Abstract
Prior research suggests an association between anhedonia—diminished interest or pleasure in rewarding activities—and stimulant use in selected samples. However, it is unclear whether this association generalizes to the overall population and is consistent across stimulant drug types (amphetamine vs. cocaine) and outcome characteristics (any lifetime use vs. dependence). Questions also remain as to whether the anhedonia–stimulant relationship is unique from covariance with depressed mood, psychiatric disorders, and nonstimulant substance use. The current study addressed these questions by examining anhedonia–stimulant relationships in a cross-sectional population-based sample of 43,093 American adults. Results indicated that lifetime anhedonia and depressed mood each were positively associated with lifetime stimulant use and lifetime dependence among those who reported stimulant use. Anhedonia–stimulant relationships were consistent across amphetamine- and cocaine-related outcomes and distinct from covariance with depressed mood, which exhibited no association over and above the effect of anhedonia. After adjusting for demographic, psychiatric, and nonstimulant substance use characteristics, anhedonia–stimulant associations remained significant, although effect sizes were partially attenuated. Lifetime anhedonia was also more prevalent among respondents who initiated use but did not eventually progress to dependence in comparison with individuals who never once used a stimulant drug. Anhedonia appears to be uniquely associated with lifetime use of cocaine and amphetamines and lifetime progression from use to dependence in the American population. Albeit cross-sectional in nature, these findings add further support to the generalizability and specificity of the anhedonia–stimulant relationship. Future research utilizing longitudinal and experimental designs are warranted to clarify the underpinnings of this association.
Keywords: anhedonia, stimulant dependence, amphetamine, cocaine, depressed mood
Use of stimulant drugs, including amphetamine and cocaine, is associated with higher rates and severity of depressive symptoms (Coffey, Dansky, Carrigan, & Brady, 2000; McGregor et al., 2005; Newton, Kalechstein, Duran, Vansluis, & Ling, 2004). Depressive symptoms are a highly heterogeneous set of features included in the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV) major depression diagnosis, such as depressed mood (i.e., extreme feelings of sadness), anhedonia (i.e., diminished interest or pleasure in rewarding activities), concentration problems, feelings of worthlessness, changes in weight and sleep, and other features (American Psychiatric Association [APA], 1994). It is important to identify specific depressive symptoms that are associated with stimulant use for the purpose of (a) informing the development of treatments that target emotional determinants of stimulant dependence and (b) clarifying the mechanisms underlying the link between affective disturbance and stimulant use.
Some investigations suggest an association between the depressive symptom of anhedonia and stimulant use disorder, which is defined as having a DSM–IV diagnosis of stimulant abuse (pattern of problematic or hazardous drug use leading to legal or social problems) or dependence (heavy pattern of use resulting in symptoms such as tolerance, withdrawal, inability to control use, and drug-induced social, psychological, or physical problems). In a study of abstinent cocaine-dependent individuals, Gawin and Kleber (1986) found that anhedonia was elevated through 10 weeks of continuous abstinence, whereas most other depressive symptoms remitted by that time. Similarly, Kalechstein, Newton, and Leavengood (2002) found that the cocaine-dependent individuals who were abstinent for 5 days demonstrated elevated levels of apathy—a syndrome including anhedonia and other related features—in comparison with a healthy control group. These effects were not accounted for by differences in overall depressive symptoms. Finally, Leventhal, Kahler, et al. (2008) demonstrated that psychiatric outpatients with a history of amphetamine or cocaine use disorder in full remission exhibited higher rates of current anhedonia than did patients with no history of stimulant use disorder. In order to further elucidate this putatively important clinical relationship, several points require clarification.
