Innovation — the discovery of ways to get more value from limited resources — is critically important for both society’s health and material standard of living. A widely held view is that there would be too little innovation without government support.1 This view is common in the drug discovery sector, which has faced declining productivity and mounting costs over the last three decades.2
Governments support drug discovery and development in several ways, including direct and indirect subsidies of basic research, clinical trials and other costs associated with research and development. The most important policy interventions, however, are patents and other forms of protection for intellectual property. Patents provide innovators a period of market exclusivity, during which manufacturers of low-priced generic copies are kept at bay. This allows an innovator to earn more sales revenue than would otherwise be possible; this revenue, in turn, can help cover the costs of research and development.
Patents have many limitations,3,4 and alternative approaches to supporting drug innovation may work better.5 Nevertheless, it appears that the patent system is not going away any time soon. So, if we are to continue to rely on patents to support drug innovation, we need a low-cost way of ensuring that market exclusivity is being sustained on the basis of valid patents; that is, patents that pass the test of novelty, utility and nonobviousness.
We argue that the current system is broken. The adjudication of patent validity (and hence the period of market exclusivity) is determined by extraordinarily costly and time-consuming litigation between generic and brand (i.e., innovator) drug companies. Part of the problem stems from the very complex set of regulations and case law governing the market entry of generic drugs, which has created legal uncertainty. The primary reason, however, is that generic and brand drug companies have opposed interests: brand drug companies wish to maximize the period of market exclusivity, whereas generic drug companies wish to minimize it. Firms will use whatever tools are available to them from the regulations to pursue these goals. In this article, we describe how adaptations by these firms to Canadian regulations have generated these costs. A key policy issue is to determine what tools brand and generic drug companies should be able to use to contest market exclusivity. We propose some solutions.
Pharmaceutical intellectual property regulations in Canada from 1969 to present
The federal government has changed its policy on pharmaceutical intellectual property many times during the last four decades. The changes that most affected market exclusivity for brand drugs, however, occurred in 1969, 1987, 1993, 1995 and 2006.
In 1969, the Patent Act was amended to allow generic drug firms to import the active ingredients needed to produce and sell copies of brand drugs that were still under patent (Box 1). This authorization was by way of a “compulsory license,” granted by the Commissioner of Patents to generic firms. Generic drug companies, in turn, were required to pay a royalty of 4% of their drug’s selling price to the innovator firm.8 This policy change is credited with the expansion of Canada’s domestic generic drug sector. However, it was not welcomed by the multinational brand drug industry. The industry exerted pressure during subsequent trade negotiations to bring Canada’s laws in line with standards in the United States. Thus, the major policy changes preceded the passage of the Free Trade Agreement in 1988 with the United States, the North American Free Trade Agreement in 1994 and the Agreement on Trade Related Aspects of Intellectual Property Rights (TRIPS) in 1995 brokered by the World Trade Organization.
Box 1: Summary of Canadian laws protecting pharmaceutical intellectual property.
The federal government uses three sets of regulations that attempt to “balance effective patent enforcement over new and innovative drugs with the timely market entry of their lower-priced generic competitors.”6
The Patent Act: Pharmaceutical firms can apply for patents to obtain 20 years of exclusivity for an invention disclosed in a patent. Examiners at the Canadian Intellectual Property Office, a division of Industry Canada, decide which innovations are worthy of patent protection on the basis of several legal criteria, most notably novelty, utility, and nonobviousness to a person “skilled in the art.”7 Typically, commercially successful drugs have numerous patents disclosing the active ingredient, coatings, therapeutic indications, dosing, manufacturing methods and other aspects of the drug.
The Patented Medicines (Notice of Compliance) regulations: In short, a firm wishing to sell a generic drug must address patents asserted to be relevant by the patent owner before it can be sold. For any given patent, a generic drug firm can either await expiry or allege that the patent is invalid or not infringed. If it chooses the latter path, the brand drug firm can trigger a judicial proceeding in which the merits of the allegations are assessed in federal court. Health Canada is prohibited from granting market approval to the generic drug until after the matter is adjudicated, or 24 months elapse, whichever comes first.
