Abstract:
Carbon monoxide (CO), a by-product of Heme metabolism, is a potent modulator of inflammation. Low dose inhaled CO has demonstrated reduced lung and kidney injury in animal models of cardiopulmonary bypass (CPB). We evaluated the impact of low dose inhaled CO on systemic, pulmonary, and myocardial inflammatory response to CPB in rats. Sixteen male Sprague-Dawley rats underwent CPB for 1 hour. The CO (n = 8) group received inhaled CO at 250 ppm for 3 hours before CPB. The Air (n = 8) group served as the control. Pulmonary mechanics were assessed pre and post CPB. The animals were recovered for 30 minutes post CPB and subsequently sacrificed. Pre CPB and post CPB serum Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-10 (IL-10) were analyzed by enzyme-linked immunosorbent assay. Gene expression array and real time quantitative polymerase chain reaction (PCR) analysis was performed on the extracted heart tissue. Baseline characteristics were similar between the groups with the expected exception of carboxyhemoglobin levels (p ≤.001) and oxyhemoglobin saturation (p ≤.01) in Air versus CO treated groups, respectively. Serum TNF-α (363 ± 278 vs. 287 ± 195; p = .13) and IL-10 (237 ± 26 vs. 302 ± 137; p = Not Significant) in Air versus CO groups respectively were not statistically different after CPB, despite showing a trend of inflammatory attenuation. Gene expression array of the myocardial tissue suggested a pattern of inflammatory modulation, which was confirmed by real time quantitative PCR demonstrating IL-10 expression 3.13 times higher (p = .02) in the CO treated group com pared to the Air group. These data demonstrate that pretreatment with CO at 250 ppm may have a modulatory effect on the inflammatory response to CPB without compromising hemodynamics or oxygen delivery. Further investigation in a survival model of CPB is warranted.
Keywords: carbon monoxide, cardiopulmonary bypass, inflammation, rat, cytokines
The robust inflammatory response triggered by cardiopulmonary bypass (CPB) is thought to account for some of the morbidity associated with cardiovascular surgery (1). Increased production of Tumor Necrosis Factor-alpha (TNF-α), nuclear factor-kappa B (NF-κB), and stimulation of the Mitogen-Activated Protein Kinase signaling pathway (particularly p38) have been attributed to CPB-induced systemic and pulmonary inflammatory response (2). Carbon monoxide (CO) has been implicated as a modulator of inflammatory processes in animal models. As a by-product of heme metabolism, CO is produced in large quantities via induction of hemeoxygenase 1 (HO-1) in injured tissue. Treatment with low dose inhaled CO at 250 ppm inhibits gene expression of proinflam matory cytokines Interleukin-1 beta,TNF-α, and Interleukin-6 (IL-6), and induces the anti-inflammatory cytokine Interleukin-10 (IL-10) without significantly altering hemodynamic parameters and oxygen carrying capacity (3). The anti-inflammatory effects of CO are correlated with improvement in outcome in small animal models of organ injury and inflammation, such as graft rejection in organ transplantation, hemorrhagic shock, and hyperoxic lung injury. Moreover, CO improves myocardial energetics (4), reduces pulmonary inflammatory response (5), and prevents acute kidney injury after CPB in pigs (6). Clinically, the excessive inflammatory response observed in some critically ill patient populations adversely affects morbidity and mortality. Carboxyhemoglobin (CO-Hb) levels correlate with the HO-1 activity and endogenous CO production. Both low and very high arterial CO-Hb levels are associated with increased mortality in critically ill patients, perhaps implicating the importance of the HO-1 axis in modulating excessive inflammation (7).
Endogenous CO is involved in important intracellular and intercellular signaling processes. It has been described as a neuromodulator, a hyperpolarizing factor (8), and is implicated in regulation of vascular tone via stimulation of soluble guanylate cyclase with formation of cyclic guanosine monophosphate (cGMP) (9). Its modulation of the p38-Mitogen-Activated Protein Kinase signaling pathway that is critical for cellu lar signal transduction in response to stress or inflammation is thought to be important in the anti-inflammatory, anti-apoptotic, and anti-proliferative effects of CO (10).
