Abstract
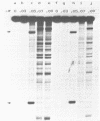
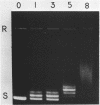
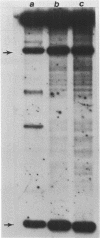
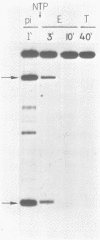
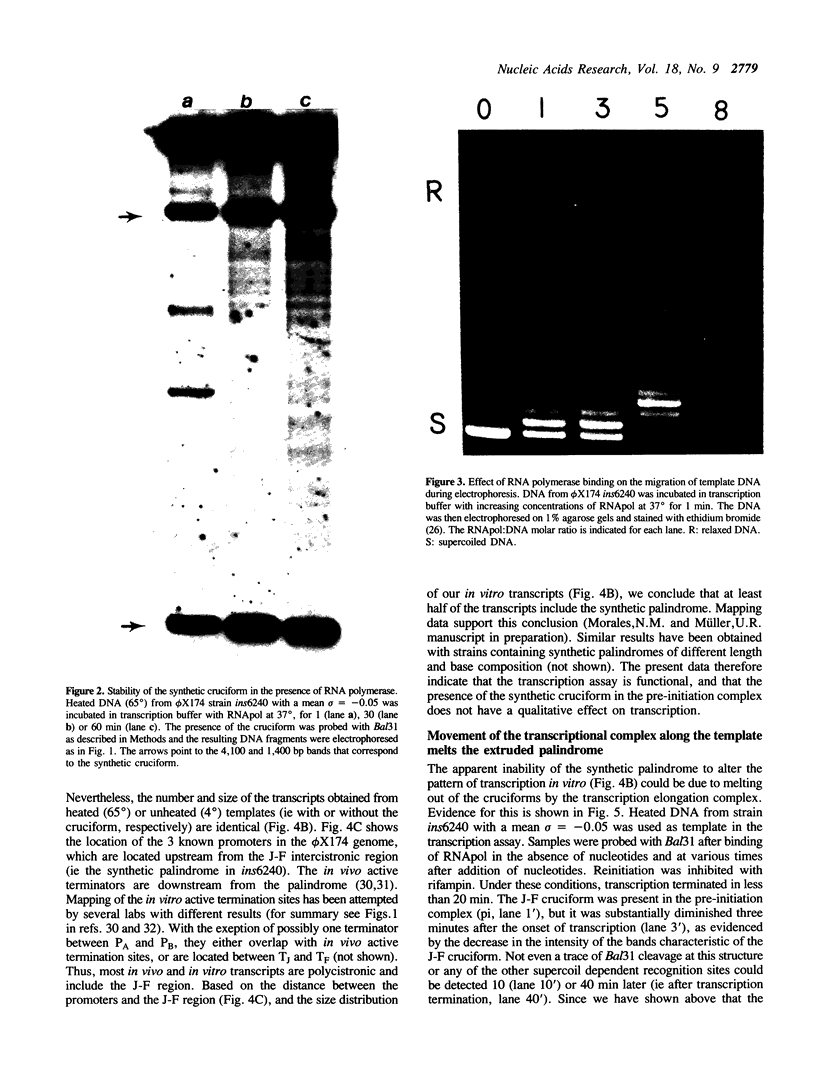
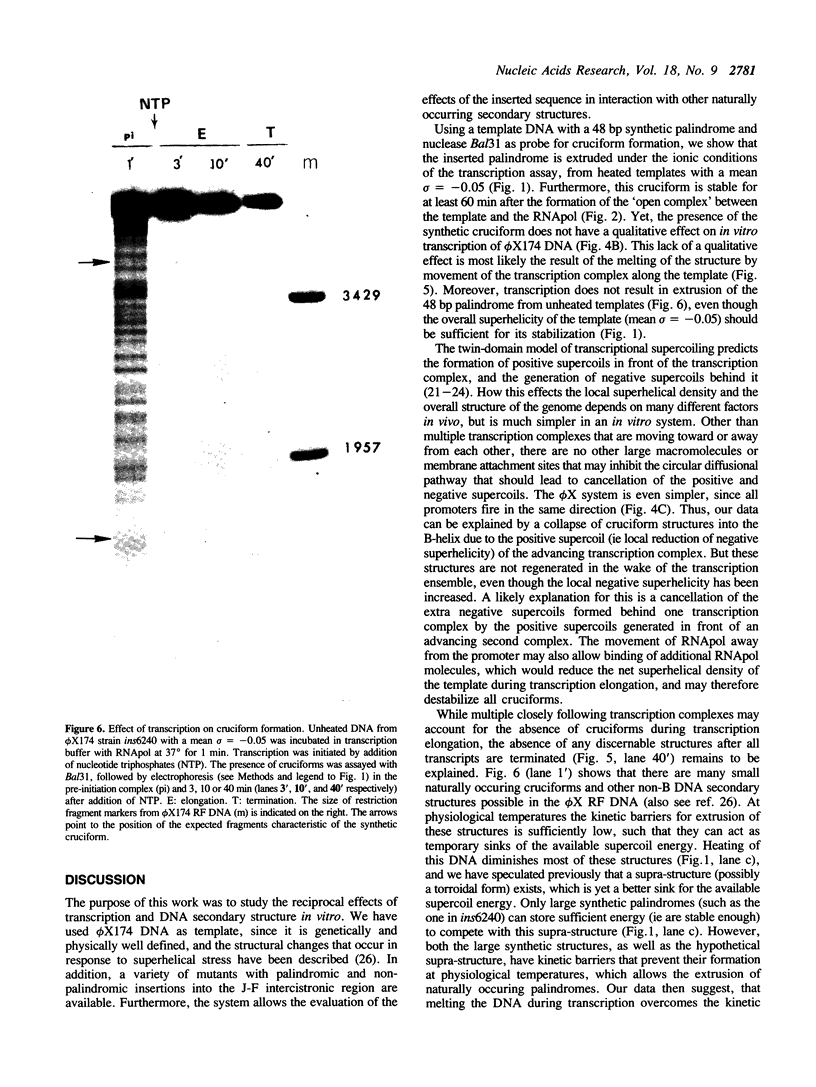
We have investigated the effect of in vitro transcription on cruciform stability. Replicative form DNA of phiX174 strain ins6240, containing a 48 bp synthetic palindrome in the J-F intercistronic region, was supercoiled in vitro to mean negative superhelical densities (sigma) ranging from 0 to 0.15. The presence of cruciforms was probed by limited digestion with the single-strand specific nuclease Bal31. The 48 bp palindrome was extruded at a mean sigma = -0.05, but only after heating the DNA. An in vitro transcription reaction with E. coli RNA polymerase and [alpha-32P]UTP gave identical transcripts with heated or unheated template DNA. The synthetic cruciform was stable upon binding of the RNA polymerase to the template, but it was destabilized upon movement of the transcription complex along the template. Transcription of unheated templates did not result in cruciform formation. We propose that cruciform structures in supercoiled template DNAs present no hindrance to RNA polymerase, and thus have no detectable effect on transcription elongation in vitro.
Full text
PDF

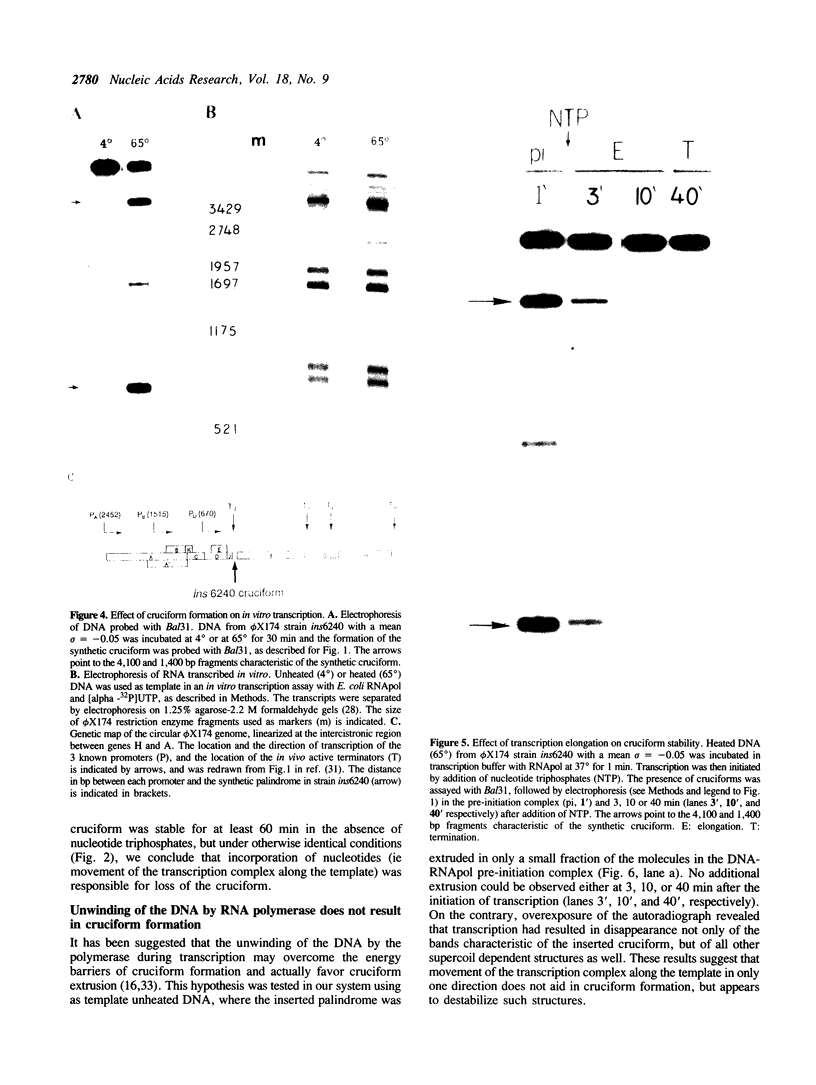

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M., Hutchison C. A., Fabricant J. D., Sinsheimer R. L. Genetic Map of Bacteriophage phiX174. J Virol. 1971 May;7(5):549–558. doi: 10.1128/jvi.7.5.549-558.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms J. G., Dargouge O., Brahms S., Ohara Y., Vagner V. Activation and inhibition of transcription by supercoiling. J Mol Biol. 1985 Feb 20;181(4):455–465. doi: 10.1016/0022-2836(85)90419-x. [DOI] [PubMed] [Google Scholar]