Canada’s unique pharmaceutical patent system means that generic drug firms may have to litigate a single brand patent twice. First, they may face litigation over their allegation of patent invalidity under the Notice of Compliance regulations. Second, after launching the generic drug, they risk being sued for infringement under the Patent Act. Similarly, a brand drug firm that wins under the Notice of Compliance regulations may be forced to defend a patent’s validity again in a patent impeachment action.
Data protection regulations: These regulations essentially guarantee brand drugs a minimum period of market exclusivity. A generic drug firm cannot apply for regulatory approval, by establishing its bioequivalence to the reference brand drug, until the brand drug has been on the market for six years. It cannot receive regulatory approval until the brand drug has been on the market for at least eight years. A six-month extension to these minimums is granted for drugs that have undergone clinical trials in pediatric populations.
The rationale for the data protection regulations is that the clinical trials mandated by Health Canada can use up much of a brand drug’s patent life. In these cases, the period of effective market exclusivity may be too short.
Data protection privileges are restricted to “innovative drugs,” defined under section C.08.004.1(1) of the Food and Drug Regulations as “A drug that contains a medicinal ingredient not previously approved in a drug by the Minister and that is not a variation of a previously approved medicinal ingredient such as a salt, ester, enantiomer, solvate or polymorph.”
Although the first set of reforms, passed in 1987, did not eliminate compulsory licensing, they provided brand drug firms with a minimum period of market exclusivity of between 7 and 10 years, depending on whether the active ingredient was imported or produced domestically. The legislation also changed the length of patent terms. Beginning in 1989, patents were for 20 years from the date on which the patent application was filed, rather than 17 years from the date the patent was issued.8
In 1993, the federal government eliminated compulsory licensing. Brand drugs thus enjoyed all the protections afforded under the Patent Act (Box 1). The Patent Act governs patents generally, including the standards of novelty that determine whether an innovation is eligible for a patent and the calculation of damages payable from patent infringement. Typically, commercially successful drugs have many patents related to them, with the filing occurring at different times, leading to a series of staggered expiry dates.9–12 Patents can be awarded for identifying a family of compounds that have therapeutic potential (genus patents) and for identifying specific members in a family with therapeutic activity (selection patents) not anticipated by genus patents. Patents may also be provided for identifying indications for use, dosing regimens and methods of drug manufacture.
Two additional sets of regulations, modelled on the system in the United States,9,13 were introduced in 1993 and 1995. The Patented Medicines (Notice of Compliance) regulations were introduced in 1993. The Notice of Compliance regulations govern the market entry of generic versions of brand drugs that are still under patent protection. In particular, a generic firm must issue a Notice of Allegation to the manufacturer of the reference brand drug that the existing patents are either invalid or not infringed by the generic drug. The brand firm, should it disagree, can petition the federal court to prohibit the generic product from entering the market until after the matter is adjudicated or 30 months have elapsed, whichever comes first. (In 1998, the 30-month stay was reduced to 24 months.) The federal court can prohibit the sale of the generic drug by ordering the Minister of Health to refrain from issuing a Notice of Compliance to the generic drug firm. A Notice of Compliance is required for a prescription drug to be marketed in Canada.