The modulatory effect of low dose inhaled CO on lung tissue inflammation after CPB has been described. We hypothesized that CO would modulate the systemic and myocardial cytokine inflammatory response to CPB. A modified version of rat CPB (11) was used to evaluate the impact of low dose inhaled CO on the pathophysiologic response to CPB.
METHODS
Animals
The study was approved by the Institutional Animal Care and Use Committee and all procedures were performed as outlined by the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals (1996). Healthy male Sprague-Dawley rats weighing 450–550 g were maintained in standard laminar flow cages at a pathogen free animal facility. The animals were fed a standard diet and water ad libitum and were placed on a 3-day quarantine period prior to use.
CO Exposure and Experimental Groups
The study animals were placed in a 40 L Plexiglas chamber that was continuously ventilated with either air or CO-air mixture with the aid of a dual precision flowmeter (N042–15 Omega, Stamford, CT). Two groups of eight rats were analyzed. One group was exposed to 250 ppm of CO for 3 hours prior to anesthesia induction. The Air group served as control.
Anesthesia and Surgical Procedure
After exposure to air or CO/air mixture, anesthesia induction was achieved with intramuscular ketamine (90 mg/kg) and xylazine (10 mg/kg). Anesthesia was maintained throughout the procedure with additional doses of ketamine/xylazine. The right femoral artery was cannulated with a 22 gauge intravenous catheter. Initial blood samples of 1 mL were drawn for arterial blood gas analysis (Radiometer ABL 700, Diamond Diagnostics, Holliston, MA), CO-Hb level, and baseline serum cytokine measurements (IL-10 and TNF-α). Direct arterial blood pressure was continuously monitored.
The trachea was cannulated with a 14 gauge intravenous catheter. A volume cycled rodent ventilator (Harvard Apparatus Model 683, South Natick, MA) was used with air, tidal volume (TV) of 6 mL/kg, respiratory rate of 60 per minute, and pre-bypass respiratory mechanics were performed.
After respiratory mechanics, the left carotid artery was cannulated with a 20 gauge intravenous catheter followed by heparin 500 units/kg infused via right femoral artery. Subsequently, the right jugular vein was cannulated with a modified, multi-orifice 18 gauge intravenous catheter.
The rat was placed on CPB for 1 hour. Blood samples were drawn upon initiation of CPB for calibration of inline blood gas monitor and CO-Hb levels. Upon CPB termination, the animals were recovered for 30 minutes after which respiratory mechanics were performed and post-CPB blood gases, CO-Hb, and serum cytokine samples were obtained. Fluorescent label propidium iodide (PI) 50 mg/estimated blood volume (12) was infused via venous cannula and allowed to perfuse for 5 minutes at which time the animals were sacrificed with intravenous pentobarbital (390 mg/kg). The heart/lung block was excised for evaluation of alveolar cell injury (13). The left ventricular apex was excised for myocardial gene expression array, quantitative polymerase chain reaction (PCR), and histologic analysis.
Respiratory Mechanics
Ten animals were randomly chosen for respiratory mechanics and alveolar cell injury analysis (Air group n = 5; CO group n = 5). After tracheostomy, peak airway pressures at TV 6 mL/kg (respiratory rate [RR] 60/min) and 20 mL/kg (RR 30/min) were obtained with the use of two Harvard Rodent ventilators (Model 683, South Natick, MA) with different piston-cylinder assembly. Static airway pressure was measured at discrete inspiratory and expiratory lung volumes with an attached 10 mL glass syringe with volume displacement sensor after 15–20 breaths of 10 mL TV, creating a pressure-volume curve. The procedure was repeated after recovery from CPB.
Alveolar Cell Injury Analysis
The subpleural lung tissue was imaged using a Carl Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY) at a depth of 20 μm with 10 μm thickness optical slice. The specimen was excited with blue laser light, and emission wavelengths were collected on two channels: PI (λ> 543 nm) on channel 1 and autofluorescence (λ< 405 nm) for alveoli on channel 2. Images were digitized at an eight-bit resolution and were stored in arrays of 512 × 512 pixels. Confocal images were taken for cellular injury index (number of PI positive cells per alveolus) within 1 hour of lung extraction. After imaging, lungs were weighed for wet lung weight and placed in drying oven (Thelco, Champaign, IL) at 55°C for 24 hours for dry lung weight. Wet to dry lung weight ratio was obtained.
