Abstract
Intact phagocytic effector function is fundamental to host defense against microbial pathogens. Concern has been raised regarding the potential that accumulation of certain agents, including cationic amphiphilic antibiotics, within macrophages could cause a mixed-lipid storage disorder, resulting in macrophage dysfunction in recipients. The ability of 2 macrophage cell lines (HL-60; RAW 264.7) to kill archetypal Gram-positive (Staphylococcus aureus), Gram-negative (Acinetobacter baumannii), and fungal (Candida albicans) pathogens was tested following exposure of the macrophages to the lipoglycopeptide antibiotic oritavancin. Oritavancin did not affect killing of C. albicans but markedly enhanced killing of S. aureus by both macrophages. Oritavancin modestly reduced killing of A. baumannii by HL-60 cells but not by RAW 264.7 cells. Thus, macrophage killing of microbes remains intact despite substantial intracellular accumulation with a lipoglycopeptide antibiotic.
Intact phagocytic effector function is fundamental to host defense against microbial pathogens, such as the Gram-positive coccus Staphylococcus aureus, the Gram-negative bacillus Acinetobacter baumannii, and the fungal pathogen Candida albicans [1–6]. Although many previous studies have focused on the role of neutrophils, the importance of macrophages in host defense against such pathogens has only been recently described [2, 7–9]. These studies are concordant with older literature confirming the early and marked clearance of extracellular pathogens, such as C. albicans, within minutes after bloodstream infection by phagocytes in the reticuloendothelial system of mammals, including rodents, lagomorphs, dogs, and humans [10].
One putative mechanism by which macrophage microbicidal function may be inhibited is the accumulation of complex lipid or carbohydrate-rich deposits within the phagocytes, as occurs in genetic metabolic storage diseases [11]. Oritavancin is an investigational lipoglycopeptide antibiotic with potent anti–S. aureus activity [12]. Oritavancin accumulates markedly within macrophages, where it causes deposition of concentric lamellar structures and finely granular material and other material, often in giant vesicles, consistent with a mixed-lipid storage disorder [13]. To determine whether marked intracellular accumulation of oritavancin alters killing of microbes, macrophage killing in the presence or absence of oritavancin was tested against S. aureus, as well as organisms against which the drug has no activity, including C. albicans and A. baumannii.
Human HL-60 cells and murine RAW 264.7 macrophage cells (both from American Type Culture Collection, Rockville, MD) were tested because they are known to be capable of killing microbes after differentiation [14–16]. The cells were cultured at 37°C in 5% CO2 in Roswell Park Memorial Institute 1640 medium (Irvine Scientific, Santa Ana, California) with 10% fetal bovine serum, 1% penicillin, streptomycin, and glutamine (Gemini BioProducts), and 50 μmol/L β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO). HL-60 cells were differentiated into macrophages by 5 days of growth in the presence of 50 μmol/L recombinant tissue plasminogen activator [14]. RAW 274.7 cells were activated by 3 days of exposure to 100 nmol/L phorbol 12-myristate 13-acetate (Sigma-Aldrich). Activated HL-60 and RAW 264.7 macrophages were harvested after scraping with BD Falcon cell scrapers (Fischer Scientific).
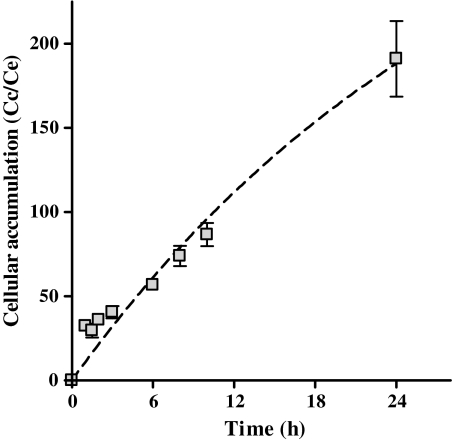
Cellular accumulation of oritavancin was first confirmed in HL-60 cells at a fixed extracellular concentration (25 μg/mL) following procedures published elsewhere [17]. This concentration was chosen because it is also reflective of the predicted maximum free drug serum concentration achieved by administration of a 1200 mg clinical dose of the drug and approximates the limit of solubility of oritavancin at physiological pH in growth medium [18, 19]. Briefly, cells incubated with [14C]-labeled oritavancin were washed 3 times in ice-cold phosphate-buffered saline, collected by scraping in distilled water, and used for radioactivity determination (liquid scintillation counting) and protein assay. The apparent cellular-to-extracellular concentration ratio was calculated by using a conversion factor of 5 μL cell volume per milligram of cell protein. Oritavancin accumulated substantially in HL-60 cells, reaching intracellular concentrations 200-fold above the extracellular concentrations after 24 hours incubation (Figure 1). This value is similar to what was observed earlier for murine J774 macrophages [20], which suggests that it corresponds to an intrinsic property of oritavancin in macrophages.
