Abstract
Polychlorinated biphenyls (PCBs) are environmental toxicants that cause vascular inflammation and facilitate the development of brain metastases. The crucial event in metastasis formation is adhesion of blood-borne tumor cells to the vascular endothelium, followed by their transcapillary migration. The aim of the present study was to examine the mechanisms of PCB118-induced brain metastasis formation at the blood-brain barrier level with the focus on tumor cell adhesion to the brain endothelium. PCB118 was administered orally to wild-type or intercellular cell adhesion molecule-1 (ICAM-1)–deficient mice, followed by an injection of Lewis lung carcinoma cells into the carotid artery. Treatment with PCB118 resulted in enhanced development of brain metastases. Injection of tumor cells induced overexpression of ICAM-1 and vascular endothelial cell adhesion molecule-1 (VCAM-1) in brain endothelium that was further potentiated in mice exposed to PCB118. PCB118 did not affect the number of adhered and extravasated tumor cells in ICAM-1–deficient mice. Additional in vitro studies indicated that VCAM-1–neutralizing antibody protected against PCB118-induced adhesion of tumor cells to cultured brain endothelial cells. These results indicate that exposure to selected PCB congeners, such as PCB118, induces adhesion and transcapillary migration of tumor cells. This process is facilitated by proinflammatory adhesion molecules and results in potentiation of brain metastasis formation.
Keywords: blood-brain barrier, ICAM-1, VCAM-1, tumor cell adhesion, vascular inflammation
Due to persistent presence in the environment and their accumulation in bio-organisms, polychlorinated biphenyls (PCBs) continue to be a significant health hazard in human populations. Chronic exposure to PCBs and their metabolites results in a variety of health effects including hepatotoxicity, neurotoxicity, immunotoxicity, and hormonal disruption (Knerr and Schrenk, 2006; Liu et al., 2010; Ndountse and Chan, 2009; Ross, 2004). In addition, environmental exposure to PCBs can contribute to cancer development, including brain cancer and malignant melanoma as well as lung, breast, and prostate cancers (Hopf et al., 2009; Knerr and Schrenk, 2006; Liu et al., 2010). Among the broad spectrum of adverse effects elicited by PCBs, chronic inflammation and increased incidence of malignant tumors are of significant concerns (Eum et al., 2006; Knerr and Schrenk, 2006; Liu et al., 2010).
Cerebral endothelial cells are a critical component of the blood-brain barrier, which actively participates in signal transduction and vascular inflammatory responses (Dietrich, 2002; Han et al., 2010; Helyar et al., 2009; Toborek et al., 1995). Brain endothelial cells produce a variety of inflammatory mediators, including adhesion molecules, such as intercellular cell adhesion molecule-1 (ICAM-1) and vascular endothelial cell adhesion molecule-1 (VCAM-1). Both these adhesion molecules are inducible surface glycoproteins, which mediate adhesion-dependent cell-to-cell interactions in the vascular system (Chen et al., 2011; Dietrich, 2002; Sumagin and Sarelius, 2007). ICAM-1 and VCAM-1 belong to the immunoglobulin supergene family and have an important influence on inflammatory reactions via mediating the attachment of leukocytes to the vascular endothelium and their transendothelial migration. This process further facilitates the release of proinflammatory mediators (Eum et al., 2009; Han et al., 2010; Sumagin and Sarelius, 2007). In addition, ICAM-1 and VCAM-1 can participate in adhesion of blood-borne tumor cells to endothelial cells (Chen et al., 2011; O'Hanlon et al., 2002).
Several studies have indicated potent vascular effects of PCBs and their interaction with endothelial cells. Exposure to PCBs effectively activates vascular endothelial cells, leading to their dysfunction (Choi et al., 2003; Eum et al., 2006, 2009; Helyar et al., 2009; Hennig et al., 2002; Seelbach et al., 2010). PCBs can increase cellular oxidative stress, induce hyperpermeability of the vascular endothelium, and upregulate adhesion molecules. These events stimulate prometastatic properties of the endothelium, leading to adhesion and transmigration of cancer cells as demonstrated in cell culture studies (Choi et al., 2003; Eum et al., 2004; Hennig et al., 2002).
Tumor metastasis is a complex cascade of events involving tumor dissemination from the primary site to growth at distant organs (Nguyen et al., 2009; Saiki, 1997; Steeg, 2006). It is generally accepted that a large number of adhesive interactions with cell adhesion molecules or receptors are involved in the arrest of tumor cells in the capillary bed and their adhesion to the vascular endothelium (Miles et al., 2008; Nguyen et al., 2009; Saiki, 1997). However, the influence of PCBs on these initial events in metastasis formation is not fully understood. In the present study, we hypothesize that exposure to PCBs can facilitate the development of brain cancer metastasis by induction of inflammatory responses in brain capillaries. Our study demonstrates that PCB118 promotes adhesion of lung tumor cells to the brain endothelium and metastatic growth in brain parenchyma.
MATERIALS AND METHODS
D122 Lewis lung carcinoma cells.
D122 Lewis lung carcinoma cells tagged with luciferase (D122-Luc cells, a gift from Dr Lea Eisenbach, Weizmann Institute of Science, Rehovot, Israel) were maintained in Dulbecco's modified Eagle's medium (DMEM) GlutaMax medium (Invitrogen, Camarillo, CA), supplemented with 10% fetal bovine serum (Thermo Scientific, Rockford, IL) and 1% penicillin/streptomycin (Invitrogen). The cells were transfected with green fluorescent protein (GFP)–encoding vector (pBMN-GFP; Orbigen, San Diego, CA) to produce D122-Luc/GFP cells. D122-Luc/GFP cells were selected by treatment with 3 μg/ml puromycin, followed by sorting of fluorescent cells. Cells were maintained in 1 μg/ml puromycin-contained media. GFP fluorescence intensity was stable over five passages; therefore, the tagged cells were used for immunostaining until the fourth passage.