Cardiopulmonary Bypass and Components
A Plexiglas® encased oxygenator with a static prime volume of 4.5 ± .5 mL was built with reference to a previously reported model. CPB was applied using a miniature extra-corporeal circuit composed of asanguinous prime (11). This assembled oxygenator differed from the previously designed oxygenator by having only one blood path and one gas path. A polypropylene sheet membrane material was used with a surface area of.014 m2 (Sorin Group, Milano, Italy). The respective paths where machined into the plastic plates. The mesh that corresponded to the sheet membrane was used in this machined space to provide support to the membrane. The membrane was kept attached to the two plastic plates by a thin layer of high pressure vacuum grease and also steel bands and bolts for added pressure. The oxygenator was pressure tested to assure integrity of the membrane.
A 30 mL plastic syringe barrel was used as reservoir and contained a stainless steel heat exchanger device (11). The reservoir was sealed and vacuum was applied (−20 to −40 mmHg) as needed. The rat was kept at normothermia during CPB using the heat exchanger and warming lamp consisting of a 60 W light bulb.
A roller pump (Minntech Renal Systems, Minneapolis, MN) was equipped with 1/4″ polyvinyl chloride tubing which contained 1/8″ tubing. This 1/8″ tubing was the actual arterial pump boot. An initial calibration was performed to find the flow calibration value since the displayed pump output was based on the use of 1/4″ tubing. During CPB, flows were maintained at about 50 mL/min to the rat. This corresponded to about a 100 mL/kg/min flow. The rest of the circuit used pressure monitoring tubing except for the venous which was 1/8″ tubing. Total prime volume of the circuit was 13 ± 1 mL. All the post-oxygenator blood flow was directed through an inline blood gas monitoring cell (CDI 500, Terumo, Ann Arbor, MI) providing continuous blood gas analysis including PaO2, PCO2, pH, temperature, and potassium level.
The circuit was primed with about 13 mL of solution taken from 50 mL of a mixture containing 25 mL PlasmaLyte A (Baxter, Deerfield, IL), 22 mL Hextend™ 6% (Hospira, Alameda, CA), 2 mL sodium bicarbonate (1 mEq/mL),.5 mL mannitol (1.25/mL),.05 mL potassium chloride (2 mEq/mL),.15 mL calcium chloride (100 mcg/mL), and.1 mL heparin (1000 units/mL). Mean arterial blood pressure was maintained in the range of 55–65 mmHg. The rats were allowed to have their own native ejection and ventilation during CPB whenever the flows were below 70 mL/kg/min. This helped to supplement the CPB machine due to the decreased efficacy of single path oxygenation.
Vital signs and hemodynamics were monitored throughout the experiment and recorded at the time of femoral cannulation, heparin administration, start and end of CPB, after weaning from CPB and 30 minutes after CPB. Measurements included heart rate, systolic, diastolic and mean arterial blood pressure, central venous pressure and temperature. These recordings were performed at the time of blood draws for arterial blood gas analysis and cytokine measurements.
Enzyme-Linked Immunosorbent Assay Measurements of TNF-α and IL-10
The serum concentration of TNF-α and IL-10 (pre and post CPB) were measured by enzyme-linked immunosorbent assay (ELISA) as described by the manufacturer (R&D Systems, Minneapolis, MN).
Gene Expression Arrays
Gene expression profiles were obtained using the rat common cytokine Oligo Gene Expression Array (GEArray) (SuperArray Bioscience Corporation, Frederick, MD). Total ribonucleic acid (RNA) was isolated using RNA-Bee (Teltest Inc., Friendswood, TX) and subsequently purified on column using the ArrayGrade Total RNA isolation kit (SuperArray Bioscience Corporation). Two to three micrograms (μg) of total RNA per sample was reverse transcribed using the TrueLabeling-AMP™ 2.0 kit (SuperArray Bioscience Corporation) according to manufacturer’s instructions. The final cRNA quantity and quality were assessed by determining the optical density at 260 nm using a DU640 spectrophotometer (Beckman Coulter Inc., Fullerton, CA).