Figure 1.
Influence of time on the cellular accumulation of oritavancin in HL-60 cells. Kinetics of the cellular accumulation of oritavancin (25 μg/mL) in HL-60 cells (complete culture medium, 10% fetal bovine serum). Results are shown as the apparent cellular to extracellular concentration ratio. Data are means ± standard deviations of 3 independent experiments.
Abbreviation: Cc/Ce, cellular-to-extracellular concentration ratio.
To test impact on cidal activity, HL-60 and RAW 264.7 cells were loaded with oritavancin (25 μg/mL) over 24 hours; as a negative control, other cells were loaded with azithromycin at 10 μg/mL, as described elsewhere [17]. After drug loading, the adherent cells were scraped and rinsed 3 times in Hanks Balanced Salt Solution (HBSS). As a positive control to suppress macrophage killing of microbes by inhibiting superoxide production, some cells were exposed to 1 nmol/L diphenylene iodonium (DPI) for 1 hour prior to cell harvesting [21–25].
To test macrophage killing, S. aureus strain LAC (methicillin-resistant clinical isolate) and A. baumannii HUMC1 (carbapenem-resistant, clinical bloodstream isolate) were cultured overnight in tryptic soy broth at 37°C, and C. albicans 15563 (clinical bloodstream isolate) was cultured overnight at 30°C in yeast peptone dextrose (YPD). The overnight cultures were passaged and organisms grown to mid log-phase prior to use in the killing assay. The killing assay was based on a modification of a method used elsewhere [26, 27]. In brief, macrophages were scraped and rinsed in HBSS as above. Microbes were cocultured in polystyrene snap cap tubes in a rotating drum at 37°C. On the basis of pilot studies, HL-60 cells were cultured at a 200:1 ratio of macrophages to microbes, and RAW 264.7 cells were cultured at a 20:1 ratio. After a 1-hour incubation, the tubes were sonicated and quantitatively plated in tryptic soy agar for S. aureus and A. baumannii or YPD agar for C. albicans. Colony-forming units (CFUs) of the cocultured tubes were compared with CFUs of growth control tubes containing only microbes with no macrophages. Percent of killing was calculated as [1−(CFUs from coculture tubes/CFUs from growth control tubes)].
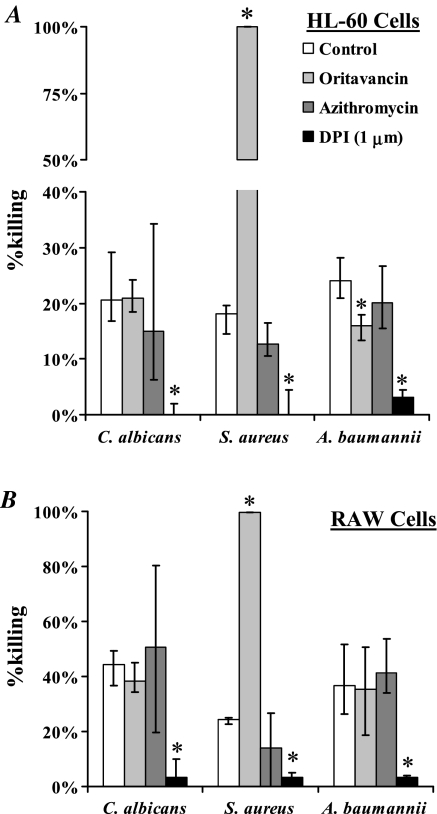
Both HL-60 and RAW 264.7 macrophages killed all 3 tested pathogens. Macrophage killing of C. albicans was not affected by oritavancin, whereas killing of S. aureus was substantially enhanced (Figure 2A). Oritavancin modestly reduced killing of A. baumannii by HL-60 cells (median [interquartile range (IQR)] killing = 24% [21%–28%] vs 16% [13%–18%], P < .01) but not by RAW 264.7 cells (median [IQR] killing = 37% [27%–52%] vs 35% [18%–50%], P = .8). DPI significantly reduced killing of all 3 organisms by both HL-60 and RAW 264.7 cells, including those compared with oritavancin-preloaded macrophages (Figure 2B).