- Brendel V. Mapping of transcription terminators of bacteriophages phi X174 and G4 by sequence analysis. J Virol. 1985 Jan;53(1):340–342. doi: 10.1128/jvi.53.1.340-342.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Kamenetskii M. Gene transcription. Waves of DNA supercoiling. Nature. 1989 Jan 19;337(6204):206–206. doi: 10.1038/337206a0. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M., Imai M. Bacteriophage phi X174-specific mRNA synthesis in cells deficient in termination factor rho activity. J Virol. 1981 Apr;38(1):198–207. doi: 10.1128/jvi.38.1.198-207.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M., Müller U. R. Role for the J-F intercistronic region of bacteriophages phi X174 and G4 in stability of mRNA. J Virol. 1983 Oct;48(1):186–196. doi: 10.1128/jvi.48.1.186-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. An E. coli promoter that regulates transcription by DNA superhelix-induced cruciform extrusion. Science. 1988 Aug 5;241(4866):703–705. doi: 10.1126/science.2456617. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Transcription regulation in vitro by an E. coli promoter containing a DNA cruciform in the '-35' region. Nucleic Acids Res. 1989 Jul 25;17(14):5537–5545. doi: 10.1093/nar/17.14.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A. Structures of DNA: a summary. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1215–1223. doi: 10.1101/sqb.1983.047.01.137. [DOI] [PubMed] [Google Scholar]
- Lau P. P., Gray H. B., Jr Extracellular nucleases of Alteromonas espejiana BAL 31.IV. The single strand-specific deoxyriboendonuclease activity as a probe for regions of altered secondary structure in negatively and positively supercoiled closed circular DNA. Nucleic Acids Res. 1979 Jan;6(1):331–357. doi: 10.1093/nar/6.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace H. A., Pelham H. R., Travers A. A. Association of an S1 nuclease-sensitive structure with short direct repeats 5' of Drosophila heat shock genes. Nature. 1983 Aug 11;304(5926):555–557. doi: 10.1038/304555a0. [DOI] [PubMed] [Google Scholar]
- McKeon C., Schmidt A., de Crombrugghe B. A sequence conserved in both the chicken and mouse alpha 2(I) collagen promoter contains sites sensitive to S1 nuclease. J Biol Chem. 1984 May 25;259(10):6636–6640. [PubMed] [Google Scholar]
