Abstract
Intranasal exposure to the heavy metal cadmium has been linked to olfactory dysfunction and neurotoxicity. Here, we combine optical imaging of in vivo neurophysiology, genetically defined anatomical tract tracing, mass spectrometry, and behavioral psychophysical methods to evaluate the persistent harmful effects of acute intranasal exposure to cadmium in a mouse model and to investigate the functional consequences of sensory rehabilitation training. We find that an acute intranasal instillation of cadmium chloride leads to an accumulation of cadmium in the brain's olfactory bulb that persists for at least 4 weeks. This is accompanied by persistent severe pathophysiology of the olfactory nerve, a gradual reduction in axonal projections from the olfactory epithelium, and complete impairment on an olfactory detection task. Remarkably, 2 weeks of odorant-guided operant conditioning training proved sufficient to restore olfactory detection performance to control levels in cadmium-exposed mice. Optical imaging from rehabilitated mice showed that this training did not cause any detectable restoration of olfactory nerve function, suggesting that the recovery of function was mediated by central neuroplasticity in which the brain learned to interpret the degraded sensory input. These data demonstrate that sensory learning can mask even severe damage from neurotoxicants and suggest that explicit sensory training may be useful in rehabilitation of olfactory dysfunction.
Keywords: rehabilitation, sensory learning, heavy metal, olfactory, neuroplasticity, neurotoxicity
The mammalian nervous system is capable of remarkable recovery after injury. The peripheral nervous system exhibits axonal regeneration, proliferation of glia, and remyelination (Chen et al., 2007; Liu et al., 2011). Although the brain and spinal cord have limited capacity for physical repair, they can reorganize to partially compensate for damage (Chen et al., 2002). For example, after a peripheral motor nerve lesion, the corresponding parts of the motor cortex are repurposed to represent other uninjured body parts (Bruehlmeier et al., 1998; Huntley, 1997; Sanes et al., 1990). These changes are typically compensatory, for instance, after traumatic injury, the spinal cord's central pattern generation circuitry can adapt to maintain proper sensorimotor integration by using load and speed information derived from local proprioceptive inputs in the absence of descending sensory input from the brain (Edgerton et al., 2004; Harkema et al., 1997). This capacity for central plasticity is a potential confound for behavioral assays of neurotoxicity (Hastings, 1990; Moser, 2011; Rohlman et al., 2008).
The olfactory system is especially vulnerable to neural damage because its primary sensory neurons are in direct contact with the external environment. Perhaps in consequence, olfactory receptor neurons (ORNs) in the nasal epithelium are replaced constantly from a local population of adult stem cells that differentiate into neurons and project their axons through the olfactory nerve to the brain's olfactory bulb (Graziadei and DeHan, 1973; Graziadei and Monti Graziadei, 1979; Schwob et al., 1995). This poses a unique challenge for the brain because it must interpret sensory information from an unstable population of neural inputs. Olfactory structures downstream of the ORNs may thus be capable of adaptive plasticity that permits the interpretation of degraded sensory inputs that survive even severe neurotoxicant exposures.
Cadmium is a heavy metal that is frequently encountered in aerosolized form in cigarette smoke and in some industrial workplaces, including battery manufacture and smelting (Agency for Toxic Substances & Disease Registry, 2008). ORNs may be particularly vulnerable to cadmium accumulation because they express high levels of metallothioneins and other metal-binding proteins (Himeno et al., 2009; Sunderman, 2001). Exposure to aerosolized cadmium compounds has been associated with olfactory dysfunction in humans, notably the elevation of olfactory thresholds and increased incidence of parosmia (Adams and Crabtree, 1961; Mascagni et al., 2003; Rose et al., 1992; Sułkowski et al., 2000). We have recently reported (Czarnecki et al., 2011) that acute intranasal instillations of cadmium chloride disrupt odorant-evoked neurotransmitter release from the olfactory nerve in a mouse model, even at modest doses (0.2–2 μg). Higher doses (20–400 μg) also induce histopathology in the olfactory epithelium in the nose and in the ORN projections to the olfactory bulb (Bondier et al., 2008; Czarnecki et al., 2011).
Behavioral studies of olfactory sensory function after intranasal cadmium exposure in rodent models have produced three different results: immediate anosmia (the inability to detect odorants; Czarnecki et al., 2011), temporary anosmia (Bondier et al., 2008), and no impairment (Sun et al., 1996). In the study that reported no impairment, the subjects were continuously trained on the olfactory discrimination task throughout the period of cadmium exposure (Sun et al., 1996). Though the authors interpreted their results as showing no harmful effect of cadmium, the design leaves open the possibility that cadmium exposure disrupted the physiology of the ORNs but that the ongoing training allowed the animals to learn how to compensate. Similarly, the study by Bondier et al. (2008) showed a complete recovery of a spontaneous odorant avoidance behavior despite an only partial recovery of the olfactory epithelium, which might also be attributable to central plasticity.
In a previous report (Czarnecki et al., 2011), we used acute intranasal cadmium exposure to illustrate the utility of in vivo optical imaging for assessing neurotoxicity in the mouse olfactory system. Here, we extend those findings to investigate the time course of cadmium-induced pathophysiology over 4 weeks and the consequences of explicit postexposure rehabilitation training. First, we use inductively coupled plasma mass spectrometry (ICPMS) to investigate transport of cadmium chloride to the olfactory bulb and its residence time therein in C57BL/6 mice. To assess the effects of cadmium exposure on ORN synaptic physiology, odorant-evoked neurotransmitter release from the olfactory nerve into olfactory bulb glomeruli was visualized in vivo at various postexposure and/or rehabilitation time points in a line of C57BL/6-background transgenic mice that express the fluorescent exocytosis indicator synaptopHluorin in the synaptic terminals of essentially all ORNs (Bozza et al., 2004; Czarnecki et al., 2011; McGann et al., 2005). After imaging, the olfactory bulbs of these mice were sectioned and ORN projection density was quantified to show effects of cadmium exposure on olfactory nerve structure. Olfactory sensory function was assessed in parallel behavioral assays in C57BL/6 and olfactory marker protein-synaptopHluorin (OMP-spH) mice that were trained to nose poke for a sucrose reward if and only if they detect an odorant at a separate sampling port. Operant conditioning continued following cadmium exposure to reveal the sensory and (in OMP-spH mice) physiological consequences of rehabilitation training.