The membranes were hybridized and washed the following day according to the Oligo GEArray Hybridization protocol (SuperArray Bioscience Corporation). The mem branes were imaged using Chemidoc XRS (BioRad, Hercules, CA) at different exposure times (5–10–20–30 minutes) and analyzed using the GEArray Expression Analysis Suite (SuperArray Bioscience Corporation). The following analysis settings were used: density = average of four spots; background correction = local; normalization against selected genes (housekeeping genes); absent/present (AP) calls threshold 1.5. The results of the membranes treated with control cRNA versus membranes treated with CO pre-treated cRNA were averaged and the ratio CO-pretreated versus Air group (control) was calculated.
Real Time Quantitative PCR (RT-qPCR)
To confirm IL-10 gene expression, SybrGreen qPCR (Applied Biosystems, Warrington, UK) was performed on total RNA from both groups. One μg of total RNA was reverse transcribed using GeneAmp Gold RNA PCR Reagent Kit (Applied Biosystems) according to manufacturer’s instructions. RT-qPCR was performed on an ABI Prism 1700 Sequence detector (Applied Biosystems). To differentiate specific amplicons from nonspecific products using SybrGreen, a DNA dissociation curve was generated after each reaction with the ABI Prism sequence detection system.
All mRNAs (IL-6, IL-10, and TNF-α) were quantified relative to β-actin mRNA using the comparative threshold cycle number (Ct) method. The Ct difference (ΔCt = Ctgene − Ctβ-actin) was taken as a relative quantity of the transcript. To ascertain the validity of the ΔCt calculation (ΔΔCt = ΔCtcontrol − ΔCtCO-pretreatment), the amplification efficiency was checked and found to be identical for all the genes measured. To facilitate interpretation of the RT-PCR results, data are reported as fold difference relative to beta-actin, and were normalized within each RT-PCR run.
Statistical Analysis
Data was analyzed by the JMP® statistical package (SAS Institute Inc., Cary, NC). Fisher’s exact test was used to assess categorical variables expressed as frequencies and percentages. Numerical data were analyzed for normality of their distribution and when appropriate, analyzed using non-parametric tests. Repeated measures multivariate anal ysis of variance was used for time-dependent continuous variables. As appropriate, Student’s t test was used to compare groups post hoc. For RT-qPCR, group differences in mRNA expression were compared using one-way analysis of variance. All data are reported as mean ± standard error, unless otherwise indicated. A p-value less than.05 was considered statistically significant.
RESULTS
Baseline characteristics, hemodynamic variables, and blood gas analyses were similar between the groups (Table 1) with the expected exception of CO-Hb levels (p ≤ .001) and baseline oxy-hemoglobin saturation (p = .01) in Air versus CO treated groups, respectively.
Table 1.
Hemodynamic parameters and arterial blood gas analyses.
| Air | CO | p | |||
|---|---|---|---|---|---|
| Weight (g) | 495 (470, 560) | 491 (493, 522) | .8 | ||