Figure 2.
Macrophage killing of bacterial and fungal pathogens is not inhibited by oritavancin. Human HL-60 (A) or mouse RAW 264.7 (B) macrophages were cocultured with Staphylococcus aureus, Acinetobacter baumannii, or Candida albicans with or without preexposure to oritavancin, azithromycin, or diphenylene iodonium (DPI; a superoxide inhibitor). Median and interquartile ranges are shown from 6 to 12 samples each, performed in duplicate from 2 to 3 separate experiments. *P < .05 versus killing by macrophages without preexposure to any substance.
Thus, in contrast to DPI, which suppressed production of reactive oxygen intermediates and which inhibited macrophage killing of all 3 extracellular pathogens, killing of C. albicans or A. baumannii remained intact after loading of macrophages with substantial levels of oritavancin or azithromycin. Although oritavancin mediated a modest reduction in killing of A. baumannii by HL-60 cells, the killing was similar to that following azithromycin; no similar reduction was seen with RAW cells, and there was no reduction against other pathogens. These data indicate that accumulation of lipoglycopeptides in macrophages does not necessarily correspond with dysfunction of macrophage killing of microbes, and provide reassurance that oritavancin accumulation does not prevent phagocytic killing of key pathogens.
Notes
Financial support.
This work was supported by NIH/NIAID R01 grants AI072052 and AI077681, and the Belgian Fonds de la Recherche Scientifique Médicale (grant 3.4.597.06 .F), in addition to funding from The Medicines Company.
Supplement sponsorship.
This article was published as part of a supplement entitled “Oritavancin for the Treatment of Serious Gram-Positive Infections,”sponsored by The Medicines Company.
Potential conflicts of interest.
F. V. B. has received research grants from the Medicines Company and RibX, his research institute has been paid consulting fees from Targanta, and he serves on the board of RibX and Morphochem. P. M. T. has received grants from Medicines Company and Cerexa, his research institute has been paid consulting fees for board membership at Bayer and Astellas, and he has received lecture fees from GlaxoSmithKline (GSK) and AstraZeneca. B. S. has received research grants or contracts from the Medicines Company, Pfizer, Cubist, and GSK, his institute has been paid consulting fees for his consulting activities from GSK, Pfizer, Achaogen, Meiji, the Medicines Company, and he has received lecture fees from Cubist, AstraZeneca, and Achaogen. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008;4:e35. doi: 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Kockritz-Blickwede M, Rohde M, Oehmcke S, et al. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol. 2008;173:1657–8. doi: 10.2353/ajpath.2008.080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu H, KuoLee R, Harris G, Chen W. High susceptibility to respiratory Acinetobacter baumannii infection in A/J mice is associated with a delay in early pulmonary recruitment of neutrophils. Microbes Infect. 2009;11:946–55. doi: 10.1016/j.micinf.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Qiu H, Kuolee R, Harris G, Chen W. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun. 2009;77:1015–21. doi: 10.1128/IAI.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun. 2007;75:5597–608. doi: 10.1128/IAI.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun. 2010;3:180–99. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian Q, Jutila MA, Van Rooijen N, Cutler JE. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994;152:5000–8. [PubMed] [Google Scholar]
- 9.Rudnicka W, Wieckowska M, van Rooijen N, Rozalska B. The immune response to staphylococcal antigens in mice depleted of macrophages by Cl2MDP-liposomes. Zentralbl Bakteriol. 1997;286:511–22. doi: 10.1016/s0934-8840(97)80054-0. [DOI] [PubMed] [Google Scholar]
- 10.Stone HH. Studies in the pathogenesis, diagnosis, and treatment of Candida sepsis in children. J Pediatr Surg. 1974;9:127–33. doi: 10.1016/0022-3468(74)90019-0. [DOI] [PubMed] [Google Scholar]
- 11.Ananworanich J, Shearer WT. Immune deficiencies in congenital and metabolic diseases: glycogen storage disease type Ib. In: Rich RR, Fleisher TA, Shearer WT, Kotzin BL, Schroeder HWJ, editors. Clinical immunology: principles and practice. 2nd ed. New York, NY: Mosby; 2001. 42.3. [Google Scholar]
