Many different terms related to fibrinogen and fibrin have come into general use in the fibrin(ogen) field, but there is much confusion and controversy over some of the terminology. The existing terminology related to fibrinogen structure and fibrin polymerization and recommendations on the standardization of commonly used nomenclature have been discussed at the 52nd and 53rd annual meetings of the ISTH Scientific and Standardization Committee (SSC), Subcommittee on Fibrinogen and Factor XIII. The recommendations on nomenclature of fibrinogen and fibrin presented here are based on numerous comments and suggestions made by the Subcommittee members and leading scientists from the fibrin(ogen) field. They have been approved by the Subcommittee at the 54th ISTH SSC meeting in Vienna in 2008.
1. Recommended terms and abbreviations to designate different levels of the structural organization of fibrinogen
1.1. Polypeptide chain composition
The fibrinogen molecule consists of two identical subunits, each of which is formed by three non-identical polypeptide chains denoted Aα, Bβ, and γ according to the recommendations of the International Committee on Haemostasis and Thrombosis in 1973 [1] (Fig. 1A). Letters “A” and “B” in the Aα and Bβ chains designate respectively fibrinopeptide A (residues 1–16) and fibrinopeptide B (residues 1–14), which are cleaved by thrombin upon conversion of fibrinogen into fibrin. The commonly used numbering of fibrinogen sequence is based on the processed mature product (polypeptide chains), however, the Human Genome Variation Society recommends using the primary translation product including signal peptide sequences. A nomenclature Working Group of the SSC is currently considering the adoption of this numbering system for all coagulation factors. The amino acid sequence is numbered from the first methionine of the protein as +1. Potential confusion can be avoided by always referring to the reference sequence and start point within it. If desired, double numbering with a residue number of the mature product in parentheses can be used.
Fig. 1. Fibrinogen structure.
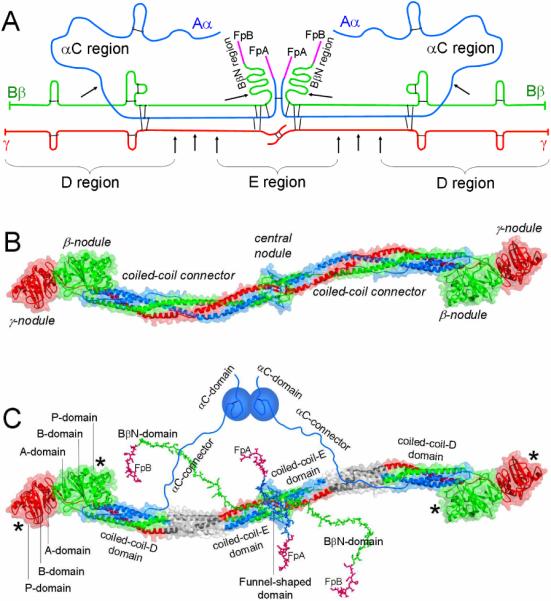
Panel A, polypeptide chain composition of fibrinogen. The individual chains, Aα, Bβ and γ, are blue, green and red, respectively; fibrinopeptides A and B (FpA and FpB) are magenta; the disulfide bonds are shown by black bars; triple arrows show proteolytic cleavages between the D and E regions, single arrows show cleavages resulting in the removal of the αC and BbN regions. Panel B, crystal structure of fibrinogen [8]. The central nodule is formed by the NH2-terminal portions of all six chains; it is connected to the distal β- and γ-nodules formed by the COOH-terminal portions of the Bβ and γ chains, respectively, by triple-helical coiled-coil connectors, each formed by the middle portions of the Aα, Bβ and γ chains. The color scheme is the same as in panel A. Panel C shows the same molecule as in panel B plus those regions that were not identified in the crystal structure, the interacting αC-domains, which are attached to the bulk of the molecule with the flexible αC-connectors, and the NH2-terminal portions of the Bβ chains forming the BbN regions (functional BβN-domains). Note, that the BβN-domains are shown in random conformation as in [23], and that this model does not present their interaction with the αC-domains identified in [24]. The funnel-shaped domain in the center contains fibrinopeptides A (colored magenta), which are also shown in random conformation as in [23]; the γN-domain [6] is located on the opposite side of the molecule and is not visible. The individual domains of the D regions, A-domain, B-domain, and P-domain, are indicated only in one subunit of the molecule. The site `a' or hole 'a' and site `b' or hole `b' in the P-domain of the γ- and β-nodules, respectively, are indicated by asterisks. The three chains are colored as in panels A and B, their protease sensitive portions between the D and E regions, which are not present in the D and E fragments, are shown in grey.