| MAP (mmHg) | Baseline | 93 (86, 100) | 87 (84, 92) | .8 | |
| T1 | 63 (57, 74) | 55 (55, 59) | |||
| T2 | 66 (56, 96) | 76 (59, 91) | |||
| HR (min−1) | Baseline | 160 (152, 175) | 160 (150, 180) | .7 | |
| T1 | 160 (156, 170) | 160 (152, 177) | |||
| T2 | 160 (146, 178) | 155 (140, 178) | |||
| CVP (mmHg) | Baseline | NA | NA | .07 | |
| T1 | 4 (3, 4) | 4 (3, 5) | |||
| T2 | 4 (3, 4) | 3 (1, 3) | |||
| Hb (g/dL) | Baseline | 15.0 (13.1, 15.2) | 14.5 (13.4, 15.4) | .26 | |
| T1 | 5.6 (5.2, 7.7) | 7.1 (5.6, 7.4) | |||
| T2 | 5.9 (5.2, 7.5) | 7.3 (5.9, 8.4) | |||
| CO-Hb (%) | Baseline | 0 (0, .8) | 3.6 (2.4, 4.8) | .001 | |
| T1 | 1 (.1, 3) | .6 (.1, 1) | |||
| T2 | 3 (0, 3.8) | 0 (0, .3) | |||
| pH | Baseline | 7.43 (7.39, 7.48) | 7.39 (7.34, 7.44) | .3 | |
| T1 | 7.35 (7.16, 7.55) | 7.20 (7.13, 7.37) | |||
| T2 | 7.36 (7.30, 7.47) | 7.21 (7.11, 7.37) | |||
| PaO2 (mmHg) | Baseline | 234 (119, 394) | 113 (101, 178) | .5 | |
| T1 | 169 (96, 288) | 161 (81, 259) | |||
| T2 | 230 (103, 362) | 208 (80, 334) | |||
| PaCO2 (mmHg) | Baseline | 35 (28, 37) | 36 (32, 44) | .36 | |
| T1 | 35 (28, 39) | 47 (31, 53) | |||
| T2 | 32 (29, 40) | 42 (38, 53) | |||
| HCO3 (mmHg) | Baseline | 22 (20, 24) | 23 (20, 23) | .8 | |
| T1 | 20 (14, 26) | 18 (16, 20) | |||
| T2 | 18 (17, 21) | 16 (14, 23) | |||
| SaO2 (%) | Baseline | 98 (97, 99) | 91 (90, 96) | .01 | |
| T1 | 97 (88, 98) | 94 (87, 97) | |||
| T2 | 98 (94, 98) | 95 (81, 98) |
Data presented as median with interquartile range (25%, 75%). Comparisons between Air and CO groups are based on repeated measures multivariate analysis of variance (see Methods for details), except for weight (analysis of variance). MAP, mean arterial blood pressure; HR, heart rate; CVP, central venous pressure; Hb, hemoglobin; Baseline, time of femoral artery cannulation; T1, Time 1 (CPB start); T2, Time 2 (CPB end).
Respiratory Mechanics and Alveolar Cell Injury Analysis
There were no differences in pre and post-CPB respiratory mechanics between the treatment arms. Dynamic compliance pre-CPB was.55 ± .12 mL/cm H2O and .52 ± .10 mL/cm H2O (p = .34) in the Air and CO treated groups, respectively. Post CPB dynamic compliance was 22% lower in both groups (.41 ± .05 mL/cm H2O and.40 ± .07 mL/cm H2O; p = .38) (Figure 1). The wet to dry lung weight ratio was 14.6 ± 3 and 13.8 ± 2.5 in the Air and CO treated groups, respectively (p = .05). The alveolar cell injury index was low averaging.086 (p = .8, Figure 2) in both groups.
Figure 1.
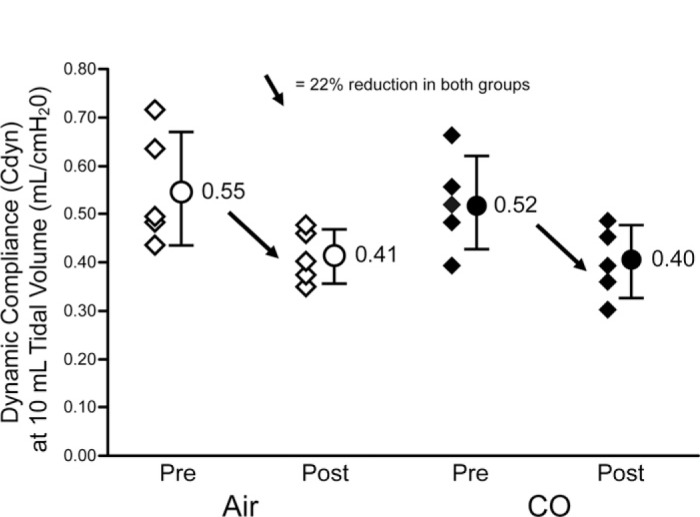
Dynamic compliance (Cdyn) at 10 mL tidal volume is similar between the Air and CO group preCPB with identical reduction (22%) in both groups post CPB.
Figure 2.

Alveolar cell injury index in subpleural alveoli is similar between the Air and CO groups. (Top) Confocal images of subpleural alveoli (left, Air group; right, CO group). The red nuclei mark the injured cells (PI–positive nuclei). (Bottom) Average number of PI-positive cells per alveolus assessed from 10 random subpleural fields (cellular injury index).
