, corresponding to 0, 3.125, 6.25, 12.5, 25, and 50 μg of total peptide, respectively. The standard curve was conducted in triplicate. For each microsphere sample, three 2–3 mg samples of lyophilized material were weighed out into separate 1.5 ml Eppendorf tubes. 150 μl of working reagent was added to each of the standard wells, and 300 μl water plus 300 μl of working reagent were added to each microsphere sample. Both the microsphere samples and the 96-well plate containing the standards were then incubated at 37 °C for 2 hours. Following incubation, both the plate and the microspheres were removed from the incubator, the microspheres were spun down at 16,100 RCF in a microfuge, and 300 μl of supernatant from each sample was added to the plate for analysis. The 300 μl of each sample collected and added to the plate for absorbance analysis represents total protein for half of the 2–3 mg of microspheres used in the assay. A plate reader was then used to read the absorbance of each standard and sample at 562 nm. The total protein in each sample was determined using the sample absorbance and the BSA standard curve (absorbance at 562 nm vs. total protein). The mass of microspheres corresponding to the analyzed 300 μl was then used in conjunction with the calculated total protein to derive the μg peptide per mg microsphere for each sample.
2.5 VEGF binding assays
Hydrogel microspheres containing varying amounts of either the VBP or the scramble peptide were incubated in a 4 ng/ml VEGF solution in 1x PBS and 0.1% BSA at a loading of 1mg dry microspheres/ml of solution. Of the VEGF in solution, 1% was radioactively labeled with 125I (Perkin Elmer). The samples were held in 1.5 ml microcentrifuge tubes that had been pre-blocked in 0.1% BSA solution, rinsed with buffer and DI water, and were subsequently lyophilized. With the exception of the binding kinetics experiment, samples were incubated for a period of four hours at 37 °C. Following incubation, the samples were spun down in a microcentrifuge at 12,000 RCF and 1 ml of liquid volume was removed from the supernatant for testing. Sample concentrations were determined using a Cobra II gamma counter (Packard). Binding was determined by calculating the difference in concentration between solutions that were not exposed to microspheres and solution that had been incubated with microspheres. A sample size of n=4 was used for each condition.
2.6 Competitive binding experiment
VBP microspheres were allowed to incubate with VEGF solution for a period of one hour at 37 °C. The microsphere-containing samples were then dosed with varying concentrations of unbound VBP. Control samples were prepared without microspheres and dosed with a corresponding volume of free VBP solution. The samples were then allowed to incubate for an additional four hours at 37 °C and the amount of bound VEGF was measured for each condition. The amount of bound VEGF was determined by taking the difference between the solution concentration of the control samples and the microsphere containing samples for each condition.
2.7 Release experiments
During VEGF release experiments, microspheres were first incubated in a solution of radio-labeled VEGF solution for one hour on a rotator held at 37 °C to allow for VEGF binding. The microspheres were subsequently centrifuged and washed three times with buffer in order to remove unbound growth factor. VEGF release was determined by spinning down each sample, collecting 1 ml of supernatant, and taking a measurement on the gamma counter. Each tube was then replenished with 1ml of fresh PBS buffer solution and placed back in the incubator.
2.8 Serum binding experiment
Hyclone fetal bovine serum (FBS) (Fisher Scientific) was used to evaluate the impact of serum on the VBP’s ability to bind VEGF. Solutions of varying FBS concentration were prepared in Cellgro alpha MEM solution (Mediatech). After adding 4 ng/ml of VEGF, 1 ml of the respective serum containing solutions was added per mg of lyhophilized microspheres and binding was evaluated as previously mentioned.
2.9 Microsphere serum stability
Microspheres were place in 10% FBS containing medium and incubated on a rotary mixer at 37 °C for either one or seven days. Following incubation, the samples were washed three times with DI water in order to remove serum components. The microspheres were then frozen, lyophilized, and used in the standard VEGF binding experimental format.