MATERIALS AND METHODS
Subjects.
Subjects in all imaging and histological experiments were a total of 27 homozygous male and female OMP-spH mice, which express the spH construct (Miesenböck et al., 1998) from the locus for olfactory marker protein (Bozza et al., 2004). The mice used in this study are on an albino C57BL/6 background as previously reported (Czarnecki et al., 2011). Subjects in behavioral and mass spectrometry experiments were 23 and 15 adult male and female C57BL/6 mice. All animals were group housed with a 12:12-h light:dark cycle with food and water available ad libitum. All procedures were performed in accordance with protocols approved by the Rutgers University Animal Care and Use Committee.
Intranasal infusions.
Mice in Cd-exposed groups received intranasal instillations (see Czarnecki et al., 2011) of 6 μl pH 7.4 buffer solution containing 200mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 0.9% NaCl, and 18.18mM CdCl2, yielding a 20 μg CdCl2 physical dose per naris. Mice in all behavioral experiments randomly received a bilateral infusion of 20 μg CdCl2 or vehicle solution (without CdCl2), whereas animals in the time course imaging experiments received unilateral instillations with side counterbalanced between subjects. Reflux of solution was not observed. The experimenter was blind to the contents of all infusates.
Inductively coupled plasma mass spectrometry.
Cadmium has been previously shown to transport down the olfactory nerve to the ipsilateral olfactory bulb following intranasal instillations (Bondier et al., 2008; Tjälve et al., 1996), but it was unknown how long Cd levels remain elevated following an acute exposure. Fifteen male C57BL/6 mice, five animals per group, were given unilateral intranasal instillations of 20 μg CdCl2. Two, 10, or 28 days after exposure, animals were lightly anesthetized with isoflurane and decapitated. The olfactory bulbs were dissected out, weighed, and stored in an Isotemp −20°C freezer until tissue samples were processed. The tissue was analyzed for Cd using ICPMS (Thermo Elemental X-5) after microwave (CEM Mars X) digestion. Approximately 0.01–0.03 g of olfactory bulb tissue was weighed into 15-ml centrifuge tubes and digested with concentrated nitric acid (0.25 ml). After approximately 15 min, the samples were sonicated for at least 1 h before introduction into the microwave. Samples were digested five times using 300 W power, 75% duty cycle, 5 min. Samples were cooled before their final dilution to 7.625 ml using 1.6% vol/vol HNO3. This gave a final acid concentration of 5%. All the standard solutions were prepared in 5% HNO3. The m/z = 111 Cd isotope was used to quantify cadmium. All of the necessary quality assurance (QA)/quality control (QC) protocols were maintained throughout the extraction and analysis process. Roughly 20% of the samples were QC samples including blanks (laboratory and solvent) spikes and standard reference materials. Recoveries of > 90% of the laboratory spike were deemed acceptable.
In vivo imaging of neurotransmitter release.
Three time points were chosen in reference to the approximate 4-week life cycle of cells in the olfactory epithelium (Graziadei and Monti Graziadei, 1979). Two, 7, or 28 days after intranasal cadmium exposure, four mice per group were anesthetized with 0.01 ml/g of 10 mg/ml pentobarbital (ip), with 0.05 ml boosters as needed to maintain anesthetic plane. Body temperature was maintained using a rectal temperature probe and feedback-regulated heating pad. Mice were administered 0.01–0.02 ml/g 0.1% atropine (sc) to reduce nasal secretions and ∼0.25 ml of 0.25% bupivacaine (sc) as a local anesthetic along the scalp. The scalp was shaved, then surgically opened with a midline incision. The periosteal membrane was removed and the skull dried with a 70% ethanol solution. A headbar was fixed to the skull using dental acrylic to rigidly mount the mouse's skull to a custom headholder. Using a micromotor dental handpiece, the skull overlying both olfactory bulbs was thinned until transparent when wet. Ringer's solution containing 140mM NaCl, 5mM KCl, 1mM CaCl2, 1mM MgCl2, 10mM HEPES, and 10mM dextrose was applied over this cranial window, then topped with a glass coverslip.
Optical imaging was performed using a custom apparatus as previously reported (Czarnecki et al., 2011). Images were acquired using a RedShirtImaging monochrome, back-illuminated CCD camera (NeuroCCD SM256) at 256 × 256 pixel resolution and frame acquisition rate of 7 Hz. The mouse was positioned under the microscope using a custom optomechanical stage. The entire apparatus floated on a TMC vibration isolation table.
Odorants were presented by a custom eight-channel air dilution olfactometer controlled by a computer running software written for MatLab (Mathworks). Nitrogen served as a carrier vapor that was saturated with odorant and then diluted into ∼500 ml/min ultrazero-humidity compressed air by computer-controlled mass flow controllers. Source gases were filtered by a hydrocarbon/moisture gas purification system (Chromatography Research Services). Stimulus onset and offset were controlled by a computer-controlled valve that shunted a vacuum from and to an odorant removal tube concentric with the odorant delivery tube. The odorant delivery tube was placed within 2 cm of the mouse's nose. Odorants included methyl valerate, 2-methyl-2-butenal, hexanone, and butyl acetate, which are known to evoke transmitter release on the dorsal aspect of the olfactory bulb (Bozza et al., 2004; Wachowiak and Cohen, 2001). Six 6-s trials of each odorant were presented at a 2–6% dilution of saturated vapor. The minimum intertrial interval was 60 s. The experimenter was blind to the experimental condition of the mouse.