Serum Cytokine Response to Cardiopulmonary Bypass
There was a significant difference between pre and post-CPB serum cytokine levels. Serum TNF-α levels changed from undetectable to 363 ± 278 pg/mL in the Air group and 287 ± 195 in the CO group. IL-10 also changed from undetectable levels to 237 ± 26 in the Air group versus 302 ± 137 in the CO group. When comparing post-CPB serum cytokines, there was no significant difference between the groups, neither TNF-α (363 ± 278 pg/mL vs. 287 ± 195 pg/mL; p = .24, Air vs. CO), nor IL-10 (237 ± 26 pg/mL vs. 302 ± 137 pg/mL; p = .2, Air vs. CO).
Myocardial Gene Expression Array and Real Time Quantitative PCR
The myocardial cytokine expression profile revealed that the pro-inflammatory cytokine IL-6, Tumor Growth Factor-β1, and Insulin-like Growth Factor-1 (IGF-1) were down regulated in CO treated group compared to the Air group; while the anti-inflammatory and regulatory cytokine IL-10 and Cardiotropin Growth Factor-1 (CTF-1) were up regulated in the CO treated group compared with controls (Table 2).
Table 2.
Myocardial gene expression array after CPB in the CO group compared to Air.
| Molecule | Air Group | CO Group | Ratio CO/Air |
|---|---|---|---|
| IL-6 | .613 | .347 | .566‡ |
| TGF-β1* | .06 | .04 | .64‡ |
| IGF-1† | .015 | .007 | .44 |
| IL-10 | .095 | .237 | 2.491‡ |
| CTF-1* | .175 | .313 | 1.8 |
Data are expressed as average with n = 3–4. CTF–1, Cardiotropin Growth Factor–1, shown to have hypertrophic and protective effects in myocytes, neurotrophic.
TGF-β1, Tumor Growth Factor-β1, multifactorial peptide, implicated as a cause of fibrosis in kidney disease and acute respiratory distress syndrome.
IGF, Insulin like growth factor, somatomedin C.
p ≤ .05.
Based on the gene expression profile and the importance of IL-10 as a potent anti-inflammatory regulator, IL-10 expression levels post-CPB were confirmed with real time quantitative PCR using the comparative threshold cycle number. The average delta Ct was 9.02 ± 1.27 versus 7.3 ± 1.1 (p = .02) in the Air and CO treated group, respectively. This correlated with a 3.13 fold up-regulation of IL-10 in the CO pretreated animals (Figure 3).
Figure 3.

Real time quantitative PCR of IL-10 using the comparative threshold cycle (delta Ct) number. Control = β-Actin. The average delta Ct was 9.02 ± 1.27 versus 7.3 ± 1.1 (p = .02) in the Air and CO treated group, respectively. This correlated with a 3.13 fold up-regulation of IL-10 in the CO pretreated animals.
DISCUSSION
We have demonstrated that CPB triggered a substantial inflammatory response in rats, and CO pre-treatment attenuated this inflammatory response by altering myocardial cytokine gene expression.
The baseline characteristics of both groups were similar with the exception of oxygen saturation and CO-Hb. However, the CO treated group was still in a safe oxygenation range of 92.7 ± 3%, precluding hypoxic preconditioning as a probable variable. The CO-Hb level was well below the toxic range. More importantly, hemodynamic parameters were not altered in the CO group pre, during, and post-CPB, demonstrating adequate oxygen delivery (Table 1). Respiratory mechanics revealed a decrease in lung compliance (Figure 1) and abnormally high wet-todry lung weight ratio was seen in both Air and CO groups after CPB, consistent with some degree of lung injury. All animals were ventilated with a low tidal volume strategy to avoid confounding ventilator induced lung injury. There were no differences in the post-CPB respiratory mechanics, wet-to-dry lung weight ratio, and alveolar cellular injury index between the CO and Air groups. This finding does not support the robust modulation in inflammatory response to CPB in the porcine lung (6). This difference can be explained by different CO-Hb levels achieved on CPB, different timing of treatments, and different species.