Recommended terms for individual chains: Aα chain, Bβ chain, γ chain.
Recommended abbreviations for fibrinopeptides A and B: FpA, FpB.
Not recommended: Aα-chain, Bβ-chain, γ-chain; fpA, FPA, fpB, FPB.
1.2. Structural regions
The very low resolution structure of fibrinogen originally obtained by electron microscopy revealed three linearly arranged nodules connected by two rod-like strands [2]. It was also shown that plasmin cleavage of fibrinogen results in a set of core fragments that were designated as A, B, C, D and E fragments based on the order of their elution upon DEAE-cellulose chromatography [3]. Among these fragments, E contains the central and D contains one of the two identical terminal regions of fibrinogen. The prevalence of studies using these fragments has led to extensive use of descriptive terms such as D domain and E domain. Respectively, the structure of fibrinogen was often presented as consisting of three linearly arranged domains, D-E-D. However, subsequent studies revealed that the D and E fragments each consist of a number of independently folded and structurally distinct domains [4–6]. Therefore, fibrin(ogen) regions corresponding to these fragments cannot be called D and E domains; it is more appropriate to denote them as D and E regions, respectively (Fig. 1A). Following this logic, it is reasonable to denote the COOH-terminal regions of the Aα chains (residues ~221–610), which are readily removed from fibrin(ogen) at the early stages of its proteolytic degradation, as aC regions. Similarly, the NH2-terminal regions of the Bβ chains (residues ~1–55), which are also readily removed upon proteolysis, can be denoted as BβN regions. Because “B” stands for FpB, in fibrin these regions should be called βN regions. Note that the D and E regions correspond to the core D and E fragments, respectively, while αC and BβN correspond to the regions that are removed from fibrinogen upon proteolysis and then degraded to smaller fragments.
Recommended terms for fibrin(ogen) regions: Fibrinogen contains the central E region and two terminal D regions, two αC regions, and two BβN regions (βN regions in fibrin).
Not recommended: D domain or terminal domain, E domain or central domain.
1.3. Overall structure
The higher resolution 3D structure of more than two-thirds of the fibrinogen molecule including the D and E regions was established by X-ray analysis of crystals of fibrinogen and its D and E fragments [5–8]. The structure revealed the overall shape and domain composition of the molecule (Fig. 1B). In each subunit, the Aα, Bβ and γ chains form a triple helical coiled-coil, which links the central nodule, composed of the disulfide-linked NH2-terminal portions of all six chains, with the distal nodules, formed by the COOH-terminal portions of the β and γ chains. The central nodule is sometimes called the central domain; however, it contains two crystallographically distinct domains [6]. Similarly, although the distal nodules are sometimes called γ or γC domain and β or βC domain, the crystal structure clearly indicates that each of these nodules consists of three crystallographically distinct domains [5,9].
Recommended terms for describing the overall structure of fibrinogen: Fibrinogen consists of the central nodule connected to the distal β- and γ-nodules by two coiled-coil connectors.
Not recommended: central domain, γ or γC domain, β or βC domain.
It should be noted that the polypeptides forming the β- and γ-nodules are homologous to each other and to fibrinogen-like sequence segments found in a number of non-related proteins [10]. Thus, each of these nodules is representative of a typical protein module and therefore they are often called β-module and γ-module [11]. This terminology is also acceptable.
1.4. Domain structure
According to the crystal structure, the fibrinogen E region contains four structural domains and each D region contains seven structural domains [5–8] (Fig. 1C). In each subunit of the E region (E fragment), the COOH-terminal portions of all three chains form a triple helical coiled-coil-E domain. The NH2-terminal portions of both γ chains meet each other at the center to form a single asymmetric domain, originally denoted as the γN-domain [6]. On the opposite side, the portions of two Aα and two Bβ chains form another structural domain, denoted as the funnel-shaped domain [6]. In the D region (D fragment), the NH2-terminal portions of all three chains form a triple helical coiled-coil-D domain. The remaining COOH-terminal portions of the β and γ chains forming the β-nodule (β-module) and γ-nodule (γ-module), respectively, each consists of three structural domains. Originally these domains were identified in the crystal structure of the recombinant γ-module and denoted as NH2-terminal A-domain, central B-domain, and COOH-terminal P-domain (P domain contains polymerization site) [9].