The patents that a generic drug firm needs to address are listed in Health Canada’s Patent Register (analogous to the Orange Book in the United States). These patents are typically a subset of all patents that could potentially apply to a drug. Not surprisingly, the type of patents eligible for listing in the Patent Register has been the subject of considerable controversy and litigation.14
The Notice of Compliance proceedings are unlike patent infringement suits. First, they are intended to be brief. They do not entail full exploration of the evidence that would otherwise be considered in patent infringement proceedings.12 Rather, litigation consists of an out-of-court exchange of affidavit evidence and cross-examination, followed by a hearing that lasts between two and five days. Various motions can precede the actual hearing, including multiple variations on what evidence is admissible. This process currently takes 18–19 months on average to resolve.14 Conservatively, the cost of litigating a Notice of Compliance proceeding to conclusion is $1–2 million per side, assuming that the proceeding runs smoothly. This is consistent with estimates from similar litigation in other jurisdictions.15–17
Second, Notice of Compliance proceedings do not determine patent validity. Even if an allegation of patent invalidity is found to be justified, the patent is still valid for every other purpose, and the patentee may still sue for infringement under the Patent Act. In some recent cases, pharmaceutical patents have been deemed either invalid or not infringed under the Notice of Compliance regulations but deemed valid or infringed in a later infringement proceeding.18
The second set of regulations concerning pharmaceutical intellectual property are the “data protection regulations” found in the Food and Drugs Act. When introduced in 1995, these provisions guaranteed brand drugs five years of market exclusivity following regulatory approval. Data protection is a binding constraint on entry of generic drugs only if the market exclusivity offered by the patent system is less than five years. This would occur, for instance, if most of a drug’s patent life were spent while the drug’s safety and efficacy was under regulatory review. The minimum term of data protection was lengthened to eight years in 2006; now, generic drugs are not allowed to apply for Notice of Compliance until year six. An international treaty currently being negotiated by Canada, which has ramifications for Canada’s patent laws similar to those of the North American Free Trade Agreement and TRIPS trade agreements in the 1990s, could extend the minimum term of data protection from 8 to 10 years.19
Strategic responses to the intellectual property regulations
Before 1993, brand drug firms had no tools at their disposal to influence the length of market exclusivity afforded to their products. Terms of exclusivity were determined by the entry decisions of generic drug firms, subject to the 7–10 year minimums established in 1987. The introduction of the Notice of Compliance regulations in 1993 changed things considerably. Brand drug firms now had several ways to extend exclusivity, namely by a) opposing a generic’s Notice of Allegation, thereby triggering the automatic stay on generic drug entry while the matter was before the courts; b) maximizing the number of patents listed on the Patent Register that the generic drug was required to address; and c) appealing court decisions that were in favour of the generic. (An appeal would be viable only if the generic drug firm needed to address additional patents on the Patent Register after a favourable Notice of Allegation decision. An appeal after the generic drug was launched would not restore exclusivity.)
Generic drug firms could expedite market entry by serving Notice of Allegations on some or all of the patents shortly after the brand drug was marketed. Indeed, some brand drugs have had relatively short periods of market exclusivity, including Fosamax (alendronate), which had 6.3 years of market exclusivity, Remeron (mirtazapine) with 2.7 years, Lamictal (lamotrigine) with 4.8 years, and Pariet (rabeprazole) with 6.2 years.
To delay entry, some brand drug firms use several strategies together for maximum effect. For example, GlaxoSmithKline received regulatory approval for its antidepressant Paxil (paroxetine) in March 1993. In August 1997, the generic drug firm Apotex served Notices of Allegation on the four patents listed on the Patent Register at the time. GlaxoSmithKline opposed the initial notices, triggering an automatic stay (which was then 30 months). While this matter was before the federal court, GlaxoSmithKline listed four additional patents on the Patent Register, so that when the court ruled in favour of Apotex 28 months later, Apotex was required to address a fresh set of patents. Apotex duly served another Notice of Allegation, which triggered another automatic stay. Meanwhile, GlaxoSmith-Kline appealed the court’s earlier ruling. The strategy of triggering an automatic stay, listing new patents and appealing an earlier unfavourable ruling was repeated several times, ultimately delaying Apotex’s market launch until October 2003.20 Had GlaxoSmithKline not engaged in this strategy, Apotex would have received its Notice of Compliance in October 1999. GlaxoSmithKline’s strategy thus prolonged Paxil’s market exclusivity by four years, earning it an estimated $300 million in extra sales revenues,21 despite the federal court siding with Apotex in its review of each of the Notice of Allegations.