2.10 Cell proliferation – VEGF sequestering and release
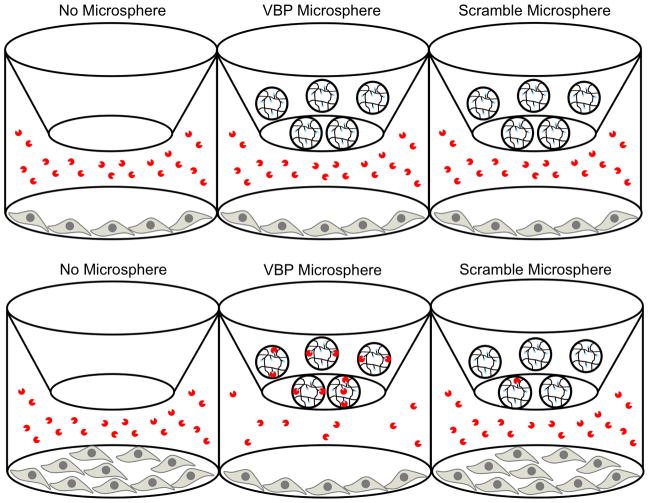
In both sequestering and release experiments, human umbilical vein endothelial cells (HUVECs) (Lonza) were seeded in gelatin-coated 24 well plates at 1.1 × 104 cells/well using Medium 199 (M199) supplemented with the EGM-2 bullet kit (Lonza). Cells were allowed to attach overnight. In addition, a final VEGF concentration of 10 ng/ml was used for all experimental conditions in both the VEGF sequestering and release assays, as this concentration resulted in the strongest response in HUVEC proliferation. Therefore, microsphere-mediated downregulation of VEGF activity in the sequestering assay and upregulation of VEGF activity in the release assay are compared to conditions that have been optimized for VEGF-dependent HUVEC proliferation.
For the sequestering experiments, ethanol-sterilized VBP or scramble peptide microspheres (5.4 mg per condition) were incubated with 10 ng/ml VEGF in M199/10% FBS (5.4 ml per condition) for 30 minutes. The microspheres were centrifuged and 0.7 ml of supernatant was added to all wells (n=6) for each experimental condition. Transwell inserts (Corning) were placed into the wells in order to separate the microspheres from the cells, facilitating faster and more efficient sphere removal necessary for downstream cell counting. For each microsphere condition, microspheres were resuspended in the remaining solution and 0.2 ml added per transwell insert, resulting in a final microsphere concentration of 1 mg microspheres/ml total solution in each well. For the no microsphere condition, 0.7 ml of 10 ng/ml VEGF in M199/10% FBS was added to each well and 0.2 ml of 10 ng/ml VEGF in M199/10% FBS was added to each transwell insert, resulting in a final VEGF concentration of 10 ng/ml in each well. Ultimately, each well corresponding to a microsphere condition contained 10 ng/ml VEGF in M199/10% FBS and 1 mg/ml microspheres, and each well corresponding to a no microsphere condition contained 10 ng/ml VEGF in M199/10% FBS. Cell counts were determined after 72 hours using the CellTiter-Blue Cell Viability Assay (Promega).
For release experiments, ethanol-sterilized VBP or scramble peptide microspheres (5.4 mg per condition) were incubated with 25 ng/ml VEGF in M199/10% FBS (5.4 ml per condition) for 30 minutes in order to load the microspheres with approximately 10 ng VEGF per mg microsphere. The microspheres were centrifuged and collected, washed three times with sterile PBS solution, and resuspended in M199/10% FBS to a concentration of 4.5 mg microspheres/ml. Then, 0.7 ml of M199/10% FBS was added to all wells (n=6) for each experimental condition and transwell inserts were placed into all wells, as in the VEGF sequestering assay. For each microsphere condition, 0.2 ml microsphere solution was added per transwell insert, resulting in 1 mg microspheres/ml total solution in each well. For the no microspheres condition, 0.2 ml of 45 ng/ml VEGF in M199/10% FBS was added to each transwell insert, resulting in a final VEGF concentration of 10 ng/ml in each well. Ultimately, each well corresponding to a microsphere condition contained 1 mg/ml microspheres in M199/10% FBS loaded with approximately 10 ng VEGF per mg microsphere, and each well corresponding to a no microsphere condition contained 10 ng/ml VEGF in M199/10% FBS. Cell counts were determined after 72 hours using the CellTiter-Blue Cell Viability Assay.
2.11 Statistical analysis
Four replicates of each condition were used for all binding and release experiments, with all data represented as the mean ± standard deviation. The Student’s t-test was used to compare different conditions within a given experiment.
Results
3.1 Microsphere characterization and ligand incorporation
The synthesis process consisted of 2 aqueous phases being emulsified, stabilized, exposed to UV light, and washed to produce microspheres formed via a step-growth mechanism (Fig. 1A). The emulsion process produced microspheres with an average size of 8.2 μm (± 2.6 μm) (Fig. 1B). A modified MicroBCA assay showed that the amount of peptide in the pre-polymer solution was proportional to the final mass of peptide incorporated into the microspheres (Fig. 2A). The data indicated that it was possible to predictably control the mass of VBP covalently incorporated by varying the mass of VBP in the emulsion used to synthesize the microspheres.