Recommended terms for the E region domains: The E region consists of the γN-domain, funnel-shaped domain, and two coiled-coil-E domains.
Recommended terms for the D region domains: The D region includes the coiled-coil-D domain and NH2-terminal A-domain, central B-domain, and COOH-terminal P-domain in each γ-and β-nodule.
Although the structure of the remaining fibrinogen regions is less defined, it is well established that each αC region consists of two structurally very distinct portions. Namely, the COOH-terminal portion (residues ~Aα392–610) contains an independently folded compact domain [12] whose structure was partially solved by NMR [13], while the NH2-terminal portion (residues ~Aα221–391) forms a flexible tether connecting this domain to the bulk of the molecule (Fig. 1C). Therefore, it was proposed to refer to the compact part as αC-domain and to the flexible part as αC-connector [12,14]. The two BβN regions contain a number of functionally important binding sites that become fully active after removal of FpB. The fact that these regions have not been identified in the crystal structure of fibrinogen [8] does not exclude a possibility that they may form ordered structures (domains). Thus, until their folding status is established, they can be considered as functional domains, which were originally denoted as BβN-domains [15].
Recommended terms for the αC and BβN region domains: Each αC region consists of the αC-domain and αC-connector; each BβN region forms the functional Bβ N-domain, which in fibrin should be called the βN-domain to reflect the absence of FpB.
2. Recommended terms and abbreviations to designate different forms of fibrin
The thrombin-mediated chemical reactions result in the removal of fibrinopeptides that triggers the process responsible for the formation of a fibrin clot. This process is designated as fibrin polymerization or fibrin assembly.
2.1. Polymerization sites
Cleavage of fibrinopeptides A and B exposes binding sites `A' and `B' in the E region that are complementary to sites `a' and `b' always exposed in the D regions [16] (Fig. 2A–B). The polymerization sites have also been called holes and knobs [17]. X-ray crystallographic studies of fibrinogen fragments revealed binding pockets, the so-called holes (Fig. 1C), in which the peptides corresponding to the newly exposed amino terminal ends of the α and β chains of fibrin, the so-called knobs, bind [5]. Since the structure of the actual complexes that occur in fibrin have not been observed, it is not known if the binding sites consist only of the peptides fitting into the holes or are more extensive.
Fig. 2. Fibrin monomer species and polymeric fibrin structures.
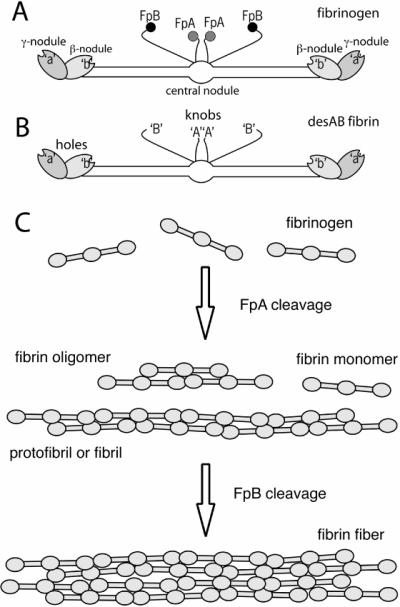
Schematic diagrams of fibrinogen (panel A), showing FpA, FpB, hole `a', hole `b', and desAB fibrin monomer (panel B), showing the exposure of knobs `A' with cleavage of FpA and the exposure of knobs `B' with cleavage of FpB. Panel C, initial steps of fibrin polymerization, showing fibrin oligomers, protofibrils or fibrils, and fibers.
Recommended terms for polymerization sites: knobs `A' and `B' and complementary holes `a' and `b'; sites `A' and `B' and complementary sites `a' and `b'.
The letters here are placed inside single quotation marks to make it clear that these are symbols, especially to avoid confusion with the article `a.' The sites may be more than just the knobs or holes. The peptides can be called synthetic knobs.