The Notice of Compliance regulations allow companies whose generic drugs are kept off the market inappropriately to sue brand drug firms for lost profits. However, the prospect of paying damages has not been a deterrent to some brand drug firms. Because a brand drug firm can earn greater profits from the sale of its drug than a generic drug could earn, the former can compensate the latter and still have money left over. (Profits from the sale of a generic drug are lower because the price is regulated to be a fraction of the cost of the brand drug. Also, generic drugs often offer discounts off the list prices to compete with other generic drug firms for pharmacy business.) The Notice of Compliance regulations do not speak to the rights, if any, of drug insurers and consumers to claim compensation for delays in the availability of generic drugs. In the case of Paxil, attempts to certify a national class action lawsuit on behalf of insurers and consumers have so far been unsuccessful.22
In 2006, the federal government amended the Notice of Compliance regulations to eliminate some strategies available to brand and generic drug companies. Now, a generic drug firm need only address patents listed on the register before it applies to Health Canada for regulatory approval. Hence, patents listed afterward cannot be used to trigger a 24-month automatic stay. But, as per the amended data protection regulations, generic drugs cannot apply for regulatory approval until after the brand drug has been on the market for six years. Brand drug firms thus have some time to list additional patents on the register. The 2006 amendments to the Notice of Compliance regulations, however, limit the types of patents eligible for inclusion on the register. In the Paxil case and others, the brand drug firm was able to list patents claiming new coatings, crystalline forms, manufacturing processes and other such modifications, even though some of these patents were unrelated to the drug being sold. Now, patents must pertain to the medicinal ingredient, formulation, dosage form or therapeutic indication of the specific drug given regulatory approval by Health Canada.23
Problems with the current regulations
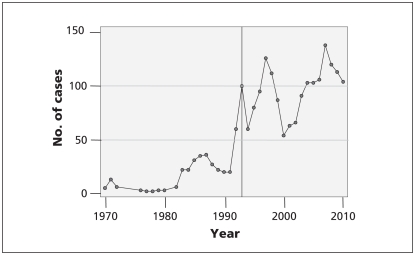
The present system has created two problems. First, it has generated an extraordinarily large amount of litigation between brand and generic drug companies. Figure 1 plots the annual number of cases considered by the federal court and the federal court of appeal in which a generic drug firm is either an applicant or respondent. “Cases” include any petitions made to the court, including applications for prohibition orders, judicial review and appeals. There has been a marked increase in litigation since the introduction of the Notice of Compliance regulations in 1993 (59 cases in 1992, 103 in 2010).
Figure 1:
Number of federal court cases in which a generic drug firm was either an applicant or a respondent, 1970–2010. The line represents the introduction of Notice of Complience regulations in 1993. Source: court index and docket of federal court and federal court of appeal (http://cas-ncr-nter03.cas-satj.gc.ca/IndexingQueries/infp_queries_e.php).
Drug companies do not normally report litigation expenses. However, Apotex recently claimed that, in the last 10 years, it has spent $300–$400 million on litigation in Canada (Jack Kay, Apotex, Toronto, Ont.: personal communication, 2011). Because brand drug firms have greater profit at stake than do generic drug firms, we can expect that they are spending at least as much as Apotex and other generic drug firms on litigation. Extrapolating the costs of litigation claimed by Apotex to other generic and brand drug firms, it appears that the costs of litigating pharmaceutical patents in Canada are well over $100 million dollars annually. These costs inevitably are passed on to drug plans and consumers in the form of higher prices.24
It is unclear to what extent the 2006 reforms will reduce the amount of litigation. Brand drug firms are now unable to trigger multiple stays by strategically listing marginal patents. However, if the drug is commercially successful, generic drug firms will continue to work around patents where possible and challenge patents perceived as being weak, and brand drug firms will likely continue to patent judiciously and contest generic entry so as to prolong exclusivity.