First, extant studies generally have been conducted in constrained samples of psychiatric patients or stimulant-dependent individuals. Second, past findings have compared individuals with and without a stimulant use disorder. Accordingly, it remains unclear whether the anhedonia–stimulant association is restricted to stimulant dependence, a syndrome associated with heavy and chronic use, or whether this association extends to any lifetime history of stimulant use, which is a more sensitive marker of at least a single instance of stimulant use over one's entire lifetime that may be relevant to understanding potential factors that influence stimulant use experimentation. Third, prior studies have not examined the concomitant role of depressed mood in the context of the anhedonia–stimulant association. Given that anhedonia often co-occurs with depressed mood among individuals suffering from depression (both are index symptoms of major depression; APA, 1994), exploring whether anhedonia and depressed mood exhibit unique or overlapping relationships to stimulant use could refine our understanding of the overall association between depression and stimulant use. Finally, prior studies of the anhedonia–stimulant relationship have not compared findings across cocaine and amphetamine use within the same sample, which is important for clarifying whether relations extend across two drugs that share common neuropharmacological properties (D'Souza & Markou, 2009), but have disparate demographic and psychosocial correlates (Rawson et al., 2000; Simon et al., 2002).
The current study examines whether presence versus absence of a lifetime history of anhedonia is associated with (a) stimulant use (i.e., at least one instance of stimulant consumption) and (b) the transition to stimulant dependence among those who report at least one episode of use. This study extends the anhedonia–stimulant literature by leveraging a nationally representative sample, examining stimulant use and dependence as discrete outcomes, disentangling the role of anhedonia versus depressed mood, and comparing findings across cocaine and amphetamine.
Method
Participants
Participants were 43,093 respondents in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC; Wave 1, 2001–2002). The purpose of the NESARC is to assess the epidemiology of alcohol and substance use, psychiatric disorders, psychosocial features, and other clinical characteristics within the U.S. adult population. All participants were civilian, noninstitutionalized, and ages 18 years or older. African Americans and Hispanic Americans were oversampled, and each group accounted for approximately 20% of the sample. Young adults between ages 18 and 24 were also oversampled by a 2.25 to 1 ratio. The data had accompanying weights to account for oversampling in order for adjustment to be representative of the 2000 U.S. Census results by age, ethnicity, and gender. Further details of the sampling, purpose of the survey, and weighting procedures have been published elsewhere (B. Grant, Moore, Shepart, & Kaplan, 2003). Of the 43,093 total respondents, individuals who could not recall whether they ever experienced depressed mood or anhedonia (n = 970, 2.3%) or used amphetamine or cocaine (n = 70, 0.2%) were eliminated from the sample.
Procedure
Potential respondents were contacted in writing by the U.S. Census Bureau and informed about the nature of the study and confidentiality. One adult from each household was selected for the interview, and the response rate was 81%. After informed consent was obtained, interviewers administered the Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS–IV; B. F. Grant, Dawson, et al., 2003) in face-to-face interviews and recorded responses onto a laptop computer. The U.S. Census Bureau and the U.S. Office of Management reviewed and approved all consent procedures, research measures, and protocol.
Assessment
The AUDADIS–IV includes questions that assess demographic information, DSM–IV criteria for psychiatric and substance use disorder diagnoses (APA, 1994), substance use patterns, and other clinical and demographic characteristics.
Lifetime stimulant use
All respondents were queried with separate items assessing lifetime cocaine use (i.e., “Have you ever used crack or cocaine?” Yes/No) and lifetime use of amphetamine or other stimulant/appetite suppressants (i.e., “Have you ever used stimulants, e.g., Preludin, Benzadrine, Methadrine, uppers, or speed?” Yes/No).
Lifetime stimulant dependence
Lifetime DSM–IV amphetamine and cocaine dependence was classified in all respondents who endorsed lifetime amphetamine (N = 1718) and cocaine (N = 2490) use, respectively, regardless of whether abuse criteria were met. Stimulant abuse was not analyzed to reduce the number of statistical tests performed (and Type I error) and because there is more extensive evidence supporting a link of anhedonia and depression to stimulant dependence (Gawin & Kleber, 1986; Kalechstein et al., 2002; Newton et al., 2004).