Animal treatment, surgical procedures, and bioluminescent imaging.
Studies were performed on male C57BL/6J mice (9 months old, 30–40 g; Harlan Laboratory, Indianapolis, IN), ICAM-1 knockout mice (ICAM−/−, 4–6 months old, 25 g; Jackson Laboratory, Bar Harbor, ME), and matched wild-type controls, in strict accordance with the University of Kentucky Animal Care and Use Committee and the National Institutes of Health guidelines. Mice were acclimatized for 7 days prior the experiments.
Mice were administered via oral gavage with PCB118 (2,3′-,4,4′-,5-pentachlorobiphenyl; > 99% pure; 150 μmol/kg; AccuStandard, New Haven, CT) dissolved in vitamin E-stripped safflower oil (Dyetts, Bethlehem, PA) or vehicle alone, as described earlier (Chen et al., 2009). The amount of administered PCB118 was based on the previous reports that indicated that such a dose activates vascular endothelium (Seelbach et al., 2010; Sipka et al., 2008) and results in plasma PCB concentration of 5μM (Choi et al., 2010) that reflects serum PCB levels in acutely exposed human populations (Jensen, 1989; Wassermann et al., 1979).
Mice were injected 48 h post-PCB118 administration with 2 × 106 D122-Luc/GFP cells in 100 μl of DMEM Glutamax serum-free medium into the internal carotid artery (ICA) using the procedure standardized in our laboratory (Chen et al., 2009). The development of brain metastatic nodules was monitored for 4 weeks using the IVIS Xenogen Bioluminescence Imager (Caliper Life Sciences, Hopkinton, MA). Prior the measurements, animals were anesthetized with isoflurane in oxygen and injected ip with D-luciferin potassium salt (2 mg/100 μl) to induce bioluminescence of D122-Luc cells. Identical instrument settings were used for all measurements. At the end of the observation period, mice were sacrificed, microvessels were isolated, as described earlier (Seelbach et al., 2010), and used for gene and protein analyses.
A typical experiment contained four experimental groups: (1) Vehicle (mice administered orally with vitamin E-stripped safflower oil and injected with DMEM medium into the ICA); (2) PCB118 (mice administered orally with PCB118 dissolved in vitamin E-stripped safflower oil and injected with DMEM medium into the ICA); (3) Tumor cells (mice administered orally with vitamin E-stripped safflower oil and injected with D122-Luc/GFP cells dissolved in DMEM medium into the ICA); and (4) PCB118 + tumor cells (mice administered orally with PCB118 and injected with D122-Luc/GFP cells into the ICA).
Real-time quantitative reverse transcription PCR.
RNA was extracted from brain microvessels 6 h after tumor cell injection using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Reverse transcription was performed using the AMV reverse transcription system (Promega). VCAM-1 and ICAM-1 messenger RNA (mRNA) levels were assessed using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). The primers and probes were obtained from Applied Biosystems. The following thermocycling conditions were used: 95°C for 10 min, followed by 95°C for 15 s, and 60°C for 60 s for 40 cycles. The standard curve was generated by plotting the threshold cycle versus the log concentration of the serial dilutions of one specific complementary DNA sample served as a calibrator. All samples were prepared in duplicate. Expression of mRNA was calculated by the comparative CT method. PCR amplification of β-actin (housekeeping gene) was performed for each sample to normalize mRNA levels of the target genes.
Immunofluorescent staining.
Immunofluorescent staining was performed on freshly isolated brain microvessels 6 h and 4 weeks posttumor cell injection, as described earlier (Seelbach et al., 2010). Microvessels were smeared on slides, fixed, and blocked with 1% bovine serum albumin in PBS for 1 h. Mouse anti-VCAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-ICAM-1 (BD Bioscience, San Diego, CA) monoclonal antibody (both at 1:500 dilution) was used for overnight incubation at 4°C, followed by incubation with Alexa Fluor 568 goat anti-mouse IgG (1:2000 dilution; Invitrogen). Slides were mounted with ProLong Gold Antifade reagent (Invitrogen), containing 4,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. Images were acquired using an Olympus BX61WI laser scanning confocal microscope (Olympus, Melville, NY). The average intensity of the fluorescent signal was measured using the FluoView software.
Immunohistochemistry.
Immunohistochemistry to evaluate adhesion and transcapillary migration of tumor cells was performed 24 h posttumor cell injection. Briefly, mice were perfused with saline, the brains were removed, and fixed in 10% paraformaldehyde. The brains were embedded into paraffin and cut into a series of 10-μm thick sections with 150-μm thick intersections. Sections were mounted onto glass slides, deparaffinized, and subjected to antigen retrieval in citrate buffer (pH 6.0). Endogenous peroxidase activity was decreased by treatment with 3% H2O2 in methanol for 10 min. The slides were rinsed in Tris-buffer saline (TBS) and TBS containing 0.1% Triton X-100 (TBST), blocked with 1.5% rabbit serum in TBST, and incubated with chicken anti-GFP monoclonal antibody (1:100; Aves Lab, Tigard, OR) at 4°C overnight, followed by incubation with rabbit anti-chicken IgY-HRP secondary antibody (1:200; Genway Biotech, San Diego, CA) for 1.5 h in room temperature. Diamino benzidine (Vector Laboratories, Burlingame, CA) was used for color developing. The sections were counterstained with hematoxylin (Sigma, St Louis, MO) and examined under a Nikon Eclipse E600 light microscope.