Another reason for increased litigation is that the reforms do little to clarify the complex legal standards that govern the conduct of the drug firms. This complexity stems from the fact that three intertwined sets of rules affect exclusivity periods: the Patent Act, the Notice of Compliance regulations and the data protection regulations. Moreover, conflicting legal standards between these rules are creating a substantial degree of legal uncertainty.16,25 Since the Notice of Compliance regulations were introduced, the Supreme Court of Canada has had to resolve nine disputes about pharmaceutical intellectual property. One can imagine that the judges on the Supreme Court are concerned about this legal quagmire. In the seminal case of Free World Trust, the Supreme Court stated that “there is a high economic cost attached to uncertainty and it is the proper policy of patent law to keep it to a minimum.”26 Referring to an earlier decision,27 the court stipulated that “The patent owner, competitors, potential infringers and the public generally are thus entitled to clear and definite rules as to the extent of the monopoly conferred.”
A second problem with the existing policy for intellectual property is only now emerging. Challenges by generic drug companies to brand drug patents, while contributing to the growing burden of litigation described above, confer a spillover benefit to drug plans and consumers. That is, these legal challenges ensure that brand drug firms are unable to extend exclusivity — and high prices — based on patent claims that fail the test of novelty, utility or nonobviousness. Generic firms perform this role, not as a public service, but in the pursuit of profits. The profits from patent challenges, however, appear to be declining. The reason is that a generic firm entering the marketplace following a favourable Notice of Compliance decision risks being sued for patent infringement (the Notice of Compliance proceedings do not determine patent validity). This exposes the firm to financial risk: generic drugs sell at a fraction of the cost of brand drugs, but damages are calculated based on the full price of the brand drug. The financial risk is thus greater the lower the price of the generic drug. As a result, recent decisions by provincial drug plans to reduce the prices of generic drugs to as low as 25% of the brand drug price28 may have inadvertently increased the financial risk to generic drug firms that challenge patents. Given the uncertainty of the outcomes of infringement suits, this risk may be deemed to be unacceptably high. The generic drug firm that successfully shows patent invalidity opens up entry for all generic competitors. In these circumstances, it may be better to wait for other generic drug firms to invest in risky patent challenges.
Another possible outcome is that generic and brand drug firms may find it profitable to settle patent disputes out of court,29,30 again leading to extended exclusivity and high prices for payers.
Possible solutions
We urge policy-makers to reform the legal and regulatory framework governing the privileges of market exclusivity afforded to brand drugs. Several options deserve consideration. The first is to repeal the existing regulations and simply guarantee innovative drugs exclusivity for 10 years or some other fixed period. This would enhance investment certainty to brand drug firms and markedly reduce litigation because brand and generic drug firms would be less able to contest market exclusivity.
Providing fixed terms of exclusivity is a compromise solution; some brand drugs would be given longer periods of exclusivity under such a system while others would have a shorter period. But, under this proposal, the nominal period of exclusivity would become less important. All public drug plans, and an increasing number of private plans, now negotiate with brand firms over the prices to be paid for new drugs that are being considered for formulary listing. Drug plans currently use evidence of cost-effectiveness and forecasted budget impact in these negotiations. Common knowledge of the term of exclusivity could just as easily be incorporated into negotiations over the price paid and would reduce the uncertainty over the budget impact of listing a new drug.
Another option is to repeal the Notice of Compliance regulations and rely solely on the Patent Act and the data protection regulations. This would reduce litigation because patents could be litigated only once. Removing the Notice of Compliance regulations would also relieve Health Canada of the task of deciding which patents should be listed on the Patent Register, a job that critics suggest it lacks the legal expertise to perform properly.13 Also, the Notice of Compliance regulations are not required by Canada’s obligations under TRIPS.31,32
The government’s stated rationale for the Notice of Compliance regulations was to prevent abuse of the patent system by generic drug firms. Apparently, there was a concern that generic drug firms found to have infringed a patent would declare bankruptcy or otherwise fail to provide compensation. This concern, however, can be addressed much more effectively by requiring firms that launch a generic before the expiry of all relevant patents to post a performance bond that is relinquished in the event that the drug is found to have infringed on the patents.