We demonstrated that 1 hour of CPB produced marked elevation of serum cytokines IL-10 and TNF-α measured 30 minutes after separation from CPB compared to pre-CPB levels, validating our model of CPB as an inflammatory stressor. However, the serum levels of TNF-α and IL-10 post-CPB were not significantly different between the groups. Yet, the myocardial gene expression profiles confirmed a pattern of inflammatory attenuation by CO, with increased IL-10 expression post-CPB in the CO treated group compared to the Air group (Table 2). IL-6, an important pro-inflammatory cytokine, (14,15) was down-regulated in the CO group post-CPB (Table 2).
Due to the recognized importance of IL-10 in buffering the development of myocardial and lung injury after cardiopulmonary bypass (16), expression of IL-10 was further confirmed with real time quantitative PCR. Increased IL-10 expression was found in the CO treated group (3.13 times compared to the Air group, p = .02) (Figure 3), corroborating the findings on the gene expression array. These results are consistent with previous studies using low dose CO in other models of inflammation (17–20), and adds to the evidence of it’s role modulating CPB mediated systemic and pulmonary inflammatory response (6).
We have detected a significant difference in the myocardial gene expression profile and quantitative PCR between CO and Air groups post-CPB, however, there was no difference in serum cytokine levels. This could be due to underpowered sample size, sensitivity of the assay, the length of CPB, relatively low CO-Hb levels during CPB, and the relatively short post-CPB recovery period chosen in this experiment. The duration of CPB exposure was restricted to 1 hour since this is the conventional duration in reported studies using this animal model (11,21–23). In humans, the peak level of inflammatory cytokines (TNF-α and IL-6) is reached at 90–120 minutes post-CPB (24,25). We postulate that prolonging the length of CPB exposure time and the post-CPB recovery period in a larger sample of animals will yield statistically significant difference in cytokine protein levels as well as enhance the small but important difference in myocardial cytokine gene expression in future experiments.
Further limitations of this study include the lack of sternotomy, cardioplegic arrest, or hypothermia, all of which can add to the inflammatory effects of cardiac surgery.
In conclusion, our findings provide additional evidence that CO modulates the inflammatory response to CPB by altering myocardial cytokine expression. This study adds to the armamentarium of possibilities for CO therapy in inflammatory states. Further investigation is needed to assess the optimal timing, method of delivery, mechanism of action, and target patient population for CO as a novel and promising therapy in cardiovascular surgery requiring cardiopulmonary bypass.
ACKNOWLEDGMENTS
We would like to thank Mira Wouters, PhD, Fawn Atchison, MD, PhD, Randolph Stroetz, De Anna Haugen, Maria Theresa Barbosa, MD, Eric Ericson, Laurie Olsen, Mayo Clinic Division of Engineering; Robert Vassallo, MD, Dylan Miller, MD, Eduardo Chini, MD, PhD, Brian Goodmanson, and Gerry Rach, Mayo Clinic Laboratory; Bruce Searles, CCP, SUNY Upstate Medical University, New York; Terumo Cardiovascular Systems for the donation of CDI 500 sensors and Sorin Group for the donation of the oxygenator membrane material.