Figure 1.
Microsphere synthesis and size characterization. (A) A water-in-water emulsion containing two distinct phases was used to create functionalized PEG microspheres. The “discontinuous phase” is comprised of prepolymer solution that will form the microspheres (4 arm PEGNB 20k and PEGDT 3.4k), while the “continuous phase” is comprised of Dextran 40k that acts as the medium within which the microspheres form. This emulsion, upon UV crosslinking, yields microspheres that are formed via a step growth mechanism of reaction between the thiol and norbornene groups. (B) Histogram of microsphere size distribution with an average microsphere diameter was 8.2 μm (±2.6 μm).
Figure 2.
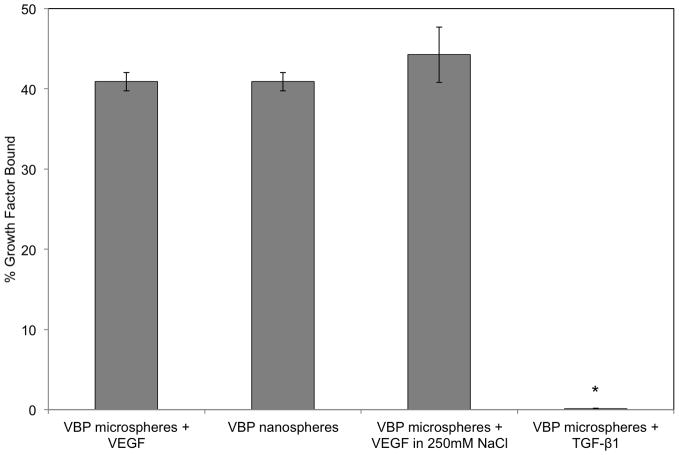
Quantification of ligand incorporation and VEGF binding characterization. (A) A modified Micro BCA was used to determine the extent of ligand incorporation in emulsions containing varying concentrations of VBP. As the concentration of ligand in the emulsion increased, the extent of ligand incorporated into the network increased as well in a linear fashion. (B) Microspheres containing varying amounts of VBP and scramble peptide ligand were used to bind VEGF. The microspheres synthesized using a peptide:norbornene ratio of 1:64 showed the highest specific binding and the lowest non-specific binding. (C) Competitive binding assay in which 1:64 VBP microspheres incubated in VEGF had varying concentrations of free VBP ligand added to solution. As the concentration of free VBP ligand increased, the amount of VEGF bound to the microspheres decreased. (D) VEGF bound by 1:64 VBP microspheres, VEGF bound by 1:64 VBP nanospheres, VEGF bound by 1:64 VBP microspheres in solution with 250 mM of NaCl added, and TGF-β1 bound by 1:64 VBP microspheres. TGF-β1 binding to 1:64 VBP microspheres was significantly lower than VEGF binding to VBP microspheres in both the presence and absence of salt (p<0.01). TGF-β1 binding to 1:64 VBP microspheres was not statistically different than TGF-β1 binding to blank microspheres (not shown). (E) VEGF binding to 1:64 VBP microspheres as a function of solution pH, showing a sharp dropoff in binding at acidic pH.
3.2 VEGF binding characterization
Binding experiments were carried out in PBS with 0.1% BSA in order to ensure that secondary molecules, such as heparin, were not mediating binding. In one set of experiments used to determine the optimal VBP concentration, a scramble version of the VBP was integrated into hydrogel microspheres at varying concentration and the binding capacity was compared to microspheres containing similar concentrations of VBP (Fig. 2B). VBP and scramble peptide microspheres both exhibited binding of VEGF at high ligand loading concentrations, indicating non-specific binding (Fig. 2B). However, as the ratio of peptide to norbornene was reduced to 1:64, non-specific binding was eliminated and specific VEGF binding to VBP microspheres was maximized (Fig. 2B). Based on this study, a VBP to norbornene group ratio of 1:64 was chosen for further evaluation in all of the VEGF binding and release experiments conducted in this study.
A competitive binding experiment demonstrated that the amount of bound VEGF was inversely related to the amount of free ligand that was introduced into the solution (Fig. 2C), consistent with a specific VEGF-VBP binding interaction. To further characterize the specificity of binding, transforming growth factor β1 (TGF-β1) (isoelectric point of 8.9 similar to 8.5 for VEGF) was incubated with the 1:64 VBP microspheres and did not exhibit any binding when compared to blank PEG microspheres, indicating that binding was not due to non-specific electrostatic interactions (Fig. 2D).