Should the Notice of Compliance regulations remain in place and we continue to rely on generic drug firms to finance the cost of patent vetting, there are several options.14,33,34 The system in the United States, on which the Canadian Notice of Compliance regulations are modelled, provides a period of market exclusivity to the first generic drug firm that successfully addresses the existing patents. We do not favour a per se adoption of the 180-day stay, given the manipulation of these rules by both brand and generic drug firms in the United States.29,30,35 Instead, we propose that a prespecified, time-limited royalty be paid to the litigating generic by all sellers of bioequivalent products for each unit they sell.36 Such a mechanism would explicitly reward the generic firm that prevails in patent litigation for the benefit it creates by enabling competition.
There also appears to be some scope to expedite the Notice of Compliance proceedings. Federal court judges have little or no expertise in the complex technical issues that arise during patent litigation. Expert witnesses hired by the respective sides educate them. But this escalates costs because the advantage is with the party that can afford to have the most authoritative witnesses (who also tend to be the most expensive) on its side.37 Even then, federal court judges have felt overwhelmed at the job of “assimilating masses of purportedly expert opinions, predominantly on scientific matters, all in written form, often comprising several volumes.”38,39 One option is to use court-appointed experts who could help the judge quickly get to the core of a dispute. Because such experts would be paid by the court, they would have no allegiance to either party.
Other countries are facing the same challenges as Canada. None, however, have attempted fundamental reform of its laws on pharmaceutical intellectual property. It is time for Canada to show leadership.
Key points
Protection laws governing pharmaceutical intellectual property in Canada have led to costly litigation between brand and generic drug companies, costing over $100 million annually.
The problem stems from legal uncertainty created by the complex sets of regulations and the legal rights of brand and generic drug companies to contest the period of market exclusivity.
Recent decisions by provincial drug plans to lower generic drug prices may reduce the incentives for generic drug firms to contest market exclusivity, possibly resulting in longer exclusivity periods and higher costs to payers.
One solution would be to offer fixed periods of market exclusivity to innovative drugs, thereby enhancing investment certainty for brand drug firms and reducing litigation.
Acknowledgement
The authors thank several pharmaceutical industry employees (who requested anonymity) for their useful comments on an earlier version of this manuscript.
Footnotes
Competing interests: Paul Grootendorst has served on the Pharmaceutical Policy Network Advisory Committee of Canada’s Research-Based Pharmaceutical Companies (Rx&D). Paul Grootendorst and Aidan Hollis have served as consultants to the Canadian Generic Pharmaceutical Association and have provided expert testimony and/or reports on behalf of both innovator and generic drug companies. Aidan Hollis has received payment from bioMérieux Foundation for lectures about drug incentives, and he owns stock in Novartis. None declared by Ron Bouchard.
This article has been peer reviewed.
Contributors: Paul Grootendorst wrote the manuscript. Ron Bouchard and Aidan Hollis reviewed multiple drafts of the article and, along with Paul Grootendorst, revised it critically for important intellectual content. All of the authors approved the final version submitted for publication.
References
- 1.Brander JA. Presidential address: Innovation in retrospect and prospect. Can J Econ 2010;43:1087–121 [Google Scholar]
- 2.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov 2010;9:203–14 [DOI] [PubMed] [Google Scholar]
- 3.Boldrin M, Levine DK. Against intellectual monopoly. Cambridge (UK): Cambridge University Press; 2008 [Google Scholar]
- 4.Bessen J, Meurer MJ. Patent failure: how judges, bureaucrats, and lawyers put innovators at risk. Princeton (NJ): Princeton University Press; 2008 [Google Scholar]
- 5.Grootendorst P, Hollis A, Levine D, et al. New approaches to rewarding pharmaceutical innovation. CMAJ 2011;183:681–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regulatory impact analysis statement accompanying the regulations amending the Patented Medicines (Notice of Compliance) regulations, (2006) C Gaz I. Available: www.gazette.gc.ca/archives/p1/2006/2006-06-17/html/reg6-eng.html (accessed 2011 Aug. 29).