Lifetime anhedonia and depressed mood
As part of the major depression module of the AUDADIS–IV, all respondents were assessed for lifetime anhedonia (i.e., “In your entire life, have you ever had a time, lasting at least 2 weeks, when you didn't care about the things that you usually cared about, or when you didn't enjoy the things you usually enjoyed?” Yes/No) and depressed mood (i.e., “In your entire life, have you ever had a time you felt sad, blue, depressed, or down most of the time for at least 2 weeks?” Yes/No). Although the onset and offset date for entire major depressive episodes are evaluated in the AUDADIS–IV, the timing of specific depressive symptoms is not assessed. Thus, we were unable to analyze timing information for anhedonia and depressed mood.
Covariates
Lifetime nondepressive psychiatric disorder was classified for participants who met DSM–IV criteria for any psychiatric disorder other than a major depressive episode or dysthymia. Nonstimulant drug use was defined as lifetime recreational use of any drug other than alcohol, tobacco, cocaine, or amphetamines. Lifetime history of non-stimulant drug use disorder and alcohol use disorder was classified on the basis of DSM–IV criteria for either respective disorder.
Data Analysis
Preliminary analyses examined correlations between lifetime anhedonia and depressed mood. Analyses also examined correlations of anhedonia, depressed mood, and each of the four stimulant outcomes (lifetime amphetamine use, cocaine use, amphetamine dependence, and cocaine dependence) with each of the demographic, psychiatric, and substance use characteristics that served as covariates in primary analyses (see below).
Primary analyses involved calculating logistic regression models to test relations of anhedonia and depressed mood to stimulant outcomes. For each outcome, three types of models were calculated: (a) a univariate model that included anhedonia as the sole predictor; (b) a univariate model that included depressed mood as the sole predictor; and (c) a combined model that included anhedonia and depressed mood as simultaneous predictors to explore whether they were uniquely associated with stimulant outcomes after controlling for their covariance. To determine the extent to which extraneous variables accounted for affect–stimulant relationships, the combined models were recalculated after adjusting for demographic covariates (age, region, ethnicity/race, marital status, sex, and education), and again after adjusting for both demographics and substance use and psychiatric characteristics (lifetime history of nondepressive psychiatric disorder, alcohol use disorder, nonstimulant drug use disorder, and nonstimulant drug use). Nondepressive psychiatric disorder was included as a covariate to determine whether relationships were not explained by anhedonia's overlap with psychiatric disorders. Major depression was not included as a covariate because anhedonia or depressed mood is required for depression classification, which could result in near quasi-complete separation of data and biased statistics. Nonstimulant substance use variables were included as covariates to examine whether anhedonia's relationships were not accounted for by a general tendency to use or misuse any type of substance. Analyses of lifetime amphetamine and cocaine use utilized the entire sample (N = 42,053) and used sampling weights to approximate the U.S. population (B. Grant, Moore, et al., 2003). Analyses of lifetime cocaine and amphetamine dependence included the subset respondents who endorsed lifetime use of amphetamine (N = 1718) and cocaine (N = 2490), respectively.
Supplemental analyses distinguished participants with anhedonia only from those who had both anhedonia and depressed mood, examined other factors that could play a role in anhedonia–stimulant associations (e.g., age of stimulant use onset, medication use), and analyzed relations between anhedonia and nondependent lifetime stimulant use. These analyses are described in greater detail below.
All analyses were conducted in SAS using the PROC SURVEY procedures (SAS Institute Inc., 2009), which account for the complex sampling methodology of the NESARC. Primary results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). All analyses utilized two-tailed tests and set alpha levels to .05.