Further, the addition of 250 mM NaCl to the buffer solution did not significantly change the amount of VEGF that was bound by the VBP microspheres (Fig. 2D). Comparing the microspheres and the nanospheres, there was no significant difference in VEGF binding based on the microsphere diameter (Fig. 2D). VBP microspheres incubated in VEGF solutions with various pH levels showed that the VEGF-VBP interaction was consistent in neutral and mildly basic solutions while binding decreased significantly in acidic conditions (Fig. 2E).
A binding isotherm demonstrated a correlation between solution VEGF concentration and the amount of VEGF that was bound by the microspheres. VBP functionalized spheres (1 mg/ml) were shown to consistently bind between 30 and 40% of the VEGF in solution over a range of concentrations (Fig. 3A). VEGF binding to the microspheres was rapid, with 91.8% (± 9.3%) of the binding occurring within 30 minutes (Fig. 3B).
Figure 3.
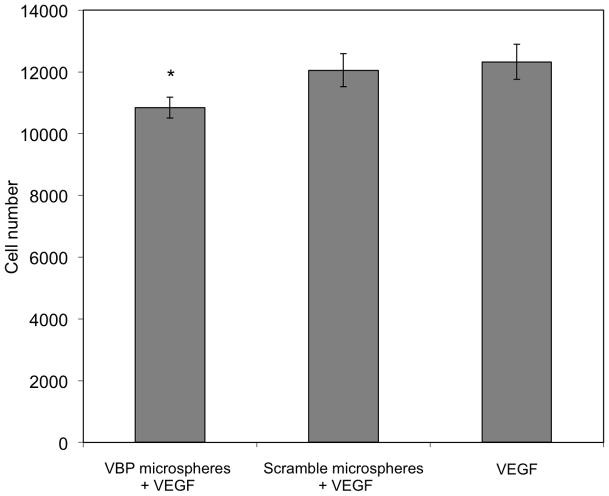
VEGF binding characterization and HUVEC proliferation assay. (A) A binding isotherm for 1 mg/ml of 1:64 VBP microspheres. In all concentrations of VEGF, the microspheres bound 30–40% of VEGF in solution, and saturation was not observed over the range of concentrations that were tested. (B) Binding kinetics showing that 1:64 VBP microspheres in 4ng/ml VEGF solution reach binding equilibrium within 30 minutes of incubation. (C) Transwell HUVEC proliferation assay testing sequestration in medium containing 10 ng/ml of VEGF with no microspheres, 1:64 scramble microspheres, and 1:64 VBP microspheres. (D) There was no difference observed in cell number between the no microsphere and scramble microsphere conditions, while the cell number in the VBP microsphere condition was significantly less compared to both the no microsphere and scramble peptide micro condition (p<0.05).
3.3 Serum influence on of VEGF binding
The presence of serum in the medium during the VEGF binding experiments decreased the amount of bound VEGF in a concentration dependent fashion, with a 46.3% (± 9.0%) decrease in binding in the presence of 10% FBS (Fig. 4A). VBP microspheres that were first incubated in 10% FBS for either one or seven days and subsequently exposed to soluble VEGF bound less VEGF when compared to microspheres that were not exposed to serum prior to binding (p<0.01); however, there was no statistical difference between VEGF binding to VBP microspheres pre-incubated in serum for 1 day when compared to microspheres pre-incubated in serum for 7 days (Fig. 4B).
Figure 4.
Serum effects on VEGF binding. (A) Percent VEGF bound from a 4ng/ml VEGF solution containing varying percent v/v FBS. (B) Percent VEGF bound from a 4 ng/ml VEGF solution using microspheres incubated in 10% FBS for varying amounts of time. VBP microspheres incubated in 10% FBS for either one or seven days and bound less VEGF compared to microspheres that were not exposed to serum prior to binding (p<0.01). No statistical difference was observed in VEGF binding between spheres that had been incubated in serum for 1 and 7 days.