- 7.Whirlpool Corp. v. Camco Inc. 2 SCR. 1067. 2000. Available: http://scc.lexum.org/en/2000/2000scc67/2000scc67.html (accessed 2011 Aug. 29).
- 8.Douglas K, Jutras C. Patent protection for pharmaceutical products in Canada — chronology of significant events. Ottawa (ON): Parliament of Canada; 2008. Available: www.parl.gc.ca/content/LOP/ResearchPublications/prb9946-e.htm (accessed 2011 Aug. 29). [Google Scholar]
- 9.Hore E. A comparison of United States and Canadian laws as they affect generic pharmaceutical market entry. Food Drug Law J 2000;55:373–88 [PubMed] [Google Scholar]
- 10.Bouchard R, Sawani J, McLelland C, et al. The pas de deux of pharmaceutical regulation and innovation: Who’s leading whom? Berkeley Technol Law J 2009;24:1461–522 [Google Scholar]
- 11.Bouchard R, Hawkins R, Clark R, et al. Empirical analysis of drug approval–drug patenting linkage for high value pharmaceuticals. Northwestern J Technol Intellectual Property 2010;8:174–227 [Google Scholar]
- 12.Bouchard R, Sawicka M. Empirical analysis of drug approval data 2001–2008: Are canadian pharmaceutical players “doing more with less”? McGill J Law Health 2009;3:85–151 [Google Scholar]
- 13.Bouchard RA, Cahoy D, Domeij B, et al. Structure–function analysis of global pharmaceutical linkage regulations. Minnesota Journal of Law Sci Technol 2011;12:391–461 [Google Scholar]
- 14.Release of the therapeutic products directorate statistical report 2010 for the Patented Medicines (Notice of Compliance) regulations and data protection. Ottawa (ON): Health Canada; 2011. Available: www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/prodpharma/applic-demande/docs/patmedbrev/patmrep_mbrevrap_2010_eng.pdf (accessed 2011 Aug. 29). [Google Scholar]
- 15.Lemley Mark A. Rational ignorance at the Patent Office. Northwestern U Law Rev. 2001;4 Available: http://ssrn.com/abstract=261400 (accessed 2011 Aug. 30).
- 16.Wagner P, Parchomovsky G. Patent portfolios. Philadelphia (PA): University of Pennsylvania Law School; 2005. Available: http://papers.ssrn.com/sol3/papers.cfm?abstract_id=582201 (accessed 2011 Aug. 30). [Google Scholar]
- 17.European Commission Pharmaceutical sector inquiry — final report. The Commission: 2009. Available: http://ec.europa.eu/competition/sectors/pharmaceuticals/inquiry/ [Google Scholar]
- 18.Bouchard R. Should scientific research in the lead-up to invention vitiate obviousness under the patented medicines (notice of compliance) regulations: To test or not to test? Can J Law Technol 2007;6:1–27 [Google Scholar]
- 19.Grootendorst P, Hollis A. The Canada–European Union comprehensive economic and trade agreement: an economic impact assessment of proposed pharmaceutical intellectual property provisions. J Gene Med 2011;8:81–103 [Google Scholar]
- 20.Hore E. Patently absurd. Toronto (ON): Hazard and Hore; 2004. Available: www.hazzardandhore.com/docs/papers/patently_absurd_04.pdf (accessed 2011 Aug. 29). [Google Scholar]
- 21.Harris v. GlaxoSmithKline Inc. ONSC 2326. 2010. Available: www.canlii.org/en/on/onsc/doc/2010/2010onsc2326/2010onsc2326.html (accessed 2011 Aug. 29).
- 22.Harris v. GlaxoSmithKline Inc. ONCA 872. 2010. Available: www.canlii.org/en/on/onca/doc/2010/2010onca872/2010onca872.html (accessed 2011 Aug. 29).
- 23.AstraZeneca Canada Inc. v. Canada (Minister of Health). 2 SCR 560. 2006. Available: http://csc.lexum.org/en/2006/2006scc49/2006scc49.pdf (accessed 2011 Aug. 29).