Imaging data were analyzed as described previously (Czarnecki et al., 2011). Briefly, blank trials on which no stimuli were presented were subtracted from each stimulus-present trial to correct for bleaching, a time-dependent reduction in fluorescence independent of stimulus presentation. Stimulus-evoked glomerular responses were measured as the average of 15 frames centered on the peak of the fluorescence increase minus the average of 15 baseline frames immediately prior to stimulus onset. Trials were treated individually for amplitude measurements and averaged within odorants to create spatial maps of odorant-evoked responses. Candidate regions of interest corresponding to olfactory glomeruli were initially selected by hand and then confirmed statistically. A glomerulus was operationally defined as responding to an odorant if its average response across trials of that odorant was at least three SEs greater than zero. The left and right nares are divided by a septum, and the ORNs on each side project exclusively ipsilaterally to the corresponding olfactory bulbs, thus permitting within-subjects comparisons following unilateral intranasal toxicant exposure (Czarnecki et al., 2011). Accordingly, within animal ratios were computed (Cd exposed/vehicle exposed) and averaged across all odorants. Analysis was performed using Neuroplex (RedShirtImaging), and custom software written in MatLab and exported to Excel, SPSS, and SigmaPlot for statistical analysis.
Olfactory bulb histology.
In histological sections, vesicular pH is neutralized, permitting spH fluorescence to serve as a green fluorescent protein (GFP)–based marker for ORN axon terminals (Czarnecki et al., 2011). Five additional animals that did not complete the full odorant battery during optical imaging and were not included in the analysis of olfactory nerve physiology were included in histological analyses. Immediately after imaging, 17 animals were intracardially perfused with 0.1M PBS followed by 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde. Tissue was transferred to PBS at least 24 h before sectioning. Brains were blocked to include both olfactory bulbs and the frontal cortex and sectioned horizontally at 50 μm on a vibratome. Sections were mounted in ProLong Gold antifade agent (Invitrogen) containing 4′,6-diamidino-2-phenylindole (DAPI) on glass slides and sealed under a glass coverslip. DAPI is a membrane permeant fluorescent nuclear stain that enables the visualization of periglomerular cell bodies that delineate the borders between glomeruli.
Photographs of olfactory bulb sections were taken at a resolution of 1360 × 1024 pixels and 14-bit analog-to-digital conversion with a Jenoptik MFcool Peltier-cooled CCD camera mounted on an Olympus BX41 microscope at ×4 (0.16 numerical aperture). Images were collected with both DAPI- and GFP-appropriate filter sets. Images were opened in ImageJ (National Institutes of Health), and the glomerular layer of each olfactory bulb was selected as a region of interest based on the rings of periglomerular interneurons visualized by the DAPI stain. The optical density of these regions of interest was then measured in the corresponding image taken using the GFP-appropriate optical filter. Optical densities were recorded in Excel and exported to SPSS for statistical analysis. Experimenters were blind to the experimental condition of the animal until after the quantification was completed.
Olfactory detection assessment.
Twenty-three C57BL/6 and eight OMP-spH mice were trained in operant conditioning chambers (Coulbourn Apparatus Habitest system) enclosed within sound-attenuating cubicles (Med Associates or Coulbourn Apparatus). Reinforcements were delivered through a reward port where 0.01 ml of 2% sucrose solution was delivered by a liquid dipper when the mouse broke a nose poke photobeam on rewarded trials. Olfactory stimuli were presented through a custom-controlled access odor port consisting of a nose poke operandum with odorant and vacuum ports and a guillotine door on the front to prevent odorant access during the intertrial interval. A house light and ventilation fan were also included. Odorants were presented using custom computer-controlled liquid dilution olfactometers, which passed room air through odorant vials containing a 1:100 dilution of the odorant in mineral oil and then on to the odor port. The rewarded olfactory stimulus was butyl acetate. Actual concentrations in the odor ports were measured and standardized across chambers and days of training using a photoionization detector (HNU DL-101; HNU Systems). Each chamber and floor was washed with 70% ethanol after every session.
Prior to training, mice were water restricted to 90% of initial weight and maintained at this level throughout training to maintain consistent motivation. Training began with conventional magazine training, in which mice received a liquid reward upon poking into the dipper port, as cued by the magazine light. The odor port remained closed throughout magazine training. Mice completed magazine training after 60 successful trials. In the second phase, mice were trained to nose poke into the odor port (in the absence of odorant) when the door opened and then move to the reward port for reinforcement. Over at least four training sessions, mice were shaped to hold the initial nose poke for at least 1 s (based on the break of a photobeam across the odor port) in order to receive a reinforcement. Each session lasted 60 successful trials or 60 min, whichever came first. After the mice achieved 60 successful trials in a single session, they were advanced to the odorant detection training. In this final phase of the training, mice were trained to nose poke in the odor port when the door opened (this poke initiated a trial) and then to poke for reward if and only if they received the odorant butyl acetate. Mice had 5 s to respond (or not) at the reward port on each trial. The intertrial interval after correct responses was 5 s, and a false alarm triggered a “time-out” punishment of 25 s added to the intertrial interval and the conclusion of the trial (only one false alarm was possible per trial). All training was performed daily. Mice received their daily ration of water at the conclusion of training to maintain body weight.
Performance was operationally defined by a discrimination metric (DM), the proportion of trials the mouse correctly entered the reward port in presence of the target odorant (“hits”) minus the proportion of trials the mouse incorrectly entered the reward port in the absence of the odorant (“false alarms”). Potential DM scores range from −1 (no hits, all false alarms) to 1 (all hits, no false alarms), with 0 representing chance responding. DMs were measured in the same odorant detection go/no-go operant procedure as described above. After reaching a DM of at least 0.5 (equivalent to 75% correct responses across trial types) for three consecutive days, animals were randomly assigned to receive a bilateral intranasal instillation of cadmium chloride or vehicle control. Animals then returned to the odorant detection task either 2 (C57BL/6 n = 11; four vehicle, seven cadmium; OMP-spH n = 8, all received cadmium) or 10 days (C57BL/6 n = 12; six vehicle, six cadmium) after infusion.
Statistical analysis.