3.4 Sustained VEGF release
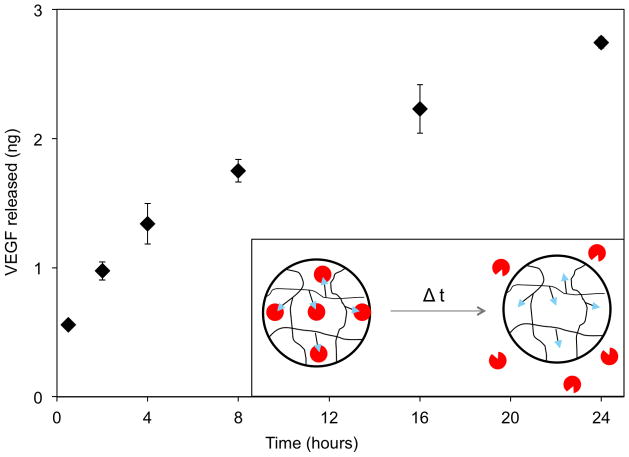
Short-term VEGF release from loaded VBP microspheres followed first order rate kinetics over a 24-hour period, with no plateau in the release curve over a 24-hour period (Fig. 5A). In a subsequent experiment, VEGF release from microspheres was measured at 24 hour intervals for 30 days in order to evaluate the sustained release kinetics. In this sustained release experiment, VEGF was released over a longer period of time from VBP microspheres when compared to microspheres containing a scramble version of VBP (Fig. 5B). The scramble spheres loaded with 2.5 ng VEGF per mg microsphere released in total 27% (0.7 ng) after 24 hours, 81% (2 ng) after 7 days, and 100% (2.5 ng) after 30 days. In contrast, the VBP microspheres loaded with 15.1 ng VEGF per mg microsphere released 14% (2.1 ng) after 24 hours, 42% (6.3 ng) after one week, 79% (11.9 ng) after 30 days, and 95% (14.4 ng) by day 50. Compared to the scramble microspheres, the VBP microspheres released more VEGF at any given time point observed throughout the release study and exhibited more sustained release of VEGF over time, by virtue of having a lower percent of total VEGF released at any given time point. In addition, the rate of VEGF release and total VEGF release were each proportional to the amount of VEGF that was initially bound within the microspheres.
Figure 5.
VEGF release characterization and HUVEC proliferation assay. (A) Release from the VBP microspheres did not reach equilibrium out to 24 hours, as shown in the inset of release over the first 24 hours. (B) Cumulative release of VEGF from the 1:64 VBP and scramble peptide microspheres incubated in 40 ng/ml shows that VEGF could be specifically bound and released in a sustained manner from the VBP microspheres. Scramble microspheres exhibit a rapid, low release corresponding to VEGF that diffused into the microspheres and did not specifically bind to a ligand. (C) HUVEC transwell proliferation assay comparing 10 ng/ml of VEGF with no microspheres to VEGF release from 1:64 scramble peptide microspheres incubated in VEGF (25 ng/ml) and 1:64 VBP microspheres incubated in VEGF (25 ng/ml). (D) There was a significant increase in cell number in both the VEGF-loaded VBP microspheres and the soluble VEGF conditions compared to the scramble microsphere condition after 72 hours (p<0.01).
3.5 VBP microsphere-mediated VEGF sequestering in HUVEC cell culture
VBP microspheres incubated in a HUVEC culture containing a soluble VEGF supplement showed a significant decrease in cell proliferation when compared to both the scramble peptide microspheres and the microsphere-free conditions. This HUVEC proliferation assay was carried out with either no microspheres, VBP microspheres, or scramble peptide microspheres placed on top of a transwell chamber situated above HUVEC cells in culture with 10 ng/ml of VEGF (Fig. 3C). After 72 hours, there was no significant difference between the number of cells in the scramble microspheres and no microsphere conditions, as expected (Fig. 3D). There was a significantly lower cell count (p < 0.05) in the VBP microsphere condition compared to both the scramble microsphere and no microsphere conditions (Fig. 3D). These data indicate that VEGF-dependent HUVEC proliferation could be inhibited by VBP microspheres, likely as a result of VEGF sequestering.
3.6 VBP microsphere-mediated VEGF release in HUVEC cell culture
VBP microspheres pre-loaded with VEGF released the loaded growth factor into HUVEC culture, resulting in cell proliferation that was significantly increased compared to the scramble peptide microspheres and equal to the effect of a soluble VEGF supplement. VBP and scramble peptide microspheres were first incubated in a VEGF solution, then placed in HUVEC culture in order to determine whether released VEGF could elicit a cellular response (Fig. 5C). An equivalent dose of soluble VEGF served as a control (Fig. 5C). After 72 hours, there was no statistical difference between the number of HUVECs in the VEGF releasing VBP microspheres and soluble VEGF conditions; however, the scramble peptide microspheres exhibited a significantly lower cell count compared to both the VBP microsphere and soluble VEGF conditions (p < 0.01) (Fig. 5D). These data indicate that VEGF-dependent HUVEC proliferation can be upregulated by VBP microspheres pre-loaded with VEGF.