In vitro tumor cell adhesion assay.
bEND.3 cells (mouse brain endothelial cell line) were grown to confluence on 24-well plates and exposed to PCB118 (10μM) or vehicle for 18 h. Then, cultures were incubated with anti-ICAM-1- or anti-VCAM-1-neutralizing antibody (both from BioLegends, San Diego, CA) for 1 h. D122-Luc/GFP cells were labeled with calcein AM (Calbiochem, La Jolla, CA), as described earlier (Eum et al., 2009), and added in the amount of 4 × 105 cells/ml onto endothelial monolayers. Cocultures were incubated for 90 min at 37°C, followed by washing with Hank’s balanced salt solution to remove nonadherent D122-Luc/GFP cells. Fluorescence intensity was then measured using a fluorescence plate reader with excitation of 490 nm and emission of 517 nm.
Statistical analysis.
Data were analyzed by one-way ANOVA, two-way repeated measured ANOVA, the pairwise comparison using Fisher's least significant difference post hoc test, or independent sample T-tests using PASW Statistic 18 or SigmaStat 2.03 (SPSS, Chicago, IL). Statistical probability of p < 0.05 was considered significant.
RESULTS
Exposure to PCB118 Stimulates Tumor Cell Adhesion to the Brain Endothelium
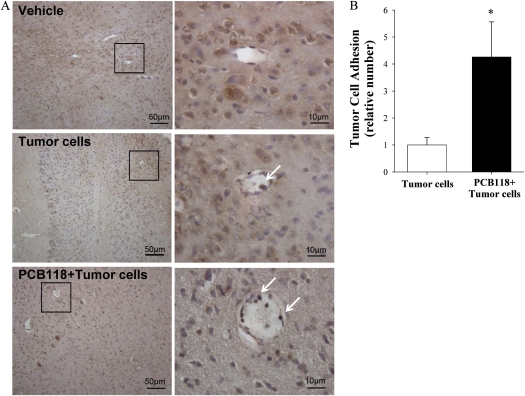
Adhesion of tumor cells to the brain endothelium was evaluated in mice exposed to PCB118 for 48 h and/or injected with D122-Luc/GFP tumor cells (Fig. 1). Analyses were performed 24 h post-injection of tumor cells by staining the brain slides for GFP-positive cells. Figure 1A illustrates representative images of tumor cell adhering to the brain endothelium with arrows indicating GFP-positive cells, distinguished from other cells by prominent brown staining. The highest number of adhered tumor cells was observed in the neocortex of the injected hemisphere; however, they were also detected in the white matter and the hippocampus. Tumor cells adhered to the capillary endothelium were counted on 14 sections per brain and quantitative data are reflected in Figure 1B. Adhesion of tumor cells was increased over four times in the PCB118 plus tumor cell group as compared with the tumor cell group.
FIG. 1.
Exposure to PCB118 stimulates tumor cell adhesion to the brain endothelium. Mice were exposed to PCB118 (150 μmol/kg) for 48 h, followed by injection of 2 × 106 D122-Luc/GFP cells into the ICA. Tumor cell adhesion was evaluated based on staining for GFP-positive cells (arrows) 24 h post-injection of tumor cells. (A) Shows representative histological images. Adhered cells were counted and quantitative data are reflected on (B). Images were acquired using ×20 (A, left panel) and ×100 (A, right panel) lens. Data are mean ± SEM, n = 6 mice per group. *Data in the PCB118 plus tumor cells are significantly different as compared with the tumor cells group at p < 0.05.
PCB118 Exposure Facilitates Formation of Tumor Metastases
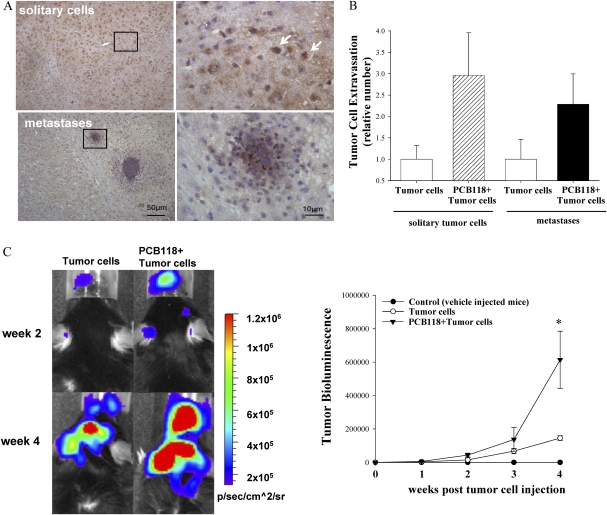
Adhesion of tumor cells to the brain endothelium is followed by their transcapillary migration and the formation of metastatic nodules in brain parenchyma. The representative images of these processes are reflected in Figure 2A. Solitary tumor cells and the number of tumor cell clusters (metastases) were counted 48 h post-tumor cells injections (Fig. 2B). These changes were observed primarily in the cortex of the injected hemisphere, with lower number present in the white matter and the hippocampus. Quantified data reflected in Figure 2B indicate a clear tendency toward an increase in both solitary tumor cells and metastases in mice exposed to PCB118 as compared with animals, which were injected with tumor cells without pre-exposure to PCB118. However, due to high variability between individual animals, these changes did not reach statistical significance.
FIG. 2.