When applicable, descriptive statistics are reported mean (± SE). ANOVA is performed to test statistically for differences between the means of experimental groups following random assignment of subjects, as described below. Along with F and p values, we also include the ηp2 estimate of effect size (proportion of variance attributable to variable of interest). To compare distributions of glomerular response amplitudes (which may differ along dimensions other than central tendency) between groups, we use the nonparametric Kolmogorov-Smirnov test to test the model that both distributions are samples from the same underlying distribution. All statistical testing was performed using SPSS.
RESULTS
Cadmium Accumulates in the Olfactory Bulb After Intranasal Instillation
We used ICPMS to measure absolute cadmium levels in the olfactory bulb ipsilateral to a unilateral intranasal instillation of pH-neutral 20 μg CdCl2 solution 2, 7, and 28 days following the instillation in 15 randomly assigned male C57BL/6 mice. Cd levels were significantly elevated in the olfactory bulb at all three time points (Fig. 1A), peaking at nearly 800 ng/g at 7 days postinstillation. Analysis of the cerebellum as a control revealed negligible levels of Cd (Fig. 1A). Remarkably, the measured olfactory bulb Cd concentration was nearly the same at the 2- and 28-day time points, and no significant effect of time was observed (one-way ANOVA; F2,12 = 1.5, p = 0.26). This indicates that cadmium persists in the olfactory bulb for at least 28 days after instillation.
FIG. 1.
Acute intranasal instillation of cadmium chloride results in elevated olfactory bulb cadmium levels and reduced neurotransmitter release from the olfactory nerve for at least 28 days after exposure. (A) ICPMS revealed elevated levels of cadmium in the olfactory bulbs at every time point measured. This accumulation was not seen in cerebellum. (B) Ratio of the number of glomeruli receiving synaptic input from ORNs on the cadmium-exposed side compared with the vehicle-exposed side. The dashed line at one represents an equal number of responding glomeruli on each side. (C) Leftmost panel shows a sample baseline fluorescence image outlining the dorsal region of the olfactory bulbs viewable during in vivo imaging through a cranial window. Three following panels are pseudocolored response maps showing the increase in fluorescence in response to an odorant compared with preodor onset baseline. Callouts show the odorant-evoked response amplitude of the selected glomerulus during baseline and odorant presentation (denoted by horizontal black bars) portions of the trial. (D) Cumulative distributions of individual glomerular response amplitudes.
Acute Cadmium Exposure Induces Persistent Disruption of Odorant-Evoked Neurotransmitter Release From the Olfactory Nerve Over 28 Days
To evaluate in vivo pathophysiology following an acute intranasal exposure to cadmium chloride, 12 adult OMP-spH mice were randomly assigned to receive unilateral intranasal instillations of pH-neutral 20 μg CdCl2 solution, with vehicle solution instilled into the contralateral naris. Two, 7, or 28 days later, odorant-evoked neurotransmitter release from the olfactory nerve into olfactory bulb glomeruli was visualized as the increase in spH fluorescence during the presentation of a panel of four odorants (six trials each). SpH signals on the cadmium-exposed and vehicle-exposed sides were compared within each mouse to reveal the effects of intranasal cadmium exposure at each time point.
As shown in Figure 1, cadmium exposure significantly reduced the number of glomeruli receiving odorant-evoked synaptic input relative to contralateral vehicle controls at all time points (Figs. 1B and C), with no significant main effect of time (one-way ANOVA; F2,9 = 1.74, p = 0.23, ηp2 = 0.28). Among the glomeruli that did still receive measurable input following cadmium exposure, the distribution of input amplitudes (Fig. 1D) were significantly smaller on the cadmium-exposed side than on the vehicle-exposed side at all three time points (Kolmogorov-Smirnov tests, p < 0.001 in each case). There was no evidence of recovery of response amplitudes over the 28 days following instillation (Fig. 1D).
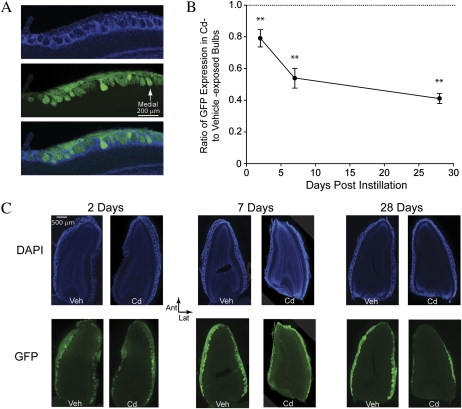
Intranasal Cadmium Exposure Causes Persistent Damage to ORN Projections From the Epithelium to the Olfactory Bulbs
After each imaging experiment, mice were fixed and histological sections through both olfactory bulbs were prepared and counterstained with DAPI. Olfactory bulb glomeruli were identified by their characteristic rings of periglomerular interneurons in the DAPI stain (Fig. 2A, upper), whereas ORN projections from the olfactory nerve were visible innervating each glomerulus from their GFP fluorescence (Fig. 2A, middle). The density of ORN projections to olfactory bulb glomeruli was measured optically and expressed as a ratio of GFP fluorescence on the cadmium-exposed side to that on the vehicle-exposed side. Two days after cadmium exposure, GFP fluorescence (Fig. 2B) was modestly reduced to 80 ± 4% of control on the cadmium-exposed side as previously reported (Czarnecki et al., 2011). However, over the following 4 weeks there was a significant time-dependent reduction in ORN axonal projections to the bulb as shown in Figures 2B and 2C (one-way ANOVA; F2,14 = 23.84, p < 0.001, ηp2 = 0.77), declining to 41 ± 3% of control by 28 days postinfusion. This indicates that significant histopathology occurred in the olfactory nerve following cadmium exposure but also that a considerable portion of the axons survived.
FIG. 2.
Reduction in ORN axonal projections from the olfactory epithelium to the olfactory bulbs following an acute exposure to cadmium chloride. (A) High-magnification (×10) image of control horizontal sections of olfactory bulb tissue. (Upper) DAPI nuclear stain (blue) marks rings of periglomerular interneurons surrounding olfactory bulb glomeruli. (Middle) ORN afferents (green) course over the surface of the olfactory bulb and project to receptor-specific glomeruli. (Lower) merge of DAPI and GFP images. (B) Ratio of GFP optical density on the cadmium-exposed compared with vehicle-exposed bulbs. Dashed line at one represents no difference between bulbs. (C) (Upper) DAPI nuclear stain in representative sections of vehicle- and cadmium-exposed olfactory bulbs at 2, 7, and 28 days after intranasal cadmium exposure. (Lower) GFP glomerular marker in vehicle- and cadmium-exposed olfactory bulbs at 2, 7, and 28 days after intranasal cadmium exposure.