4. Discussion
Synthetic hydrogel microspheres were shown to modulate the VEGF concentration in solution via specific interactions. Non-specific VEGF binding to the microspheres was observed in conditions with high peptide concentration within the microspheres (Fig. 2B). However, synthesizing VBP microspheres with a peptide:norbornene ratio of 1:64 enabled maximum VEGF binding while eliminating non-specific interactions with VEGF (Fig 2B). The high nonspecific binding observed at higher ratios of peptide to norbornene group was potentially due to clustering of hydrophobic residues in the VBP molecules (Fig. 2B). The resulting hydrophobic clusters may have promoted stronger hydrophobic interactions than single peptides, leading to non-specific binding observed at higher ratios of peptide to norbornene groups. This is further supported by the fact that high salt concentrations were unable to affect the interactions between VBP and VEGF (Fig. 2D), indicating that non-specific electrostatic interactions are not the primary cause of the VBP-VEGF interaction. Importantly, non-specific VEGF binding was abolished and specific VEGF binding was maximized by simply decreasing the amount of peptide in the microspheres.
The binding interaction was stable under neutral and slightly basic pH conditions; however, binding dropped off significantly in acidic conditions; VEGF binding was reduced to 20% at a pH of 6 and there was no significant binding at pH 5.3 (Fig. 2E). The sharp decrease in VEGF binding at acidic pH may be a result of peptide aggregation in the microsphere network as the pH approaches the VBP isoelectric point of 4.4. The peptide contains several acidic residues whose negative charge at a pH well above the isoelectric point may provide a repulsive electrostatic force sufficient to prevent the densely hydrophobic region of different peptides (FD-AD-YD-LD-I) from interacting with one another and forming aggregates that are incapable of interacting with VEGF. While the specific mechanism for decreased VEGF binding at acidic pH is unknown, it is important to note that this phenomenon may impact the use of this material in highly acidic environments both in vitro and in vivo. Interestingly, the amount of growth factor bound by the microspheres was not found to be dependent on microsphere diameter (Fig 2E). This result strongly suggests that the binding of VEGF to VBP microspheres is independent of the surface area of the microspheres, and is instead dependent upon the total amount of ligand present throughout the microsphere network.
Binding of VEGF from solution was found to be inversely proportional to the amount of serum in solution (Fig 4A). In addition, VBP microspheres pre-incubated in serum for one day and one week showed a moderate reduction in VEGF binding, binding 25% (± 3.8%) and 20.9% (± 2.3%) of VEGF in solution respectively (Fig 4B). While VEGF binding was reduced post microsphere incubation in serum, the spheres still retained over half of their original binding capability after 7 days of serum incubation (Fig. 4B). These data, coupled with our observation that VEGF-VBP was clearly a reversible interaction (Fig. 2C), suggest that the loss of binding in serum may be due to competition with other serum-derived molecules known to bind VEGF (e.g. heparin, fibronectin[21–24]). Another possible mechanism involves proteolytic degradation of the ligand, as one would expect VEGF binding to decrease as ligand is cleaved from the network or partially degraded in a manner that reduces its binding affinity for VEGF. However, the similar VEGF binding for microspheres pre-incubated in serum for 1 day and 7 days suggests that proteolytic degradation is not a dominant mechanism, since this mode of ligand degradation would be expected to continue with time. Further studies will be necessary to elucidate the specific mechanism of serum-dependent VEGF binding and to minimize serum effects. Nevertheless, VEGF sequestering decreased HUVEC proliferation in serum-containing cell culture medium (Fig. 3D), and VEGF released from VBP microspheres promoted HUVEC proliferation in serum-containing cell culture medium (Fig. 5D). In contrast, microspheres containing a scramble version of the peptide and prepared in the same manner were unable to significantly influence HUVEC proliferation (Fig. 3D and Fig. 5D). Therefore, VEGF-binding microspheres were biologically active in serum-containing environments.