PCB118 exposure facilitates formation of tumor metastases. Mice were exposed as in Figure 1. The experiment was terminated 24 h (A and B) or 4 weeks (C) post-tumor cell injection. Extravasated solitary tumor cells and cell cluster (metastases) were evaluated by staining of brain sections for GFP-positive cells (arrows). (A) Shows representative histological images and the right panels reflect quantitative data from these experiments. Images were acquired using ×20 (left panel) and ×100 (right panel) lens. (B) Extravasated cells and metastases were counted on 14 brain sections (10 μm each). (C) The growth of brain metastatic tumors was measured based on the intensity of the bioluminescent signal. Left panel shows representative images of the brains of the animals at weeks 2 and 4 postinjection of D122-Luc/GFP cells. Right panel reflects quantified results of brain tumor bioluminescence. Data are mean ± SEM, n = 4–6 mice per group. *Data in the PCB118 + tumor cells are significantly different as compared with the tumor cells group at p < 0.05.
Live imaging was then employed to monitor the progress of brain metastasis (Fig. 2C). During the first 2 weeks, brain metastases progressed at the same rate in both the tumor cell group and the PCB118 plus tumor cell group. When compared with the tumor cell group, mice additionally exposed to PCB118 (i.e., the PCB118 plus tumor cell group) revealed enhanced metastatic tumor growth by the third week of monitoring. These changes reached statistical significance at week 4, when the study was terminated. Mice that were not injected with tumor cells exhibited bioluminescence at the background levels.
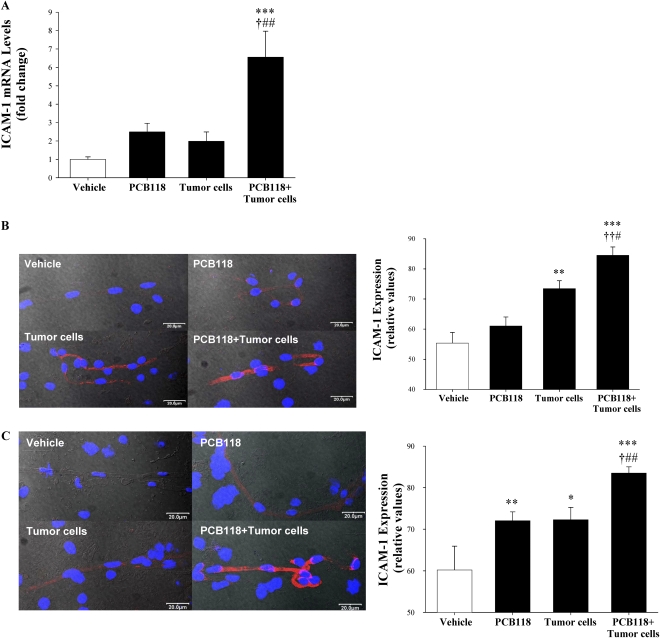
Oral Exposure to PCB118 Potentiates Tumor Cell–Mediated Induction of ICAM-1 Expression in Brain Microvessels
The effects of PCB118 exposure and/or tumor cell injection on ICAM-1 expression in brain microvessels were assessed by quantitative reverse transcription PCR and immunofluorescence microscopy (Fig. 3). In experiments reflected in Figures 3A and 3B, mice were pretreated with vehicle or PCB118 for 48 h, followed by injection with D122-Luc/GFP cells. Six hours posttumor cell injection, ICAM-1 mRNA level was increased over sixfold in the PCB118 plus tumor cell group as compared with the vehicle group. These changes were also statistically significant when compared with the tumor cell group or PCB118 group. Although there was a distinct tendency toward an increase in ICAM-1 mRNA levels in mice exposed to PCB118 or tumor cells alone, these changes did not reach statistical significance (Fig. 3A). Consistent with gene expression results, exposure to PCB118 plus tumor cells significantly increased ICAM-1 protein expression as compared with the vehicle, PCB118, or tumor cell group (Fig. 3B). Treatment with PCB118 alone did not affect ICAM-1 protein level; however, administration of tumor cells resulted in a significant upregulation of ICAM-1 protein expression 6 h postinjection.
FIG. 3.
Exposure to PCB118 potentiates tumor cell–mediated induction of ICAM-1 expression in brain microvessels. Mice were exposed as in Figure 1. The experiment was terminated 6 h (A and B) or 4 weeks (C) post-tumor cell injection. (A) ICAM-1 mRNA levels were evaluated in isolated brain microvessels by real-time quantitative reverse transcription PCR. (B and C) show overlapping images of ICAM-1 immunofluorescence staining in brain microvessels (red staining), DAPI staining to visualize the nuclei (blue), and microvessels imaged by Nomarski differential interference contrast mode. Images were acquired using a ×60 oil immersion lens and confocal microscopy. Data are mean ± SEM, n = 3–5 mice per group. *Significantly different as compared with vehicle-treated mice at *p < 0.05, **p < 0.01, or ***p < 0.001. †Values in the group PCB118 + tumor cells are significantly different as compared with those in the PCB118 group at †p < 0.05 or ††p < 0.01. #Values in the group PCB118 + tumor cells are significantly different as compared with those in the tumor cells group at #p < 0.05 or ##p < 0.01.
ICAM-1 protein expression was also measured 4 weeks after the initial exposure to PCB118 and/or injection with tumor cells (Fig. 3C). As compared with vehicle-treated controls, ICAM-1 levels were elevated in microvessels of all experimental groups, indicating chronic inflammatory reactions affecting cerebral capillaries. Importantly, ICAM-1 was expressed at a higher level in mice exposed to PCB118 plus tumor cells as compared with the animals administered with PCB118 or tumor cells alone.