2.2. Intermediates in fibrin polymerization
2.2.1. Products of thrombin cleavage of the fibrinopeptides
The two pairs of fibrinopeptides are cleaved from fibrinogen whose structure may be described as (Aα,Bβ,γ)2, to yield fibrin with structure (α,β,γ)2 (Fig. 2A–B). Initially, there are soluble species of fibrin molecules [18,19]. There are different forms of fibrin monomer depending on the particular fibrinopeptides removed. Ordinarily, fibrinopeptide A is cleaved more rapidly from fibrinogen to produce desA fibrin, (α,Bβ,γ)2. Then, fibrinopeptide B is cleaved to produce desAB fibrin, (α,β,γ)2. Under certain circumstances only or primarily fibrinopeptide B is cleaved to produce desB fibrin, (Aα,β,γ)2.
Recommended terms for fibrin molecules: fibrin monomer or monomeric fibrin
Recommended terms for fibrin monomer species: desA fibrin – molecules missing only FpA, desB fibrin – molecules missing only FpB, desAB fibrin – molecules missing both FpA and FpB
Not recommended: α-fibrin, fibrin 1, β-fibrin, αβ-fibrin, fibrin 2
In special cases where it is necessary to distinguish between cleavage of one or more of each fibrinopeptide, the terminology, desA fibrin, desAA fibrin, desAAB fibrin, desAABB fibrin, can be used.
2.2.2. Polymeric fibrin structures
Fibrin polymerization proceeds through specific interactions via these binding sites to make dimers, trimers, and larger polymers (Fig. 2C). These structures are two-stranded and half-staggered. Smaller structures are fibrin oligomers. As they grow in length, they are called protofibrils or fibrils, which can aggregate laterally to form fibers that associate with each other to make fiber bundles [19,20].
Recommended terms for fibrin polymer species: fibrin oligomer – a polymer consisting of only a few fibrin monomer units
protofibril or fibril – a two-stranded polymer made up of many fibrin monomers
fiber – a group of protofibrils associated with each other laterally
fiber bundle – several individual fibers associated with each other laterally
2.3. Factor XIIIa-mediated cross-linking
Fibrin structure is reinforced by Factor XIIIa [21], which forms covalent bonds between the α and α chains resulting in γ-γ dimers and α polymers. To a chemist, transglutaminases like Factor XIIIa catalyze ligation rather than cross-linking [22]. Since the `cross-linking' nomenclature is so commonly used, it is unlikely to be changed, but appropriate reference to ligation is also encouraged.
Acknowledgements
We thank the members of the Fibrinogen and Factor XIII Subcommittee of the Scientific Standardization Committee of the International Society on Thrombosis and Haemostasis and all other scientists who made suggestions on this nomenclature. We acknowledge support of NIH grants HL-30954 (to J.W.) and HL-56051 (to L.M.).
References
- 1.Henschen A, McDonagh J. Fibrinogen, fibrin and factor XIII. In: Zwaal RFA, Hemker HC, editors. Blood Coagulation. Elsevier Science Publishers; Amsterdam: 1986. pp. 171–241. [Google Scholar]
- 2.Hall CE, Slayter HS. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959;5:11–6. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussenzweig V, Seligmann M, Pelimont J, Grabar P. Les produits de degradation du fibrinogene humain par la plasmine. Ann Inst Pasteur. 1961;100:377–89. [PubMed] [Google Scholar]
- 4.Privalov PL, Medved LV. Domains in the fibrinogen molecule. J Mol Biol. 1982;159:665–83. doi: 10.1016/0022-2836(82)90107-3. [DOI] [PubMed] [Google Scholar]
- 5.Spraggon G, Everse SJ, Doolittle RF. Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature. 1997;389:455–62. doi: 10.1038/38947. [DOI] [PubMed] [Google Scholar]