It is noteworthy that serum effects on growth factor binding and release observed here are likely to apply to other affinity based approaches that target specific soluble factors. This competition can affect applications related to both growth factor upregulation and downregulation. With respect to upregulation, serum proteins may cause faster growth factor release by binding released proteins and altering the equilibrium concentration of the free soluble molecule. Molecules from serum may also prevent downregulation by binding growth factor in a manner that does not prevent activation of cell receptors while at the same time inhibiting the ability of microspheres to bind the growth factor. In order to account for this competition in vivo and in vitro, there is a need to develop ligands with tailored affinity in specific target environments, and there is a particular need to develop ligands with high affinity while maintaining specificity.
While the biological activity of VEGF released from the VBP microspheres was only tested over a period of 72 hours in HUVEC culture and found to active, we can speculate that the remaining VEGF in the microspheres that had not been released is also likely to be biologically active. This is based upon the fact that in the extended VEGF release study (Fig. 5B), there is sustained release of VEGF throughout the length of time the assay was conducted. This sustained release of VEGF in which only 13.6% is released after 24 hours and 78.8% is released after 30 days relies on the ability of VEGF to engage in binding interactions with the receptor-derived VBP peptide, and these interactions are highly dependent upon the native, receptor-binding structure of VEGF. If VEGF were denatured to a degree that it no longer retained its native conformation and decreased in biological activity, it follows that binding to the VBP peptide (modeled after VEGF receptor-2) would also decrease, resulting in a faster “burst” release similar to what is observed in the scramble microspheres in which VEGF-peptide interactions are minimal.
A potential challenge that is common to many biomaterials in “affinity-based” applications is specificity. For example, prior approaches have primarily focused on using glycosaminoglycans (e.g. heparin) or ECM proteins (e.g. fibronectin) to sequester growth factors[25]. In one illustrative example, porous poly(D,L-lactic-co-glycolic acid) (PLGA) microspheres containing immobilized heparin were used to modulate binding and release of the heparin-binding growth factor bFGF[26] In this previous study, microspheres were loaded with bFGF by incubating at a loading concentration of 1.11 μg bFGF per mg microsphere, 27.8 times the loading concentration of VEGF in the VBP microspheres in the current study. Total growth factor loading onto the microspheres was determined to be 1124 (± 103) pg bFGF per mg microsphere, which is less than 10% of the growth factor loading observed in the VBP microspheres in the current study. In addition, ~40% of the bound bFGF was released during the first hour of release, compared to only 13.6% VEGF released from the VBP microspheres in the current study. This comparison highlights the potential practical advantages of a high affinity growth factor-binding ligand when compared to heparin, as well as the potential advantages of ligand-containing hydrogel microspheres when compared to surface-modified microspheres.
In addition, while glycosaminoglycans and ECM proteins are capable of regulating growth factor signaling, they interact with a wide range of distinct growth factors with variable affinity, which may not be ideal for regulating release or activity of one specific growth factor in complex, multi-component biological systems. To address this issue, peptide ligands have been previously used in one study to specifically upregulate recombinant nerve growth factor (NGF)[27] and in another study to specifically downregulate tumor necrosis factor-α (TNF)[28]. The current study demonstrates that peptide-functionalized microspheres can be used to either upregulate VEGF signaling (via sustained release) or downregulate VEGF signaling (via sequestering) with high specificity. The use of microspheres is potentially enabling, as microspheres can be readily incorporated into a variety of biomaterials or injected into a localized site. In addition, our approach modified a short VEGF binding ligand by adding a cysteine to the c-terminal end for conjugation into the PEG network. This c-terminal modification results in a high degree of control over ligand orientation, as the ligand only has a single functional thiol by which it could be crosslinked into the network. Collectively, approaches that exploit specific ligand-growth factor interactions could result in new, broad mechanisms for regulating both growth factor release and local growth factor activity. One can envision ultimately generating hydrogels that mimic the ability of the natural ECM to intricately regulate growth-factor activity during physiological processes, but perhaps with higher levels of specificity.
VBP microspheres were able to bind VEGF with specificity and were able to bind VEGF in serum-containing medium and affect cellular outcomes. Taken together, these data suggest the possibility of specifically binding VEGF from natural biological solutions for subsequent therapeutic use. For example, commonly used autologous products such as platelet-rich plasma fraction and blood serum are known to contain growth factors, including VEGF. However, the cadre of growth factor types and dosages are poorly defined, which complicates therapeutic use of autologous products. One can envision developing more well-defined therapeutic approaches by capturing specific growth factors from autologous products. In addition, it may be possible to use growth factor binding microspheres to regulate growth factor concentrations in vivo, resulting in a useful tool for fundamental biology and clinical therapy. Further studies will be necessary to explore the feasibility of these concepts.