Results
Preliminary Analyses
Anhedonia and depressed mood were associated in the overall sample (N = 42,053) and in the samples of lifetime amphetamine users (N = 1718) and cocaine users (N = 2490) (φs = .76–.78). Table 1 presents correlations of anhedonia, depressed mood, and the four stimulant outcomes (lifetime amphetamine use, cocaine use, amphetamine dependence, and cocaine dependence) with each of the demographic variables (age, region, ethnicity/race, marital status, sex, and education) and substance use and psychiatric characteristics (lifetime history of nondepressive psychiatric disorder, alcohol use disorder, nonstimulant drug use disorder, and nonstimulant drug use) that were included as covariates in adjusted primary analyses (see below). These analyses indicated negligible to moderatesized associations between these sets of variables (rs < .46).
Table 1.
Bivariate Associations Among Key Variables
| Demographics |
Psychiatric |
Substance use |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor/Outcome Variables | Age | Region | Sex | Ethnicity/race | Marital status | Education | Income | Lifetime nondepressive psychiatric disorder | Lifetime nonstimulant drug use | Lifetime nonstimulant drug use disorder | Lifetime alcohol use disorder |
| Overall sample (N = 42,053) | |||||||||||
| Depressed mood | .01* | .03*** | .13*** | .09*** | .11*** | .04*** | .05*** | .32*** | .14*** | .11*** | .11*** |
| Anhedonia | .03*** | .03*** | .10*** | .10*** | .10*** | .05*** | .03*** | .35*** | .17*** | .14*** | .15*** |
| Lifetime amphetamine use | .07*** | .07*** | –.06*** | .10*** | .01* | .04*** | .04*** | .12*** | .38*** | .42*** | .26*** |
| Lifetime cocaine use | .10*** | .08*** | –.08*** | .06*** | .03*** | .05*** | .07*** | .10*** | .46*** | .45*** | .30*** |
|
Lifetime amphetamine users (N = 1718) | |||||||||||
| Depressed mood | –.03 | .05 | .16*** | .08* | .15*** | .05 | .13*** | .33*** | .05 | .09*** | .04 |
| Anhedonia | –.01 | .03 | .15*** | .10** | .15*** | .04 | .14*** | .38*** | .05* | .08*** | .05* |
| Lifetime amphetamine dependence | –.10*** | .10*** | .11*** | .05 | .05 | .05 | .15*** | .16*** | .03 | .11*** | .05* |
|
Lifetime cocaine users (N = 2490) | |||||||||||
| Depressed mood | –.02 | .02 | .15*** | .07* | .13*** | .03 | .13*** | .32*** | .04* | .09*** | .04* |
| Anhedonia | –.003 | .05 | .11*** | .08** | .13*** | .03 | .12*** | .36*** | .04 | .10*** | .09*** |
| Lifetime cocaine dependence | –.03 | .07** | .03 | .12*** | .03 | .06* | .11*** | .19*** | .02 | .13*** | .11*** |
Note. All associations are reported as phi-coefficient, except for age, which is a point-biserial r.
p < .05.
p < .01.
p < .001.
Primary Analyses
Stimulant use
As is illustrated in Table 2, both anhedonia and depressed mood were significantly associated with lifetime amphetamine use in univariate models including the overall sample (N = 42,053). However, when both symptoms were simultaneously entered into a combined model, only anhedonia was uniquely associated with amphetamine use, and the association involving depressed mood was eliminated. The ORs for anhedonia were slightly diminished when adjusting for demographic variables, and more considerably reduced after adding substance use and psychiatric characteristics to the model. Importantly, anhedonia retained significance in each adjusted combined model (see Table 2).
Table 2.