- 6.Madrazo J, Brown JH, Litvinovich S, Dominguez R, Yakovlev S, Medved L, Cohen C. Crystal structure of the central region of bovine fibrinogen (E5 fragment) at 1.4- Å resolution. Proc Natl Acad Sci U S A. 2001;98:11967–72. doi: 10.1073/pnas.211439798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JH, Volkmann N, Jun G, Henschen-Edman AH, Cohen C. The crystal structure of modified bovine fibrinogen. Proc Natl Acad Sci USA. 2001;97:85–90. doi: 10.1073/pnas.97.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Kollman JM, Pandi L, Doolittle RF. Crystal structure of native chicken fibrinogen at 2.7 Å resolution. Biochemistry. 2001;40:12515–23. doi: 10.1021/bi011394p. [DOI] [PubMed] [Google Scholar]
- 9.Yee VC, Pratt KP, Cote HC, Trong IL, Chung DW, Davie EW, Stenkamp RE, Teller DC. Crystal structure of a 30 kDa C-terminal fragment from the γ chain of human fibrinogen. Structure. 1997;5:125–38. doi: 10.1016/s0969-2126(97)00171-8. [DOI] [PubMed] [Google Scholar]
- 10.Doolittle RF. A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Sci. 1992;1:1563–77. doi: 10.1002/pro.5560011204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medved L, Litvinovich S, Ugarova T, Matsuka Y, Ingham K. Domain structure and functional activity of the recombinant human fibrinogen γ-module (γ148–411) Biochemistry. 1997;36:4685–93. doi: 10.1021/bi962795l. [DOI] [PubMed] [Google Scholar]
- 12.Tsurupa G, Tsonev L, Medved L. Structural organization of the fibrin(ogen) αC-domain. Biochemistry. 2002;41:6449–59. doi: 10.1021/bi025584r. [DOI] [PubMed] [Google Scholar]
- 13.Burton RA, Tsurupa G, Hantgan RR, Tjandra N, Medved L. NMR solution structure, stability, and interaction of the recombinant bovine fibrinogen αC-domain fragment. Biochemistry. 2007;46:8550–60. doi: 10.1021/bi700606v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medved LV, Gorkun OV, Privalov PL. Structural organization of C-terminal parts of fibrinogen Aα-chains. FEBS Lett. 1983;160:291–5. doi: 10.1016/0014-5793(83)80985-5. [DOI] [PubMed] [Google Scholar]
- 15.Gorlatov S, Medved L. Interaction of fibrin(ogen) with the endothelial cell receptor VE-cadherin: mapping of the receptor-binding site in the NH2-terminal portions of the fibrin β chains. Biochemistry. 2002;41:4107–16. doi: 10.1021/bi0160314. [DOI] [PubMed] [Google Scholar]
- 16.Budzynski AZ, Olexa SA, Pandya BV. Fibrin polymerization sites in fibrinogen and fibrin fragments. Ann NY Acad Sci. 1983;408:301–314. doi: 10.1111/j.1749-6632.1983.tb23253.x. [DOI] [PubMed] [Google Scholar]
- 17.Laudano AP, Doolittle RF. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci USA. 1978;75:3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hantgan RR, Hermans J. Assembly of fibrin. A light scattering study. J Biol Chem. 1979;254:11272–11281. [PubMed] [Google Scholar]
- 19.Weisel JW. Fibrinogen and Fibrin. In: Parry DAD, Squire JM, editors. Advances in Protein Chemistry. vol. 70. Fibrous Proteins: Coiled-coils, Collagen and Elastomers; San Diego: Elsevier: 2005. pp. 247–299. [DOI] [PubMed] [Google Scholar]
- 20.Fowler WE, Hantgan RR, Hermans J, Erickson HP. Structure of the fibrin protofibril. Proc Natl Acad Sci USA. 1981;78:4872–4876. doi: 10.1073/pnas.78.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muszbek L, Ariens RA, Ichinose A. Factor XIII: recommended terms and abbreviations. J Thromb Haemost. 2006;5:181–183. doi: 10.1111/j.1538-7836.2006.02182.x. [DOI] [PubMed] [Google Scholar]
- 22.Ferry JD. The conversion of fibrinogen to fibrin: events and recollections from 1942 to 1982. Ann NY Acad Sci. 1983;408:1–10. doi: 10.1111/j.1749-6632.1983.tb23229.x. [DOI] [PubMed] [Google Scholar]
- 23.Pechik I, Yakovlev S, Mosesson MW, Gilliland GL, Medved L. Structural basis for sequential cleavage of fibrinopeptides upon fibrin assembly. Biochemistry. 2006;45:3588–97. doi: 10.1021/bi0525369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litvinov RI, Yakovlev S, Tsurupa G, Gorkun OV, Medved L, Weisel JW. Direct evidence for specific interactions of the fibrinogen αC-domains with the central E region and with each other. Biochemistry. 2007;46:9133–42. doi: 10.1021/bi700944j. [DOI] [PMC free article] [PubMed] [Google Scholar]