REFERENCES
- 1.Wan IY, Arifi AA, Wan S, et al. Beating heart revascularization with or without cardiopulmonary bypass: Evaluation of inflammatory response in a prospective randomized study. J Thorac Cardiovasc Surg. 2004;127:1624–31. [DOI] [PubMed] [Google Scholar]
- 2.Dong X, Liu Y, Du M, et al. P38 mitogen-activated protein kinase inhibition attenuates pulmonary inflammatory response in a rat cardiopulmonary bypass model. Eur J Cardiothorac Surg. 2006;30:77–84. [DOI] [PubMed] [Google Scholar]
- 3.Ryter SW, Morse D, Choi AM.. Carbon monoxide: To boldly go where NO has gone before. Sci STKE 2004:RE6. [DOI] [PubMed] [Google Scholar]
- 4.Lavitrano M, Smolenski RT, Musumeci A, et al. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004;18:1093–5. [DOI] [PubMed] [Google Scholar]
- 5.Goebel U, Siepe M, Mecklenburg A, et al. Carbon monoxide inhalation reduces pulmonary inflammatory response during cardiopulmonary bypass in pigs. Anesthesiology. 2008;108:1025–36. [DOI] [PubMed] [Google Scholar]
- 6.Goebel U, Siepe M, Schwer CI, et al. Inhaled carbon monoxide prevents acute kidney injury in pigs after cardiopulmonary bypass by inducing a heat shock response. Anesth Analg. 2010;111:29–37. [DOI] [PubMed] [Google Scholar]
- 7.Melley DD, Finney SJ, Elia A, et al. Arterial carboxyhemoglobin level and outcome in critically ill patients. Crit Care Med. 2007;35:1882–7. [DOI] [PubMed] [Google Scholar]
- 8.Farrugia G, Lei S, Lin X, et al. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2003;100:8567–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furchgott RF, Jothianandan D.. Endothelium-dependent and -independent vasodilation involving cyclic GMP: Relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28(1–3):52–61. [DOI] [PubMed] [Google Scholar]
- 10.Otterbein LE, Otterbein SL, Ifedigbo E, et al. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol. 2003;163:2555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You XM, Nasrallah F, Darling E, et al. Rat cardiopulmonary bypass model:Application of a miniature extracorporeal circuit composed of asanguinous prime. J Extra Corpor Technol. 2005;37:60–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HB, Blaufox MD.. Blood volume in the rat. J Nucl Med. 1985;26:72–6. [PubMed] [Google Scholar]
- 13.Gajic O, Lee J, Doerr CH, et al. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med. 2003;167: 1057–63. [DOI] [PubMed] [Google Scholar]
- 14.Wan S, Arifi AA, Wan IY, et al. Cytokine responses to myocardial revascularization on cardiopulmonary bypass: Intermittent cross-clamping versus blood cardioplegic arrest. Ann Thorac Cardiovasc Surg. 2002;8:12–7. [PubMed] [Google Scholar]
- 15.Li P, Sanders J, Hawe E, et al. Inflammatory response to coronary artery bypass surgery: Does the heme-oxygenase-1 gene microsatellite polymorphism play a role? Chin Med J (Engl). 2005;118:1285–90. [PubMed] [Google Scholar]
- 16.Giomarelli P, Scolletta S, Borrelli E, et al. Myocardial and lung injury after cardiopulmonary bypass: Role of interleukin (IL)-10. Ann Thorac Surg. 2003;76:117–23. [DOI] [PubMed] [Google Scholar]
- 17.Zuckerbraun BS, McCloskey CA, Gallo D, et al. Carbon monoxide prevents multiple organ injury in a model of hemorrhagic shock and resuscitation. Shock. 2005;23:527–32. [PubMed] [Google Scholar]
- 18.Dolinay T, Szilasi M, Liu M, et al. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–20. [DOI] [PubMed] [Google Scholar]
- 19.Nakao A, Toyokawa H, Abe M, et al. Heart allograft protection with low-dose carbon monoxide inhalation: Effects on inflammatory mediators and alloreactive T-cell responses. Transplantation. 2006;81:220–30. [DOI] [PubMed] [Google Scholar]
- 20.Otterbein LE, Mantell LL, Choi AM.. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276: L688–94. [DOI] [PubMed] [Google Scholar]
- 21.Dong GH, Xu B, Wang CT, et al. A rat model of cardiopulmonary bypass with excellent survival. J Surg Res. 2005;123:171–5. [DOI] [PubMed] [Google Scholar]
- 22.Mackensen GB, Sato Y, Nellgard B, et al. Cardiopulmonary bypass induces neurologic and neurocognitive dysfunction in the rat. Anesthesiology. 2001;95:1485–91. [DOI] [PubMed] [Google Scholar]
- 23.Senra DF, Katz M, Passerotti GH, et al. A rat model of acute lung injury induced by cardiopulmonary bypass. Shock. 2001;16:223–6. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg JB, Kapelanski DP, Olson JD, et al. Cytokine and complement levels in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;106:1008–16. [PubMed] [Google Scholar]
- 25.Ashraf S, Butler J, Tian Y, et al. Inflammatory mediators in adults undergoing cardiopulmonary bypass: Comparison of centrifugal and roller pumps. Ann Thorac Surg. 1998;65:480–4. [DOI] [PubMed] [Google Scholar]