Weighted Logistic Regression Analyses Predicting Lifetime Stimulant Use From Depressed Mood and Anhedonia
| Univariate |
Combineda |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjustedb |
Adjustedc |
||||||
| Predictor | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Outcome: Amphetamine use | ||||||||
| Depressed mood | 2.54 (2.26–2.86) | <.0001 | 1.00 (0.82–1.22) | .99 | 1.09 (0.89–1.35) | .40 | 1.00 (0.81–1.23) | .99 |
| Anhedonia | 3.31 (2.95–3.72) | <.0001 | 3.31 (2.71–4.06) | <.0001 | 3.03 (2.46–3.73) | <.0001 | 1.56 (1.27–1.92) | <.0001 |
|
Outcome: Cocaine use | ||||||||
| Depressed mood | 2.10 (1.90–2.32) | <.0001 | 1.06 (0.89–1.26) | .54 | 1.20 (1.00–1.44) | .054 | 1.09 (0.90–1.31) | .39 |
| Anhedonia | 2.56 (2.31–2.83) | <.0001 | 2.44 (2.04–2.92) | <.0001 | 2.31 (1.92–2.78) | <.0001 | 1.24 (1.02–1.50) | .03 |
Note. N = 42,053. OR = odds ratio; CI = confidence interval.
Models include anhedonia and depressed mood as simultaneous predictors.
Adjusted for demographics (age, region, income, ethnicity/race, marital status, sex, and education).
Adjusted for demographics and lifetime history of nondepressive psychiatric disorder, alcohol use disorder, nonstimulant drug use disorder, and nonstimulant drug use.
A similar pattern was found with cocaine use as the outcome variable (see Table 2); anhedonia and depressed mood both evidenced univariate effects, but only anhedonia exhibited a unique association in the combined models. ORs for anhedonia were smaller in models predicting cocaine use than in models predicting amphetamine use, though the associations remained statistically significant in all models.
Stimulant dependence
As is demonstrated in Table 3, among lifetime amphetamine users (N = 1718), both anhedonia and depressed mood were significantly associated with lifetime amphetamine dependence in univariate models. Results from combined models incorporating both mood symptoms demonstrated that the relation between depressed mood and amphetamine dependence was eliminated, whereas the association involving anhedonia remained significant in combined models. The ORs for anhedonia were reduced slightly after adjusting for demographics, and more substantially after also adjusting for substance use and psychiatric characteristics, although they remained statistically significant in all models (see Table 3).
Table 3.
Logistic Regression Analyses Predicting Lifetime Stimulant Use Disorders From Depressed Mood and Anhedonia Among Respondents With Lifetime History of Stimulant Use
| Univariate |
Combineda |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Unadjusted |
Adjustedb |
Adjustedc |
|||||
| Predictor | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Outcome: Amphetamine dependence | ||||||||
| Depressed mood | 2.30 (1.71–3.10) | <.0001 | 1.20 (0.76–1.90) | .44 | 1.29 (0.80–2.08) | .30 | 1.15 (0.72–1.84) | .56 |
| Anhedonia | 2.69 (1.99–3.65) | <.0001 | 2.34 (1.47–3.73) | .0004 | 2.20 (1.36–3.57) | .001 | 1.85 (1.15–3.00) | .01 |
|
Outcome: Cocaine dependence | ||||||||
| Depressed mood | 2.30 (1.84–2.87) | <.0001 | 1.03 (0.73–1.48) | .84 | 1.11 (0.77–1.60) | .56 | 1.04 (0.72–1.50) | .76 |
| Anhedonia | 2.87 (2.29–3.59) | <.0001 | 2.79 (1.95–3.98) | <.0001 | 2.81 (1.95–4.06) | <.0001 | 2.22 (1.53–3.22) | <.0001 |
Note. N = 1718 for amphetamine analyses; N = 2490 for cocaine analyses. OR = odds ratio; CI = confidence interval.
Models include anhedonia and depressed mood as simultaneous predictors.
Adjusted for demographics (age, region, ethnicity/race, marital status, sex, and education).
Adjusted for demographics and lifetime history of nondepressive psychiatric disorder, disorder, alcohol use disorder, nonstimulant drug use disorder, and nonstimulant drug use.
Similarly, in the subset of lifetime cocaine users (N = 2490), anhedonia and depressed mood both evidenced univariate associations with lifetime cocaine dependence. Anhedonia, but not depressed mood, was significantly associated with cocaine dependence in combined models both with and without adjusting for demographic, psychiatric, and substance use characteristics (see Table 3).