- Mizuuchi K., Mizuuchi M., Gellert M. Cruciform structures in palindromic DNA are favored by DNA supercoiling. J Mol Biol. 1982 Apr 5;156(2):229–243. doi: 10.1016/0022-2836(82)90325-4. [DOI] [PubMed] [Google Scholar]
- Müller U. R., Wilson C. L. The effect of supercoil and temperature on the recognition of palindromic and non-palindromic regions in phi X174 replicative form DNA by S1 and Bal31. J Biol Chem. 1987 Mar 15;262(8):3730–3738. [PubMed] [Google Scholar]
- Panayotatos N., Fontaine A. A native cruciform DNA structure probed in bacteria by recombinant T7 endonuclease. J Biol Chem. 1987 Aug 15;262(23):11364–11368. [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Transcriptional block caused by a negative supercoiling induced structural change in an alternating CG sequence. Cell. 1985 Jan;40(1):129–137. doi: 10.1016/0092-8674(85)90316-2. [DOI] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. DNA supercoiling and prokaryotic transcription. Cell. 1989 Feb 24;56(4):521–523. doi: 10.1016/0092-8674(89)90574-6. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. Topoisomerase I mutants: the gene on pBR322 that encodes resistance to tetracycline affects plasmid DNA supercoiling. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8952–8956. doi: 10.1073/pnas.83.23.8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Smith G. R. DNA supercoiling: another level for regulating gene expression. Cell. 1981 Jun;24(3):599–600. doi: 10.1016/0092-8674(81)90085-4. [DOI] [PubMed] [Google Scholar]
- Tsao Y. P., Wu H. Y., Liu L. F. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989 Jan 13;56(1):111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- Williams W. L., Müller U. R. Effects of palindrome size and sequence on genetic stability in the bacteriophage phi X174 genome. J Mol Biol. 1987 Aug 20;196(4):743–755. doi: 10.1016/0022-2836(87)90401-3. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]