- 24.Church JR, Ware R. Industrial organization: a strategic approach. New York(NY): McGraw-Hill; 2000 [Google Scholar]
- 25.Bouchard R. Living separate and apart is never easy: inventive capacity of the PHOSITA as the tie that binds obviousness and inventiveness in pharmaceutical litigation. U Ottawa Law Technol J 2007;4:1–57 [Google Scholar]
- 26.Free World Trust v. Electro Sante Inc. 2 SCR 1024. 2000. Available: http://scc.lexum.org/en/2000/2000scc66/2000scc66.html (accessed 2011 Aug. 29).
- 27.R. v. Nova Scotia Pharmaceutical Society. 2 S.C.R. 606. 1992. Available: http://scc.lexum.org/en/1992/1992scr2-606/1992scr2-606.html (accessed 08/29, 2011).
- 28.Grootendorst P, Hollis A. Managing pharmaceutical expenditure: overview and options for Canada. Ottawa (ON): Canadian Health Services Research Foundation; 2011. Available: www.chsrf.ca/PublicationsAndResources/ResearchReports/ArticleView/11-02-18/85553e6f-379f-47d7-8817-4056e69360b7.aspx (accessed 2011 Aug. 29). [Google Scholar]
- 29.Caffrey A, Rotter J. Consumer protection, patents and procedure: generic drug market entry and the need to reform the Hatch–Waxman Act. 2004. Available: www.vjolt.net/vol9/issue1/v9i1_a01-Caffrey.pdf (accessed 2011 Aug. 29).
- 30.Bulow J. The gaming of pharmaceutical patents. Stanford (CT): Stanford Business School; 2004. Available: https://faculty-gsb.stanford.edu/bulow/articles/6.27.1036%20Pharmaceutical.pdf (accessed 2011 Aug. 29). [Google Scholar]
- 31.Harrison C. Protection of pharmaceuticals as foreign policy: the Canada–US trade agreement and Bill C-22 versus the North American Free Trade Agreement and Bill C-91. North Carol J Int Law Commer Regul 2001;26(2) [Google Scholar]
- 32.Tancer RS. Foreign investment in North America and the pharmaceutical industry in Canada. Int Exec 2007;39:283–97 [Google Scholar]
- 33.Bouchard RA. I’m still your baby: Canada’s continuing support of US linkage regulations for pharmaceuticals. Marquette Intellect Prop Law Rev 2011;15:72–146 [Google Scholar]
- 34.Bouchard R.A.Qualifying intellectual property II: a novel Innovation index for pharmaceutical products. Santa Clara Comput High Technol Law J 29:78 In Press. [Google Scholar]
- 35.Avery M. Continuing abuse of the Hatch–Waxman Act by pharmaceutical patent holders and the failure of the 2003 amendments. Hasting Law J 2008;60:171–200 Available: www.mavery.com/academic/Avery_Continuing_Abuse_Hatch-Waxman.pdf (accessed 2011 Aug. 29). [Google Scholar]
- 36.Hollis A. Generic drug pricing and procurement: a policy for Alberta. Calgary (AB): University of Calgary; 2009. Available: http://policyschool.ucalgary.ca/files/publicpolicy/Hollis%20ONLINE%20(Feb%2009).pdf (accessed 2009 Apr. 7). [Google Scholar]
- 37.Kingston W. Reducing the cost of resolving intellectual property disputes. Eur J Law Econ 1995;2:85–92 [Google Scholar]
- 38.Eli Lilly Canada Inc. v. Novopharm Ltd. FC 596. 2007. Available: www.canlii.org/en/ca/fct/doc/2007/2007fc596/2007fc596.html (accessed 2011 Sept. 1).
- 39.McNish J. As patent cases clog courts, drugs are a lawyer’s best friend, Globe and Mail [Toronto] 2008. March 12 Available: www.theglobeandmail.com.myaccess.library.utoronto.ca/report-on-business/as-patent-cases-clog-courts-drugs-are-a-lawyers-best-friend/article26641 (accessed 2011 Aug. 31).