Supplemental Analyses
Four-group analysis
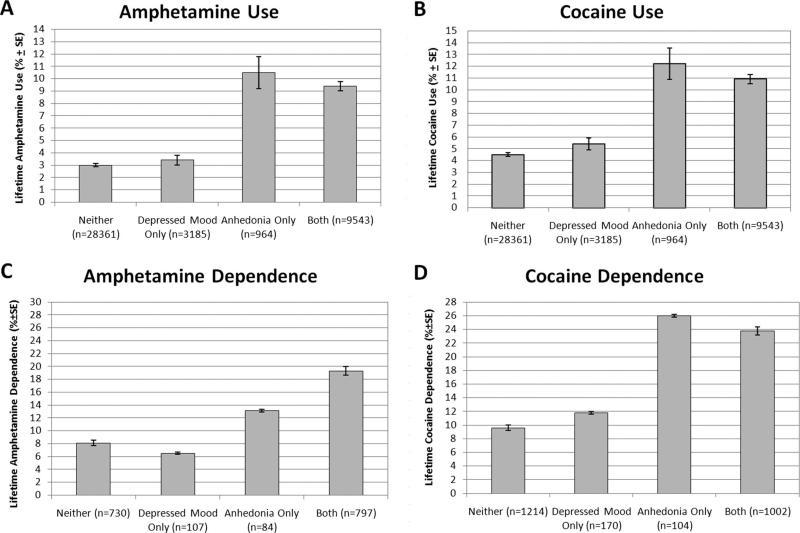
Because anhedonia and depressed mood were strongly associated, we divided the samples into four groups based on whether participants endorsed (a) neither symptom; (b) depressed mood but not anhedonia; (c) anhedonia but not depressed mood; and (d) both symptoms. The prevalence of amphetamine use, cocaine use, amphetamine dependence, and cocaine dependence across these four groups are presented in Figure 1. Analyses comparing these four groups using pairwise chi-square tests demonstrated that participants with anhedonia only and those with both symptoms did not differ in stimulant outcomes from each other, but both groups had higher rates than did those with neither symptom and those with depressed mood only, which did not differ from each other. These results were consistent across all outcomes with the exception of comparisons of participants with anhedonia only (n = 84) to those with neither symptom and those depressed mood only in rates of amphetamine dependence among those with a lifetime history of amphetamine use. Although participants with anhedonia only exhibited higher rates of amphetamine dependence, these tests failed to reach statistical significance (ps = .12; see Figure 1).
Figure 1.
(A) Weighted percentage (±SE) of lifetime amphetamine use by depressive symptom group in overall sample (n = 42,053). (B) Weighted percentage (±SE) of lifetime cocaine use by depressive symptom group in overall sample (n = 42,053). (C) Percentage (±SE) of lifetime amphetamine dependence by depressive symptom group within lifetime amphetamine users (n = 1718). (D) Percentage (±SE) of lifetime cocaine dependence by depressive symptom group within lifetime cocaine users (n = 2490).
Exploratory analyses testing the interaction between depressed mood and anhedonia outcomes yielded nonsignificant interactions for all stimulant outcomes. Thus, the relation between anhedonia and stimulant use and dependence was not influenced on concomitant depressed mood.
Lifetime stimulant use without dependence
It is possible that the anhedonia–stimulant use relationships could have been primarily driven by respondents who were dependent. To address this potential explanation, we conducted analyses predicting stimulant use after eliminating participants who had a lifetime stimulant-dependence diagnosis. As in the primary analysis, both symptoms were associated with stimulant use in univariate models, whereas combined models adjusting for demographic, psychiatric, and substance use characteristics demonstrated that anhedonia predicted lifetime use of cocaine, OR (95% CI) = 1.27 (1.04–1.54), p = .02, and amphetamine, OR (95% CI) = 1.53 (1.24–1.898), p < .0002, whereas depressed mood did not uniquely associate with lifetime use of either cocaine or amphetamine (ORs ≤ 1.05, ps ≥ .60).