Exposure to PCB118 and/or Tumor Cells Stimulates VCAM-1 Overexpression in Brain Microvessels
VCAM-1 mRNA and protein expression was evaluated in the same microvessel preparations as those used for ICAM-1 studies. VCAM-1 mRNA levels were highly increased in mice exposed to PCB118 for 48 h plus injected with tumor cells for 6 h as compared with the vehicle, PCB118, or tumor cell group (Fig. 4A). Within the same time frame, exposure to PCB118 alone but not to tumor cells alone also significantly upregulated VCAM-1 mRNA levels as compared with vehicle-treated mice.
FIG. 4.
VCAM-1 is overexpressed in brain microvessels of mice exposed to PCB118. Mice were exposed to PCB118 and/or D122-Luc/GFP as in Figure 1. The experiment was terminated 6 h (A and B) or 4 weeks (C) posttumor cell injection. (A) VCAM-1 mRNA levels were evaluated in isolated brain microvessels by real-time quantitative reverse transcription PCR. (B and C) show overlapping images of VCAM-1 immunofluorescence staining in brain microvessels (red staining), DAPI staining to visualize the nuclei (blue), and microvessels imaged by Nomarski differential interference contrast mode. Data are mean ± SEM, n = 3–7 mice per group. *Significantly different as compared with vehicle-treated mice at **p < 0.01 or ***p < 0.001. †Values in the group PCB118 plus tumor cells are significantly different as compared with those in the PCB118 group at †††p < 0.001. #Values in the group PCB118 plus tumor cells are significantly different as compared with those in the tumor cells group at ###p < 0.001.
VCAM-1 protein levels were evaluated by immunofluorescence. At the time points used for gene expression studies, VCAM-1 protein was upregulated in all experimental groups as compared with vehicle-treated control mice (Fig. 4B). The same measurements performed 4 weeks post-PCB118 and/or tumor cells administration revealed consistently upregulated VCAM-1 levels in mice that received tumor cell injections with or without PCB118 pre-exposure (Fig. 4C). VCAM-1 protein levels were within the control range in mice exposed to PCB118 alone.
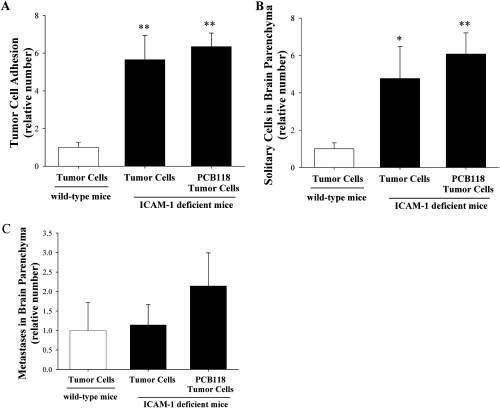
Exposure to PCB118 Does Not Affect Tumor Cell Adhesion and Metastasis Formation in ICAM-1-Deficient Mice
In order to further evaluate the role of ICAM-1 in PCB118-mediated adhesion of tumor cells to the brain endothelium and formation of brain metastases, ICAM-1-deficient mice and wild-type controls were exposed to PCB118 for 48 h and injected with D122-Luc/GFP cells (Fig. 5). Adhesion of tumor cells to the brain endothelium, tumor cell extravasation, and metastasis formation were analyzed as in Figures 1 and 2, respectively. Exposure to PCB118 plus tumor cells did not affect the number of adhering tumor cells in ICAM-1-deficient mice as compared with mice administered with tumor cells alone, indicating the importance of this adhesion molecule in PCB-induced tumor cell adhesion (Fig. 5A). Similarly, the number of solitary tumor cells and metastases in brain parenchyma analyzed in ICAM-1-deficient mice was the same in the PCB118 plus tumor cell group as compared with the tumor cell group (Figs. 5B and C). However, the total number of adhering and extravasated cells in ICAM-1-deficient mice was higher than in wild-type controls, suggesting the development of compensatory mechanisms that increase adhesiveness of the endothelium.
FIG. 5.
Exposure to PCB118 does not affect tumor cell adhesion and metastasis formation in ICAM-1 deficient mice. ICAM-1–deficient and age-matched wild-type mice were exposed to PCB118 and/or tumor cells as in Figure 1. Tumor cell adhesion (A), extravasation (B), and metastasis formation (C) were evaluated as in Figures 1 and 2, respectively. Adhered cells were counted on 14 brain sections (10 μm each). Data are mean ± SEM, n = 6 mice per group. *Significantly different as compared with wild-type control at *p < 0.05 or **p < 0.01.
VCAM-1 Is Involved in PCB118-Induced Adhesion of Tumor Cells to Brain Endothelial Cells In Vitro
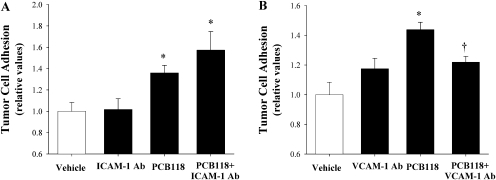
In the final series of experiments, mouse brain endothelial cells (bEND.3 cells) were treated with PCB118 (10μM) or vehicle for 18 h. Then, adhesion of tumor cells to the stimulated endothelial cells was evaluated in the presence or absence of ICAM-1– or VCAM-1–neutralizing antibody. As compared with the vehicle group, exposure to PCB118 increased adhesion of tumor cells to endothelial monolayers. Interestingly, this effect was not altered by adding ICAM-1–neutralizing antibody (Fig. 6A). In contrast, PCB118-induced adhesion of tumor cells was blocked in the presence of VCAM-1–neutralizing antibody (Fig. 6B), suggesting that VCAM-1 may also be involved in PCB118-induced adhesion of tumor cells to the brain endothelium.