Cadmium-Induced Olfactory Deficits Are Reversed by Odorant Detection Training
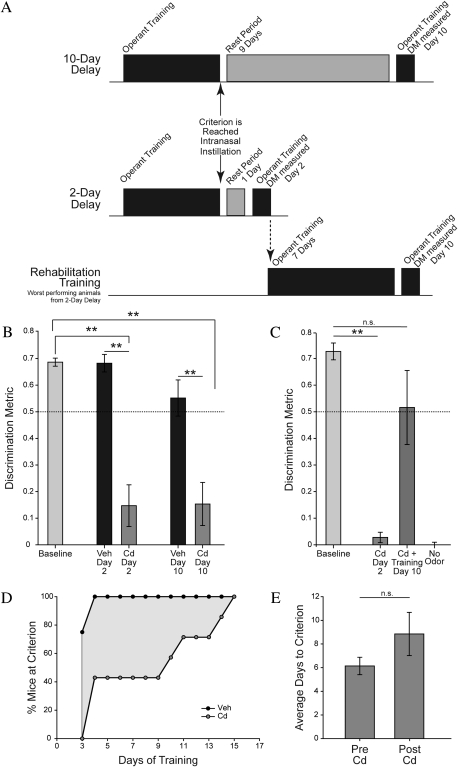
In order to investigate the perceptual impairments corresponding to the severe cadmium-induced pathophysiology, animals were trained to perform an operant go/no-go odorant detection task. After achieving criterion performance of three consecutive days with a DM above 0.5 (equivalent to 75% correct), mice were randomly assigned to receive bilateral instillations of either 20 μg CdCl2 or vehicle solution and to be tested either 2 days or 10 days postinstillation (Fig. 3A).
FIG. 3.
Cadmium-induced deficits in odorant detection performance persist following acute exposure but can be reversed by sensory rehabilitation training. (A) Experimental design schematic. In the 2-day and 10-day delay groups, animals were given one or nine rest days after intranasal instillation, respectively. In the rehabilitation training group, the worst performing mice from the 2-day delay group were given eight additional days of training before their detection metric was again assessed on day 10 after intranasal instillation. (B) Odorant detection ability of cadmium- and vehicle-exposed animals before and either 2 or 10 days after intranasal instillation. (C) Odorant detection metric of worst performing mice from the 2-day delay group from panel B measured before and 2 days following intranasal exposure to cadmium, then after eight additional days of training and without odors presented to ensure animals were not responding to an additional cue. (D) Percentage of animals to reach criterion after intranasal instillation. Criterion is defined as three consecutive days with a DM over 0.5; therefore, day 3 is the first eligible day to reach criterion. (E) Average number of days to reach criterion before and after cadmium exposure.
Cd-exposed C57BL/6 animals were significantly impaired on the odorant detection task at both time points (Fig. 3B). A mixed-model ANOVA (with assessment day as a within-subjects variable and instillation solution and time postinstillation as between subjects variables) revealed a significant assessment day by instillation solution interaction (F1,19 = 27.12, p < 0.001, ηp2 = 0.59), a significant effect of assessment day (F1,19 = 39.42, p < 0.001, ηp2 = 0.68) and a significant effect of instillation solution (F1,19 = 27.45, p < 0.001, ηp2 = 0.59). At both the 2 day and 10-day postinstillation time points, Cd-exposed mice exhibited no ability to detect the odorant, with a DM that was significantly reduced compared with both their own baseline (paired t-test, t(6) = 5.08, p = 0.002 and t(5) = 6.64, p = 0.001, respectively) and vehicle-exposed mice (independent samples t-test, t(9) = 4.06, p = 0.003 and t(10) = 3.77, p = 0.004, respectively) and not significantly different from zero, indicating chance performance (one-sample t-test, t(6) = 1.88, p = 0.11 and t(5) = 1.89, p = 0.12, respectively). The pattern of errors in Cd-exposed mice indicated a large increase in the false alarm rate (i.e., guessing), which is the optimum strategy to maximize reward for an anosmic subject in this paradigm. Vehicle-exposed mice exhibited no change in discrimination performance relative to their own baseline at either time point (paired t-test, t(3) = −0.02, p = 0.99 for 2 day group and t(5) = 1.77, p = 0.14 for 10 day group).
To assess the effect of rehabilitation training on olfactory abilities, cadmium-exposed mice from the day 2 test group received daily odorant detection training until they again achieved criterion performance of DM > 0.5 for three consecutive sessions or up to 15 days. Despite their initial inability to discriminate between odorant-present and odorant-absent trials, cadmium-exposed mice continued to initiate trials and to receive rewards when they guessed correctly. As shown in Figure 3D, after 3 days of postinstillation training, 75% of vehicle-exposed animals had reached criterion, whereas 0% of cadmium-exposed animals had reached criterion. By day 4, 100% of vehicle-exposed animals and only 43% of cadmium-exposed animals reached criterion. By 15 days of postinstillation training, 100% of cadmium-exposed animals had been rehabilitated to criterion. The average number of days for cadmium-exposed animals to reach criterion after infusion was not significantly different than the number of days to reach criterion when first beginning the task (Fig. 3E, paired samples t-test, t(6) = −1.21, p = 0.27), consistent with the idea that rehabilitated mice may be relearning the task using whatever sensory inputs survived cadmium exposure.