- 12.Belley A, McKay GA, Arhin FF, et al. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant Enterococci to effect rapid bacterial killing. Antimicrob Agents Chemother. 2010;54:5369–71. doi: 10.1128/AAC.00760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Bambeke F, Saffran J, Mingeot-Leclercq MP, Tulkens PM. Mixed-lipid storage disorder induced in macrophages and fibroblasts by oritavancin (LY333328), a new glycopeptide antibiotic with exceptional cellular accumulation. Antimicrob Agents Chemother. 2005;49:1695–700. doi: 10.1128/AAC.49.5.1695-1700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nusing R, Goerig M, Habenicht AJ, Ullrich V. Selective eicosanoid formation during HL-60 macrophage differentiation: regulation of thromboxane synthase. Eur J Biochem. 1993;212:371–6. doi: 10.1111/j.1432-1033.1993.tb17671.x. [DOI] [PubMed] [Google Scholar]
- 15.Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis. 2010;201:1718–28. doi: 10.1086/652407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arana DM, Alonso-Monge R, Du C, Calderone R, Pla J. Differential susceptibility of mitogen-activated protein kinase pathway mutants to oxidative-mediated killing by phagocytes in the fungal pathogen Candida albicans. Cell Microbiol. 2007;9:1647–59. doi: 10.1111/j.1462-5822.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 17.Seral C, Van Bambeke F, Tulkens PM. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob Agents Chemother. 2003;47:2283–92. doi: 10.1128/AAC.47.7.2283-2292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhavnani SM, Passarell JA, Owen JS, Loutit JS, Porter SB, Ambrose PG. Pharmacokinetic-pharmacodynamic relationships describing the efficacy of oritavancin in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50:994–1000. doi: 10.1128/AAC.50.3.994-1000.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubino CM, Van Wart SA, Bhavnani SM, Ambrose PG, McCollam JS, Forrest A. Oritavancin population pharmacokinetics in healthy subjects and patients with complicated skin and skin structure infections or bacteremia. Antimicrob Agents Chemother. 2009;53:4422–8. doi: 10.1128/AAC.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Bambeke F, Carryn S, Seral C, et al. Cellular pharmacokinetics and pharmacodynamics of the glycopeptide antibiotic oritavancin (LY333328) in a model of J774 mouse macrophages. Antimicrob Agents Chemother. 2004;48:2853–60. doi: 10.1128/AAC.48.8.2853-2860.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decleva E, Menegazzi R, Busetto S, Patriarca P, Dri P. Common methodology is inadequate for studies on the microbicidal activity of neutrophils. J Leukoc Biol. 2006;79:87–94. doi: 10.1189/jlb.0605338. [DOI] [PubMed] [Google Scholar]
- 22.Ellis JA, Mayer SJ, Jones OT. The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem J. 1988;251:887–91. doi: 10.1042/bj2510887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampton MB, Winterbourn CC. Modification of neutrophil oxidant production with diphenyleneiodonium and its effect on bacterial killing. Free Radic Biol Med. 1995;18:633–9. doi: 10.1016/0891-5849(94)00181-i. [DOI] [PubMed] [Google Scholar]
- 24.Takao S, Smith EH, Wang D, Chan CK, Bulkley GB, Klein AS. Role of reactive oxygen metabolites in murine peritoneal macrophage phagocytosis and phagocytic killing. Am J Physiol. 1996;271:C1278–84. doi: 10.1152/ajpcell.1996.271.4.C1278. [DOI] [PubMed] [Google Scholar]
- 25.Woo CH, Yoo MH, You HJ, et al. Transepithelial migration of neutrophils in response to leukotriene B4 is mediated by a reactive oxygen species-extracellular signal-regulated kinase-linked cascade. J Immunol. 2003;170:6273–9. doi: 10.4049/jimmunol.170.12.6273. [DOI] [PubMed] [Google Scholar]
- 26.Spellberg BJ, Collins M, Avanesian V, et al. Optimization of a myeloid cell transfusion strategy for infected neutropenic hosts. J Leukoc Biol. 2007;81:632–41. doi: 10.1189/jlb.0906549. [DOI] [PubMed] [Google Scholar]
- 27.Spellberg BJ, Collins M, French SW, Edwards JE, Jr, Fu Y, Ibrahim AS. A phagocytic cell line markedly improves survival of infected neutropenic mice. J Leukoc Biol. 2005;78:338–44. doi: 10.1189/jlb.0205072. [DOI] [PubMed] [Google Scholar]