FIG. 6.
VCAM-1 is involved in PCB118-induced adhesion of tumor cells to brain endothelial cells in vitro. Confluent mouse brain endothelial cells (bEND.3 cell line) were exposed to PCB118 (10μM) or vehicle for 18 h, followed by incubation with anti-ICAM-1– (30μM) or anti-VCAM-1–neutralizing (10μM) antibody (Ab) for 1 h. Then, calcein-labeled D122 cells were added to endothelial cultures for additional 90 min. Adhesion of D122 cells was evaluated by fluorescence measurement. Data are mean ± SD from three independent experiments, n = 6 cultures per group. *Significantly different as compared with controls at p < 0.05. †Data in the PCB118 plus VCAM-1 Ab are significantly different as compared with the PCB118 group at p < 0.05.
DISCUSSION
Environmental toxicants, such as PCBs, have neurotoxic, carcinogenic, and prometastatic properties (Faroon et al., 2001; Knerr and Schrenk, 2006; Liu et al., 2010; Ndountse and Chan, 2009; Seelbach et al., 2010). The food chain is the most common route of environmental exposure to PCBs. Indeed, meat and fish remain the primary source of PCB exposure and account for consistent PCB accumulation in human tissues (Schneider et al., 2007). Studies performed on population cohorts in the Great Lakes region consistently report a higher PCB body burden in high fish eaters that may be related to the alterations of prenatal and postnatal cognitive development, behavior, and childhood IQ (Li et al., 2009; Stewart et al., 2008). To mimic this route of exposure, we administered PCB118 via oral gavage in the present study. Because cancer and metastases typically develop in humans of middle to advanced age, majority of the experiments reported in the present study were completed using 9-month-old mice.
Although detailed mechanisms of prometastatic effects of PCBs are not fully understood, it appears that specific PCB congeners may enhance tumor cell extravasation and metastatic growth through induction of inflammatory responses. Using in vitro approaches, we demonstrated that individual PCBs can upregulate production of inflammatory mediators, which then increase adhesion of tumor cells to vascular endothelial cells (Choi et al., 2003). In addition, PCBs may facilitate metastasis formation by disrupting the integrity of the endothelium via increased vascular endothelial growth factor levels, altering expression of tight junction proteins, and activating specific matrix metalloproteinases (Eum et al., 2004; Seelbach et al., 2010). Our previous study (Seelbach et al., 2010) indicated that PCB118 may be the most active among different PCB congeners in facilitating the development of brain metastases. In addition, PCB118 is a major environmental contaminant, which is frequently detected in PCB-contaminated sites (Park et al., 2007). Chemically, it belongs to the so-called mono-ortho-substituted PCBs and is a weak aryl hydrocarbon receptor agonist (Hestermann et al., 2000). Experimental studies also indicated that PCB118 can stimulate proliferation of human MCF-7 breast cancer cells (Radice et al., 2008) and induce genotoxic damage (Marabini et al., 2011).
The main emphasis of the present study was placed on an early morphological event in metastasis formation, namely, adhesion of tumor cells to the vascular endothelium. Invading cells exhibit motility by changing cell adhesion properties, rearranging the extracellular matrix environment, and reorganizing cytoskeleton (Chen et al., 2011; Li and Feng, 2011; Mierke et al., 2008; Noda et al., 2011). Growing evidence indicates that the critical step in the formation of blood-borne metastases is adhesion of tumor cells to the surface of the endothelium (Chen et al., 2011; Makrilia et al., 2009). Binding of tumor cells to the vascular endothelium is regulated by adhesion molecules expressed by endothelial cells. Adhesion molecules then facilitate the movement or retention of cells through the extracellular matrix; therefore, they are directly implicated in the progression of metastatic growth (Chen et al., 2011; Li and Feng, 2011; O'Hanlon et al., 2002; Simiantonaki et al., 2002).
Our novel results indicate that PCB118-mediated development of brain metastases may result from increased adhesion of tumor cells to the brain capillary endothelium due to upregulation of ICAM-1 and VCAM-1. These adhesion molecules are typically induced upon acute and chronic inflammatory conditions and participate in tissue infiltration with inflammatory leukocytes; however, they are also involved in tumor cell adhesion to the endothelium (Chen et al., 2011; Makrilia et al., 2009; Simiantonaki et al., 2002). Injection with tumor cells rapidly induced expression of these adhesion molecules in brain capillaries, with PCB118 markedly potentiating this effect. Although the mechanisms of induction of ICAM-1 or VCAM-1 in response to PCB118 exposure are not fully understood, earlier in vitro experiments may suggest the role of oxidative stress, alterations of cellular redox status (Eum et al., 2009), and activation of nuclear factor-κB (Hennig et al., 2002). The importance of the integrity of endothelial lipid rafts in PCB-induced stimulation of ICAM-1 and VCAM-1 was also demonstrated (Eum et al., 2009; Han et al., 2010). Interestingly, expression of both ICAM-1 and VCAM-1 in brain microvessels was preserved throughout a 4-week observation period and progression of metastatic growth. Overexpression of ICAM-1 and VCAM-1 was especially enhanced in animals exposed to PCB118 prior to injection with tumor cells, indicating a strong link between persistent inflammation and tumor growth. These effects are important because chronic vascular inflammation may be involved in the growth of metastatic tumors by its contribution to vessel and extracellular matrix remodeling.