To decouple the effects of the passage of time from the effects of rehabilitation training, the performance of retrained mice was also assessed on day 10 postinstillation (Fig. 3A). To control for the size of the initial performance deficit, only the mice that exhibited no hint of discrimination between the odorant-present and odorant-absent conditions on day 2 were included (DM < 0.1, n = 5). As shown in Figure 3C, on day 10 following cadmium exposure, mice that underwent rehabilitation training exhibited significantly increased detection performance compared with their own performance 2 days after instillation (paired samples t-test, t(4) = −3.37, p = 0.03). Detection performance after rehabilitation did not significantly differ from baseline performance (paired samples t-test, t(4) = 1.61, p = 0.18), although cadmium-exposed mice who received no additional training after intranasal infusion until day 10 remained just as impaired as animals that were tested on day 2 (Fig. 3B). To confirm that these rehabilitated mice were indeed using their olfactory systems to perform the task, they were run for one additional session that was identical to the others except that no odorants were placed in the olfactometer. Performance on this control task declined to zero (Fig. 3C).
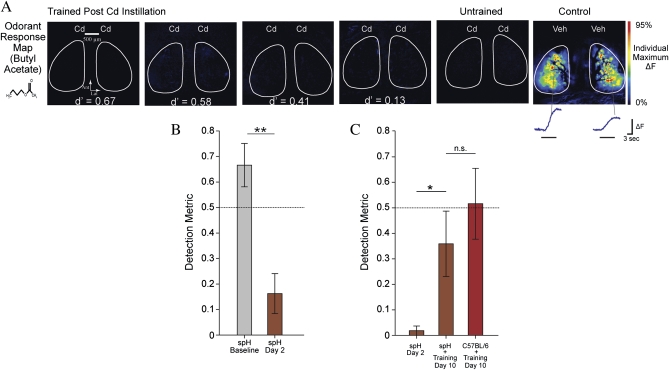
Odorant Detection Ability Recovers Without Increased Neurotransmitter Release From the Olfactory Nerve
The cadmium-induced pathophysiology of the olfactory nerve does not spontaneously recover during the 2 weeks after exposure (Fig. 1B) and ORN innervation of the olfactory bulb declines (Fig. 2B), yet all mice could be fully rehabilitated on the olfactory detection task during this time (Fig. 3D). This raised the question of whether the improved sensory performance of mice that received rehabilitation training reflected sensory learning or an actual reversal of the neural damage. To investigate this possibility, eight OMP-spH mice were trained to perform the olfactory detection task as described above. After reaching criterion, they received bilateral instillations of 200 μg cadmium chloride and their performance on the detection task was assessed on day 2 after the instillation. Similar to wild-type mice, OMP-spH mice exhibited a detection performance that was greatly reduced (Fig. 4B) relative to their baseline performance (paired samples t-test, t(7) = 6.59, p = 0.001) and not significantly different from zero (one-group t-test, t(7) = 2.07, p = 0.08). To standardize the size of the initial sensory deficit, the worst performing mice (DM < 0.1, n = 5) were selected to undergo eight additional training sessions, during which time their average detection performance significantly improved from a prerehabilitation baseline of 0.02 ± 0.02 to 0.36 ± 0.13 (paired samples t-test, t(4) = −2.89, p = 0.045). As shown in Figure 4C, this improvement was comparable with the postrehabilitation DM exhibited by a subset of the worst performing wild-type mice at the same time point (independent samples t-test, t(8) = 0.83, p = 0.43). As with the wild-type mice (Fig. 3D), after 8 days of training some of the cadmium-exposed OMP-spH mice were performing well on the detection task, whereas others were not. All mice underwent optical imaging of olfactory nerve function as described above. Surprisingly, the patterns of odorant-evoked neurotransmitter release observed in these mice were similar to those observed at this time point in untrained mice (Figs. 1 and 4A), including a limited number of glomeruli receiving odorant-evoked synaptic input from the olfactory nerve and greatly reduced amplitudes. There was no evidence that rehabilitation training improved the function of the olfactory nerve.
FIG. 4.
Olfactory detection performance was restored without recovery of olfactory nerve function. (A) Pseudocolored maps of glomeruli receiving odorant-evoked neurotransmitter release from the olfactory nerve in response to a butyl acetate presentation. The leftmost four panels are from animals measured on day 10 after bilateral intranasal instillation of cadmium and run continually after postinstillation rest day. Detection metrics from each mouse's final training session appear inset in the pseudocolored maps. The map of a cadmium exposed but behaviorally untrained animal appears second from the right as a control. A map of the response to butyl acetate in a mouse that received bilateral instillation of vehicle solution appears in the last column for comparison. (B) Detection metric of OMP-spH mice before and 2 days after cadmium exposure. (C) Detection metric of worst performing OMP-spH mice 2 days after intranasal instillation and C57BL/6 and spH mice on day 10 after rehabilitation training.
DISCUSSION
These experiments demonstrated that cadmium accumulates in the olfactory bulb after intranasal exposure and persists for at least 4 weeks. Throughout this time, odorant-evoked neurotransmitter release from ORN synaptic terminals into the olfactory bulb remains severely disrupted (< 20% of control levels), and the density of olfactory nerve projections into the olfactory bulb gradually declines until leveling off at about 40% of control levels. Two days after cadmium infusion, performance on a behavioral olfactory detection task was not significantly different from chance. However, after 8 days of explicit odorant detection training, cadmium-exposed mice performed no differently from vehicle-exposed controls (whereas untrained cadmium-exposed animals continued to perform at chance, showing no evidence of spontaneous recovery of function). These cadmium-exposed rehabilitated mice were confirmed to be using their olfactory systems to perform the task because their performance returned to chance in a control test session without odorant. Physiological imaging of odorant-evoked neurotransmitter release from the ORNs of cadmium-exposed mice at various levels of rehabilitation showed no evidence that training improved ORN function. We conclude that this recovery of olfactory ability must be the result of learning in a brain structure downstream from the ORNs. These findings demonstrate that learning can mask even severe pathological effects of neurotoxicants and, conversely, that basic olfactory function can be rehabilitated despite persistent damage.