Age of stimulant onset
Perhaps anhedonia was associated with an earlier age of onset of stimulant use and use escalation, which could account for its overlap with dependence. Results showed that anhedonia did not associate with age of first amphetamine use, anhedonia+ M (SD) = 19.3 (5.1) years versus anhedonia– M (SD) = 19.31 (5.2) years, p = .73, age of first cocaine use, 22.2 (6.4) versus 22.1 (6.0), p = .66, age when began using amphetamine the heaviest, 20.8 (6.0) versus 20.5 (5.9), p = .39, and age when began using cocaine the heaviest, 22.2 (6.4) versus 22.1 (6.0), p = .66. Age of use initiation and onset of heaviest use did not associate with depressed mood.
Psychiatric medications
It is possible that participants with depressed mood may have been more likely to be prescribed medications that could have reduced their relative stimulant use risk. Thus, combined models adjusted for demographic, psychiatric, and substance use characteristics were recalculated after eliminating respondents who reported being prescribed medication for mood disturbance at some point in their life (N = 3847). Results showed that anhedonia was uniquely associated with amphetamine use (OR = 1.50, p = .004), cocaine use (OR = 1.24, p = .049), amphetamine dependence (OR = 2.03, p = .005), and cocaine dependence (OR = 2.37, p < .0001). Depressed mood did not associate with any stimulant outcome ( ps ≥ .56).
Discussion
This study found that lifetime history of anhedonia was associated with increased prevalence of lifetime stimulant use as well as transition from use to dependence in a nationally representative sample. These relationships were consistent across amphetamine- and cocaine-related outcomes and remained significant after accounting for covariance with depressed mood and other demographic and psychiatric characteristics.
The present results extend previous research demonstrating an association between anhedonia and stimulant use disorder in several ways (Gawin & Kleber, 1986; Kalechstein et al., 2002; Leventhal, Kahler, et al., 2008; Newton et al., 2004). By exploring stimulant use and dependence as discrete outcomes, these data provide novel information relevant to understanding the underpinnings of the anhedonia–stimulant link. Analyses demonstrating that anhedonia was more prevalent among respondents who initiated use but did not eventually progress to dependence in comparison with individuals who never initiated stimulant use potentially suggest that anhedonic people may perhaps be more willing to experiment with stimulant drugs, which could be motivated by a desire to overcome reward deficits (Khantzian, 1985, 1997; Markou, Kosten, & Koob, 1998). Evidence that anhedonia was also associated with transition from use to dependence may suggest that anhedonic individuals are more vulnerable to dependence after initial experimentation with stimulants. Interestingly, results from laboratory drug administration studies show that stimulantnaive individuals with higher levels of anhedonia are more sensitive to the subjective reinforcing effects of acute amphetamine administration (Tremblay, Naranjo, Cardenas, Herrmann, & Busto, 2002; Tremblay et al., 2005). Thus, greater sensitivity to the addictive properties of stimulant drugs could explain the association between anhedonia and stimulant dependence. Given that this study design was cross-sectional, it is also plausible that heavy stimulant use, typical of stimulant dependence, induces persistent neuroadaptations that produce a chronic state of anhedonia (D'Souza & Markou, 2009; Markou & Koob, 1991).
This was the first study to distinguish amphetamine and cocaine use as correlates of anhedonia within the same sample. The consistency of the findings across both substances and the use of a nationally representative sample suggest that anhedonia–stimulant relationships generalize, despite the fact that amphetamine and cocaine use associate with distinct psychosocial factors, demographic characteristics, and drug use patterns in past research (Rawson et al., 2000; Simon et al., 2002). Accordingly, characteristics that are common between these two drugs may play a role in their link to anhedonia. Both drugs stimulate activity within the mesolimbic dopamine system, and anhedonia is associated with dysregulation within that neural pathway and other systems, including glutamate, GABA, acetylcholine, and other systems (D'Souza & Markou, 2009). Hence, the common neuropharmacological effects of these substances may account for their shared link to anhedonia.