To evaluate in more details the role of ICAM-1 in PCB-mediated adhesion of tumor cells to the brain endothelium, PCB118 was administered to ICAM-1–deficient mice, followed by injection with tumor cells. Exposure to PCB118 did not affect the number of adhered and extravasated tumor cells in ICAM-1–deficient mice, confirming an important role of this adhesion molecule in PCB118-mediated metastasis development. At the same time, ICAM-1 deficiency increased the overall adherence and extravasation of tumor cells as compared with wild-type controls. These results suggest that ICAM-1–deficient mice may express other and undefined molecules as compensatory mechanisms, which result in increased adhesiveness of the vascular endothelium to tumor cells. This suggestion is consistent with the hypothesis that individual adhesion molecules do not work independently but rather express extensive and overlapping network in order to accomplish a metastatic cascade (Li and Feng, 2011). Indeed, ICAM-1–neutralizing antibody did not affect adhesion of tumor cells to cultured brain endothelial cells exposed to PCB118.
VCAM-1 was another adhesion molecule upregulated in brain microvessels by PCB118 exposure, and thus, we evaluated its involvement in facilitation of brain metastasis. Because VCAM-1 knockout mice are not viable, these experiments were limited to in vitro studies. VCAM-1–neutralizing antibody effectively decreased PCB118-induced tumor adhesion, suggesting that VCAM-1 may play a role in PCB-mediated brain metastasis formation. This observation is consistent with the report on the correlation between VCAM-1 expression and metastatic pattern of several types of cancer, including melanoma (Makrilia et al., 2009).
In conclusion, oral administration of PCB118 facilitates adhesion of lung tumor cells to the brain endothelium, followed by their extravasation and growth to metastatic tumors in brain parenchyma. Our results indicate that PCB118-induced adhesion of tumor cells to the brain endothelium is dependent on ICAM-1 and VCAM-1; however, it may also include other adhesion molecules or cell surface receptors. Overall, these findings demonstrate the importance of adhesion molecules in mediating of the PCB-induced formation of brain metastases.
FUNDING
This work was supported in part by grants from the National Institutes of Health (P42 ES 07380, MH63022, MH072567, DA027569) and from the American Heart Association Scientist Development Grant (09SDG2300037).
Acknowledgments
The authors declare that there are no conflicts of interest.
References
- Chen L, Swartz KR, Toborek M. Vessel microport technique for applications in cerebrovascular research. J. Neurosci. Res. 2009;87:1718–1727. doi: 10.1002/jnr.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Kuo CH, Lai TY, Lin YM, Su CC, Hsu HH, Tsai FJ, Tsai CH, Huang CY, Tang CH. RANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulation. J. Cell. Biochem. 2011;112:933–941. doi: 10.1002/jcb.23009. [DOI] [PubMed] [Google Scholar]
- Choi W, Eum SY, Lee YW, Hennig B, Robertson LW, Toborek M. PCB 104-induced proinflammatory reactions in human vascular endothelial cells: Relationship to cancer metastasis and atherogenesis. Toxicol. Sci. 2003;75:47–56. doi: 10.1093/toxsci/kfg149. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Seelbach MJ, Pu H, Eum SY, Chen L, Zhang B, Hennig B, Toborek M. Polychlorinated biphenyls disrupt intestinal integrity via NADPH oxidase-induced alterations of tight junction protein expression. Environ. Health Perspect. 2010;118:976–981. doi: 10.1289/ehp.0901751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich JB. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J. Neuroimmunol. 2002;128:58–68. doi: 10.1016/s0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- Eum SY, Andras I, Hennig B, Toborek M. NADPH oxidase and lipid raft-associated redox signaling are required for PCB153-induced upregulation of cell adhesion molecules in human brain endothelial cells. Toxicol. Appl. Pharmacol. 2009;240:299–305. doi: 10.1016/j.taap.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Lee YW, Hennig B, Toborek M. VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Exp. Cell Res. 2004;296:231–244. doi: 10.1016/j.yexcr.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Eum SY, Rha GB, Hennig B, Toborek M. c-Src is the primary signaling mediator of polychlorinated biphenyl-induced interleukin-8 expression in a human microvascular endothelial cell line. Toxicol. Sci. 2006;92:311–320. doi: 10.1093/toxsci/kfj194. [DOI] [PubMed] [Google Scholar]
- Faroon O, Jones D, de Rosa C. Effects of polychlorinated biphenyls on the nervous system. Toxicol. Ind. Health. 2001;16:305–333. doi: 10.1177/074823370001600708. [DOI] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol. Appl. Pharmacol. 2010;1-2:74–82. doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyar SG, Patel B, Headington K, El Assal M, Chatterjee PK, Pacher P, Mabley JG. PCB-induced endothelial cell dysfunction: Role of poly(ADP-ribose) polymerase. Biochem. Pharmacol. 2009;78:959–965. doi: 10.1016/j.bcp.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: In vitro and in vivo evidence. Toxicol. Appl. Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hestermann EV, Stegemanm JJ, Hahnm ME. Relative contributions of affinity and intrinsic efficacy to aryl hydrocarbon receptor ligand potency. Toxicol. Appl. Pharmacol. 2000;168:160–172. doi: 10.1006/taap.2000.9026. [DOI] [PubMed] [Google Scholar]