Our mass spectrometry data indicate that cadmium accumulates in the olfactory bulb following acute intranasal instillation, consistent with previous reports in rodent models (Bondier et al., 2008; Evans and Hastings, 1992; Hastings and Evans, 1991; Sun et al., 1996; Tjälve et al., 1996). Surprisingly, we found that cadmium levels remain elevated for at least 28 days following a single acute instillation, perhaps because of the high concentration of metallothioneins and other metal-binding proteins in ORNs (Himeno et al., 2009; Sunderman, 2001). As a divalent cation, cadmium can disrupt neuronal calcium signaling, including blockade of transmembrane calcium channels (Chow, 1991; Wang et al., 2008). We have previously shown that neurotransmitter release from the ORN presynaptic terminals in the olfactory nerve requires the conduction of calcium ions through N-type calcium channels and is a power-law function of extracellular calcium concentration (Wachowiak et al., 2005). This suggests if cadmium causes even a modest reduction in presynaptic calcium influx it would greatly impact the total transmitter release from the nerve. The harmful action of cadmium in the olfactory system may thus be a combination of persistent pharmacological effects and outright damage to the olfactory nerve (see Figs. 1 and 2).
In these experiments, we observed rehabilitation of olfactory detection abilities despite a reduction of ORN projections to the olfactory bulb and sustained, severe pathophysiology of the olfactory nerve in those very animals (Fig. 4). In principle, even just a single intact ORN could be sufficient to detect an odorant, which is likely why rehabilitation is possible at all in this circumstance. However, in practice, the population of surviving functional ORNs may not initially be sufficient to activate downstream brain regions without adaptive plasticity to “recalibrate” the olfactory system to its new, weakened input. It is not clear where in the brain this plasticity occurs, but it may well be downstream of the olfactory bulbs because rodents can learn to perform odorant detection and discrimination tasks even after large bulbar lesions (Lu and Slotnick, 1998; Slotnick et al., 2004).
The present findings help to explicate inconsistent previous reports of olfactory deficits after cadmium exposure. In the study by Sun et al. (1996) that found no olfactory impairment even after 20 weeks of cadmium oxide exposure, the subjects were constantly being trained on odorant-guided tasks throughout the exposure period. The data presented here demonstrate that one training session a day for 8 days can be sufficient to rehabilitate odorant detection performance all the way from chance to baseline levels, suggesting that the ongoing training in that study could have prevented the authors from detecting cadmium's pathophysiological effects. Similarly, Bondier et al. (2008) reported that after an acute intranasal instillation of cadmium, mice no longer spontaneously avoided the unpleasant odorant butanol but returned to normal avoidance behavior by 18 days postexposure. In light of the present findings, it is likely that the olfactory systems of those mice had not returned to “normal,” but rather had undergone some form of compensatory plasticity to adapt to ongoing peripheral damage.
Although the masking of peripheral pathology by sensory learning is a confounding problem for behavioral assessment and toxicant screening, it would be a desirable consequence of rehabilitation therapy after insult to the olfactory system. The clinical loss of olfactory function is widespread and can have many causes, both peripheral and central, including upper respiratory tract infections, physical trauma, toxicant exposure, aging, and neurodegenerative diseases such as Parkinson's diseases and Alzheimer's disease (Haehner et al., 2009; Kern et al., 2000; Mascagni et al., 2003; Mesholam et al., 1998; Rose et al., 1992; Schubert et al., 2009). Interestingly, olfactory impairments sometimes seem to improve spontaneously, even in cases of Parkinson's disease where the underlying neuropathology is likely progressing (Herting et al., 2008). Moreover, exposure-based rehabilitation programs, where dysosmic patients are exposed to labeled odorants twice daily, have been shown to increase olfactory function in individuals with olfactory loss (Hummel et al., 2009). Without epithelial or bulbar histology from patients, it is unknown whether this rehabilitation therapy is facilitating peripheral recovery or triggering central plasticity, but the data presented here demonstrate that functional recovery can occur despite continued peripheral pathology.
In a previous paper, we illustrated the experimental power of our in vivo physiological imaging technique, showing that it could detect pathophysiological effects of cadmium instillation at 1/100th of the minimum dose required to detect histological changes in the same animals (Czarnecki et al., 2011). The present data from rehabilitated mice similarly reinforce the value of using a physiological assay such as imaging, which showed a massive loss of neural function, in conjunction with behavioral assays that seemed to show no effect of cadmium in those same animals. Outside the laboratory, some toxicant exposures may be extended in time while being accompanied by constant sensory use (complete with feedback on errors). Our data suggest that under these circumstances simple behavioral assays can miss real toxicant-induced damage to sensory systems. Consequently, sensory testing should be supplemented by physiological measures when possible. In the human olfactory system, this means that if sensory learning could be masking underlying neural deficits, then behavioral measures of sensory function like the University of Pennsylvania Smell Identification Test (Sensonics, Inc., Haddon Heights, NJ), and Sniffin' Sticks (Burghart Instruments, Wedel, Germany) tasks could be profitably followed up by physiological measures like intranasal electro-olfactograms, olfactory event–related potentials, and functional magnetic resonance imaging (Lötsch and Hummel, 2006; Scott and Scott-Johnson, 2002; Zald and Pardo, 2000).
FUNDING
National Institute on Deafness and Other Communication Disorders (R00 DC009442 to J.P.M.); National Institute of Environmental Health Sciences at the National Institutes of Health (P30 ES005022 and T-32 ES07148); Busch Biomedical Research Program to J.P.M.
Acknowledgments
We thank Ken Reuhl and Jason Richardson for their advice throughout this project.