5. Conclusions
This work demonstrated that a peptide ligand derived from VEGF receptor 2 could be incorporated into PEG microspheres in order to modulate the concentration of VEGF in solution. The material bound up to 40% of the VEGF out of solution over a range of concentrations. The interaction between the growth factor and the microspheres was specific and reversible in nature. Importantly, the same material was shown to downregulate HUVEC proliferation by sequestering soluble VEGF, and upregulate HUVEC proliferation by releasing VEGF that was previously bound by the microspheres. The approach described here may be broadly useful to upregulate or downregulate growth factor signaling in the cellular microenvironment, and may find particular utility in therapeutic angiogenesis and anti-angiogenesis schemes.
Acknowledgments
The authors acknowledge support from the National Science Foundation (CAREER award 0745563), the National Institutes of Health (R01HL093282 and T32 DC009401) and the Wisconsin Stem Cell and Regenerative Medicine Center (postdoctoral fellowship to SLL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tabata T, Takei Y. Morphogens, their identification and regulation. Development (Cambridge, UK) 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 2.Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature (London) 2008;451:340–348. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzucco L, Borzini P, Gope R. Platelet-derived factors involved in tissue repair-from signal to function. Transfus Med Rev. 2010;24:218–234. doi: 10.1016/j.tmrv.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. P Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A, et al. Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am J Pathol. 2010;176:496–503. doi: 10.2353/ajpath.2010.080642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 10.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophth Vis Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- 11.Saint-Geniez M, Maharaj ASR, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, et al. endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. Plos One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamba T, Tam BYY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol-Heart C. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 13.Wadman M. London’s disastrous drug trial has serious side effects for research. Nature. 2006;440:388–389. doi: 10.1038/440388a. [DOI] [PubMed] [Google Scholar]
- 14.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 15.Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1:461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Cushing MC, Liao JT, Jaeggli MP, Anseth KS. Material-based regulation of the myofibroblast phenotype. Biomaterials. 2007;28:3378–3387. doi: 10.1016/j.biomaterials.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Control Release. 2007;122:287–296. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudalla GA, Koepsel JT, Murphy WL. Surfaces that sequester serum-borne heparin amplify growth factor activity. Adv Mater. 2011 doi: 10.1002/adma.201103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudalla GA, Kouris NA, Koepsel JT, Ogle BM, Murphy WL. Harnessing endogenous growth factor activity modulates stem cell behavior. Integr Biol (Camb) 2011;3:832–842. doi: 10.1039/c1ib00021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piossek C, Thierauch KH, Schneider-Mergener J, Volkmer-Engert R, Bachmann MF, Korff T, et al. Potent inhibition of angiogenesis by D,L-peptides derived from vascular endothelial growth factor receptor 2. Thromb Haemostasis. 2003;90:501–510. doi: 10.1160/TH03-02-0106. [DOI] [PubMed] [Google Scholar]
- 21.Goerges AL, Nugent MA. pH regulates vascular endothelial growth factor binding to fibronectin: a mechanism for control of extracellular matrix storage and release. J Biol Chem. 2004;279:2307–2315. doi: 10.1074/jbc.M308482200. [DOI] [PubMed] [Google Scholar]
- 22.Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SH, Kiick KL. Heparin-mimetic sulfated peptides with modulated affinities for heparin-binding peptides and growth factors. Peptides. 2007;28:2125–2136. doi: 10.1016/j.peptides.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maynard HD, Hubbell JA. Discovery of a sulfated tetrapeptide that binds to vascular endothelial growth factor. Acta Biomater. 2005;1:451–459. doi: 10.1016/j.actbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Hudalla GA, Murphy WL. Biomaterials that regulate growth factor activity via bioinspired interactions. Adv Funct Mater. 2011;21:1754–1768. doi: 10.1002/adfm.201002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung HJ, Kim HK, Yoon JJ, Park TG. Heparin immobilized porous PLGA microspheres for angiogenic growth factor delivery. Pharm Res. 2006;8:1835–1841. doi: 10.1007/s11095-006-9039-9. [DOI] [PubMed] [Google Scholar]
- 27.Willerth SM, Johnson PJ, Maxwell DJ, Parsons SR, Doukas ME, Sakiyama-Elbert SE. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J Biomed Mater Res A. 2007;80:13–23. doi: 10.1002/jbm.a.30844. [DOI] [PubMed] [Google Scholar]
- 28.Lin CC, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials. 2009;30:4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]