The results of this study should be interpreted within the context of its limitations. Anhedonia and depressed mood were assessed at the lifetime level and based on single-item measures. Even though research supports the reliability and validity of single-item symptom ratings (Zimmerman, McGlinchy, Young, & Chelminski, 2006), multi-item severity scales are better positioned to capture a greater domain of anhedonic features (Leventhal, Chasson, Tapia, Miller, & Pettit, 2006). Additionally, no data on the putative mechanisms underlying anhedonia–stimulant relations were available (e.g., biological measures, assessments of motivation to use stimulants to alleviate affective deficits, and measures of subjective drug effects); thus, extrapolation of putative underpinnings of this relationship are speculative. Information on the timing of anhedonia and depressed mood were not available in this survey, which limited analyses to lifetime associations. Most important, these data are cross-sectional and retrospective, and do not provide information about the timing of anhedonia symptoms in relation to stimulant use and dependence. Therefore, definitive conclusions regarding the temporal or causal aspects of the anhedonia–stimulant relationship cannot be made. Finally, the current study cannot account for individuals who had no history of anhedonia or stimulant use/dependence up to the time of assessment, but who may be at risk of future onset of anhedonia or stimulant use and dependence. Future research utilizing prospective and experimental designs are warranted to clarify the underpinnings of the anhedonia–stimulant relationship to potentially inform the development of effective mood-targeting interventions for individuals suffering from stimulant dependence.
Acknowledgments
This research was supported in part by National Institutes of Health Grant DA01618. The authors report no competing interests related to the submission of this article.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; 1994. [Google Scholar]
- Coffey SF, Dansky BS, Carrigan MH, Brady KT. Acute and protracted cocaine abstinence in an outpatient population: A prospective study of mood, sleep and withdrawal symptoms. Drug and Alcohol Dependence. 2000;59:277–286. doi: 10.1016/s0376-8716(99)00126-x. [DOI] [PubMed] [Google Scholar]
- D'Souza M, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. In: Self D, Staley J, editors. Behavioral neuroscience of drug addiction. Vol. 3. Springer Berlin Heidelberg; New York, NY: 2009. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: Clinical observations. Archives of General Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Grant B, Moore T, Shepart J, Kaplan K. Source and accuracy statement for Wave 1 of the 2001–2202 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2003. [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule—IV (AUDADIS–IV): Reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug and Alcohol Dependence. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Leavengood AH. Apathy syndrome in cocaine dependence. Psychiatry Research. 2002;109:97–100. doi: 10.1016/s0165-1781(01)00354-7. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. American Journal of Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: A psychometric analysis of three anhedonia scales. Journal of Clinical Psychology. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, Zimmerman M. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. American Journal on Addictions. 2008;17:218–223. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: Preliminary findings. American Journal on Addictions. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- Rawson R, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, Ling W. Methamphetamine and cocaine users: Differences in characteristics and treatment retention. Journal of Psychoactive Drugs. 2000;32:233–238. doi: 10.1080/02791072.2000.10400234. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS system for Windows (version 9.2) Author; Cary, NC: 2009. [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W. A comparison of patterns of methamphetamine and cocaine use. Journal of Addictive Diseases. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: Altered response to dextroamphetamine. Archives of General Psychiatry. 2002;59:409–417. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Young D, Chelminski I. Diagnosing major depressive disorder I: A psychometric evaluation of the DSM–IV symptom criteria. Journal of Nervous and Mental Disease. 2006;194:158–163. doi: 10.1097/01.nmd.0000202239.20315.16. [DOI] [PubMed] [Google Scholar]