References
- Adams RG, Crabtree N. Anosmia in alkaline battery workers. Br. J. Ind. Med. 1961;18:216–221. doi: 10.1136/oem.18.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances & Disease Registry (ATSDR) Toxicological Profile for Cadmium. 2008. Division of Toxicology and Environmental Medicine, Atlanta, GA. Chemical Abstract Service Registry No. 7440-43-9. [Google Scholar]
- Bondier JR, Michel G, Propper A. Harmful effects of cadmium on olfactory system in mice. Inhal. Toxicol. 2008;20:1169–1177. doi: 10.1080/08958370802207292. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Bruehlmeier J, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur. J. Neurosci. 1998;10:3918–3922. doi: 10.1046/j.1460-9568.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Chen Z-L, Yu W-M, Strickland S. Peripheral regeneration. Annu. Rev. Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Chow RH. Cadmium block of squid calcium currents. Macroscopic data and a kinetic model. J. Gen. Physiol. 1991;98:751–770. doi: 10.1085/jgp.98.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki LA, Moberly AH, Rubinstein T, Turkel DJ, Pottackal J, McGann JP. In vivo visualization of olfactory pathophysiology induced by intranasal cadmium instillation in mice. Neurotoxicology. 2011;32:441–449. doi: 10.1016/j.neuro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJK, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Evans J, Hastings L. Accumulation of Cd(II) in the CNS depending on the route of administration: Intraperitoneal, intratracheal, or intranasal. Fundam. Appl. Toxicol. 1992;19:275–278. doi: 10.1016/0272-0590(92)90161-a. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, DeHan RS. Neuronal regeneration in frog olfactory system. J. Cell Biol. 1973;59:525–530. doi: 10.1083/jcb.59.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, Silburn PA, Johnston AN, Mellick GD, Herting B, Reichmann H, et al. Prevalence of smell loss in Parkinson's disease—A multicenter study. Parkinsonism Relat. Disord. 2009;15:490–494. doi: 10.1016/j.parkreldis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Hastings L. Sensory neurotoxicology: Use of the olfactory system in the assessment of toxicity. Neurotoxicol. Teratol. 1990;12:455–459. doi: 10.1016/0892-0362(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Hastings L, Evans JE. Olfactory primary neurons as a route of entry for toxic agents into the CNS. Neurotoxicology. 1991;12:707–714. [PubMed] [Google Scholar]
- Herting B, Schulze S, Reichmann H, Haehner A, Hummel T. A longitudinal study of olfactory function in patients with idiopathic Parkinson's disease. J. Neurol. 2008;255:367–370. doi: 10.1007/s00415-008-0665-5. [DOI] [PubMed] [Google Scholar]
- Himeno S, Yanagiya T, Fujishiro H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie. 2009;91:1218–1222. doi: 10.1016/j.biochi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Hummel T, Rissom K, Reden J, Hähner, Weidenbecher M, Hüttenbrink K-B. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119:496–499. doi: 10.1002/lary.20101. [DOI] [PubMed] [Google Scholar]
- Huntley GW. Correlation between patterns of horizontal connectivity and the extent of short-term representational plasticity in rat motor cortex. Cereb. Cortex. 1997;7:143–156. doi: 10.1093/cercor/7.2.143. [DOI] [PubMed] [Google Scholar]
- Kern RC, Quinn B, Rosseau G, Farbman AI. Post-traumatic olfactory dysfunction. Laryngoscope. 2000;110:2106–2109. doi: 10.1097/00005537-200012000-00025. [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Hummel T. The clinical significance of electrophysiological measures of olfactory function. Behav. Brain Res. 2006;170:78–83. doi: 10.1016/j.bbr.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Lu X-CM, Slotnick BM. Olfaction in rats with extensive lesions of the olfactory bulbs: Implications for odor coding. Neuroscience. 1998;84:849–866. doi: 10.1016/s0306-4522(97)00520-4. [DOI] [PubMed] [Google Scholar]
- Mascagni P, Consonni D, Bregante G, Chiappino G, Toffoletto F. Olfactory function in workers exposed to moderate airborne cadmium levels. Neurotoxicology. 2003;24:717–724. doi: 10.1016/S0161-813X(03)00024-X. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by feedback but not lateral presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfactory in neurodegenerative disease: A meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch. Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Miesenböck G, DeAngelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Moser VC. Functional assays for neurotoxicity testing. Toxicol. Pathol. 2011;39:36–45. doi: 10.1177/0192623310385255. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Lucchini R, Anger WK, Bellinger DC, van Thriel C. Neurobehavioral testing in human risk assessment. Neurotoxicology. 2008;29:556–567. doi: 10.1016/j.neuro.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CS, Heywood PG, Costanzo RM. Olfactory impairment after chronic occupational cadmium exposure. J. Occup. Med. 1992;34:600–605. [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp. Brain Res. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Murphy C, Huang G-H, Klein BEK, Klein R, Nieto FJ, Pankow JS, Tweed TS. Olfactory impairments in adults: The Beaver Dam experience. Ann. N. Y. Acad. Sci. 2009;1170:531–536. doi: 10.1111/j.1749-6632.2009.04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J. Comp. Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Scott JW, Scott-Johnson PE. The electroolfactogram: A review of its history and uses. Microsc. Res. Tech. 2002;58:152–160. doi: 10.1002/jemt.10133. [DOI] [PubMed] [Google Scholar]
- Slotnick B, Cockerham R, Pickett E. Olfaction in olfactory bulbectomized rats. J. Neurosci. 2004;24:9195–9200. doi: 10.1523/JNEUROSCI.1936-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sułkowski W, Rydzewski B, Miarzyńska M. Smell impairment in workers occupationally exposed to cadmium. Acta Otolaryngol. 2000;120:316–318. doi: 10.1080/000164800750001161. [DOI] [PubMed] [Google Scholar]
- Sun TJ, Miller ML, Hastings L. Effects of inhalation of cadmium on the rat olfactory system: Behavior and morphology. Neurotoxicol. Teratol. 1996;18:89–98. doi: 10.1016/0892-0362(95)02013-6. [DOI] [PubMed] [Google Scholar]
- Sunderman FW. Nasal toxicity, carcinogenicity and olfactory uptake of metals. Ann. Clin. Lab. Sci. 2001;31:3–24. [PubMed] [Google Scholar]
- Tjälve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol. Toxicol. 1996;79:347–356. doi: 10.1111/j.1600-0773.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC, Shipley MT. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J. Neurophysiol. 2005;94:2700–2712. doi: 10.1152/jn.00286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Shih YL, Ko WC, Wei YH, Shih CM. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell. Mol. Life Sci. 2008;65:3640–3652. doi: 10.1007/s00018-008-8383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int. J. Psychophysiol. 2000;36:165–181. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]