- Hopf NB, Waters MA, Ruder AM. Cumulative exposure estimates for polychlorinated biphenyls using a job-exposure matrix. Chemosphere. 2009;76:185–193. doi: 10.1016/j.chemosphere.2009.03.058. [DOI] [PubMed] [Google Scholar]
- Jensen AA. (1989). Background levels in humans. In Halogenated Biphenyls, Terphenyls, Naphthalenes, Dibenzodioxines and Related Products, 2nd ed. (R. D. Kimbrough and A. A. Jensen, Eds.), pp. 345–364. Elsevier Science Publishers, New York, NY. [Google Scholar]
- Knerr S, Schrenk D. Carcinogenicity of “non-dioxinlike” polychlorinated biphenyls. Crit. Rev. Toxicol. 2006;36:663–694. doi: 10.1080/10408440600845304. [DOI] [PubMed] [Google Scholar]
- Li A, Rockne KJ, Sturchio N, Song W, Ford JC, Wei H. PCBs in sediments of the Great Lakes-distribution and trends, homolog and chlorine patterns, and in situ degradation. Environ. Pollut. 2009;157:141–147. doi: 10.1016/j.envpol.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Li DM, Feng YM. Signaling mechanism of cell adhesion molecules in breast cancer metastasis: Potential therapeutic targets. Breast Cancer Res. Treat. 2011;128:7–21. doi: 10.1007/s10549-011-1499-x. [DOI] [PubMed] [Google Scholar]
- Liu S, Li S, Du Y. Polychlorinated biphenyls (PCBs) enhance metastatic properties of breast cancer cells by activating Rho-associated kinase (ROCK) PLoS One. 2010;5:e11272. doi: 10.1371/journal.pone.0011272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrilia N, Kollias A, Manolopoulos L, Syrigos K. Cell adhesion molecules: Role and clinical significance in cancer. Cancer Invest. 2009;27:1023–1037. doi: 10.3109/07357900902769749. [DOI] [PubMed] [Google Scholar]
- Marabini L, Calò R, Fucile S. Genotoxic effects of polychlorinated biphenyls (PCB 153, 138, 101, 118) in a fish cell line (RTG-2) Toxicol. In Vitro. 2011;25:1045–1052. doi: 10.1016/j.tiv.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Mierke CT, Zitterbart DP, Kollmannsberger P, Raupach C, Schlötzer-Schrehardt U, Goecke TW, Behrens J, Fabry B. Breakdown of the endothelial barrier function in tumor cell transmigration. Biophys. J. 2008;94:2832–2846. doi: 10.1529/biophysj.107.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: Capillary extravasation in cancer metastasis. Clin. Exp. Metastasis. 2008;25:305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- Ndountse LT, Chan HM. Role of N-methyl-D-aspartate receptors in polychlorinated biphenyl mediated neurotoxicity. Toxicol. Lett. 2009;184:50–55. doi: 10.1016/j.toxlet.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massagué J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Noda M, Seike T, Fujita K, Yamakawa Y, Kido M, Iguchi H. Role of immune cells in brain metastasis of lung cancer cells and neuron-tumor cell interaction. Neurosci. Behav. Physiol. 2011;41:243–251. [PubMed] [Google Scholar]
- O'Hanlon DM, Fitzsimons H, Lynch J, Tormey S, Malone C, Given HF. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur. J. Cancer. 2002;38:2252–2257. doi: 10.1016/s0959-8049(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Park H, Lee SJ, Kang JH, Chang YS. Congener-specific approach to human PCB concentrations by serum analysis. Chemosphere. 2007;68:1699–1706. doi: 10.1016/j.chemosphere.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Radice S, Chiesara E, Fucile S, Marabini L. Different effects of PCB101, PCB118, PCB138 and PCB153 alone or mixed in MCF-7 breast cancer cells. Food Chem. Toxicol. 2008;46:2561–2567. doi: 10.1016/j.fct.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Ross G. The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol. Environ. Saf. 2004;59:275–291. doi: 10.1016/j.ecoenv.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Saiki I. Cell adhesion molecules and cancer metastasis. Jpn. J. Pharmacol. 1997;75:215–242. doi: 10.1254/jjp.75.215. [DOI] [PubMed] [Google Scholar]
- Schneider AR, Porter ET, Baker JE. Polychlorinated biphenyl release from resuspended Hudson River sediment. Environ. Sci. Technol. 2007;41:1097–1103. doi: 10.1021/es0607584. [DOI] [PubMed] [Google Scholar]
- Seelbach M, Chen L, Powell A, Choi YJ, Zhang B, Hennig B, Toborek M. Polychlorinated biphenyls disrupt blood-brain barrier integrity and promote brain metastasis formation. Environ. Health Perspect. 2010;118:479–484. doi: 10.1289/ehp.0901334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiantonaki N, Jayasinghe C, Kirkpatrick CJ. Effect of pro-inflammatory stimuli on tumor cell-mediated induction of endothelial cell adhesion molecules in vitro. Exp. Mol. Pathol. 2002;73:46–53. doi: 10.1006/exmp.2002.2440. [DOI] [PubMed] [Google Scholar]
- Sipka S, Eum SY, Son KW, Xu S, Gavalas VG, Hennig B, Toborek M. Oral administration of PCBs induces inflammatory and prometastatic responses. Environ. Toxicol. Pharmacol. 2008;25:251–259. doi: 10.1016/j.etap.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg SP. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect. 2008;116:1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumagin R, Sarelius IH. A role of ICAM-1 in maintenance of leukocyte-endothelial cell rolling interaction in inflamed arterioles. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2786–H2798. doi: 10.1152/ajpheart.00720.2007. [DOI] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J. Biochem. Toxicol. 1995;10:219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- Wassermann M, Wassermann D, Cucos S, Miller HJ. World PCBs map: Storage and effects in man and his biologic environment in the 1970s. Ann. N. Y. Acad. Sci. 1979;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]