Abstract
The FA translocase cluster of differentiation 36 (CD36) facilitates FA uptake by the myocardium, and its surface recruitment in cardiomyocytes is induced by insulin, AMP-dependent protein kinase (AMPK), or contraction. Dysfunction of CD36 trafficking contributes to disordered cardiac FA utilization and promotes progression to disease. The Akt substrate 160 (AS160) Rab GTPase-activating protein (GAP) is a key regulator of vesicular trafficking, and its activity is modulated via phosphorylation. Our study documents that AS160 mediates insulin or AMPK-stimulated surface translocation of CD36 in cardiomyocytes. Knock-down of AS160 redistributes CD36 to the surface and abrogates its translocation by insulin or the AMPK agonist 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR). Conversely, overexpression of a phosphorylation-deficient AS160 mutant (AS160 4P) suppresses the stimulated membrane recruitment of CD36. The AS160 substrate Rab8a GTPase is shown via overexpression and knock-down studies to be specifically involved in insulin/AICAR-induced CD36 membrane recruitment. Our findings directly demonstrate AS160 regulation of CD36 trafficking. In myocytes, the AS160 pathway also mediates the effect of insulin, AMPK, or contraction on surface recruitment of the glucose transporter GLUT4. Thus, AS160 constitutes a point of convergence for coordinating physiological regulation of CD36 and GLUT4 membrane recruitment.
Keywords: fatty acid metabolism, diabetic heart disease, scavenger receptors
FAs serve an important role as an energy fuel for the myocardium, and their uptake by cardiomyocytes is regulated by multiple factors, including insulin and contraction (1, 2). Several proteins have been proposed to facilitate FA uptake by the myocardium, and these include the scavenger receptor cluster of differentiation 36 (CD36), the FA transport proteins, caveolin1, and plasma membrane-associated FA binding protein (as reviewed in Ref. 1). In addition to these proteins, acyl-CoA ligases that activate FA by generating the FA acyl-CoA, couple FA entry to metabolism and help maintain the gradient for FA influx across the plasma membrane (1, 3)
CD36 is abundantly expressed in the heart and facilitates a significant part of myocardial FA uptake measured in vivo in humans and rodents (4, 5). Unlike other proteins implicated in FA uptake, CD36 cycles between intracellular stores and the plasma membrane, similar to the glucose transporter GLUT4. CD36 trafficking is regulated by a number of factors including insulin, muscle contraction (1, 2), purinergic agonists such as uridine-5′-triphosphate (UTP) (6), and FAs (7–9). It is believed that the effect of muscle contraction on CD36 surface translocation is mediated via activation of AMP-dependent protein kinase (AMPK) (10), although this notion was recently challenged in skeletal muscle (11).
Insulin and contraction increase CD36 surface expression on cardiomyocytes to enhance myocardial extraction of circulating FA. The internalized FA is either directly oxidized in mitochondria (AMPK and contraction) or partitioned first into triglycerides (insulin) that can later be hydrolyzed to provide FA for oxidation (as reviewed in Ref. 2). The regulated trafficking of CD36 is essential for maintaining the appropriate balance between FA uptake and utilization by the cardiomyocyte, as evidenced from findings under conditions in which it is dysregulated. For example, persistent relocation of CD36 from intracellular stores to the sarcolemma is observed in the diabetic myocardium and is proposed to result in accumulation of lipid moieties with negative effects on heart metabolism and function (1, 5, 12–14). The permanent relocation of CD36 to the plasma membrane is an early event in insulin resistance that precedes and strongly correlates with intracellular retention of the insulin-sensitive glucose transporter GLUT4 (2). This highlights the importance of a better understanding of the events that regulate surface CD36 trafficking, an area that remains little studied.
In the heart, CD36 and GLUT4 are proposed to reside in separate endosomal subcompartments and to be recruited via transporter-specific signals (15). However, as reviewed recently (2), translocation of GLUT4 and CD36 in muscle is responsive to the same signaling pathways that are important to muscle physiology, namely those involving insulin and contraction. The cellular machinery involved in GLUT4 trafficking has been well studied. In contrast, relatively little is known related to the machinery controlling the cycling of CD36.
The small GTP-binding proteins, Rab GTPases, control many crucial steps of intracellular traffic, including formation, movement, and membrane fusion of transport vesicles. More than 60 Rab GTPases have been identified in humans to date. They interact with specific effectors to control vesicle traffic and to define the identity of trafficking organelles (16). Rabs are activated [switched from guanosine diphosphate (GDP) to guanosine-5’-triphosphate (GTP) binding] by the guanine exchange factors (GEFs) and are inactivated by the GTPase-activating proteins (GAPs). Prominent among GAPs is the master regulator Rab GAP TBC1D4 or Akt substrate 160 kDa (AS160) that can be phosphorylated following insulin or AMPK signaling.
AS160 controls the activity of several Rabs, mediating the effect of insulin and AMPK on GLUT4 membrane translocation (17–22). Insulin/AMPK-induced phosphorylation reduces AS160 GAP inhibition of its target Rab GTPases. Subsequent activation of the Rabs allows translocation of GLUT4 vesicles to the surface.
Rab8a, Rab10, and Rab14 are among the AS160-targeted Rab GTPases that regulate GLUT4 cycling (23, 24) in a cell-specific manner. Rab8a and Rab14 were shown to function in GLUT4 recycling in L6 myotubes (25), whereas Rab10 was implicated in 3T3-L1 adipocytes (26, 27).
The potential involvement of AS160 in CD36 translocation by either insulin or AMPK remains unexplored. Such information is critical for understanding the molecular pathways that control stimulated CD36 recruitment to the cell surface. In this study, we examined whether AS160 mediates the effects of insulin or AMPK in regulation of CD36 vesicular trafficking in cardiomyocytes. Our findings implicate AS160 and its substrate, GTPase Rab8a, in CD36 recruitment and suggest that AS160 may function as a hub that integrates regulation of CD36 and GLUT4 trafficking in cardiomyocytes.
MATERIALS AND METHODS
Antibodies and chemicals
Antibodies were obtained from the following sources: AS160 and Rab10 (Cell Signaling Technology); Rab8 (BD Biosciences); CD36 (R and D Systems); Rab14, green fluorescent protein (GFP), and tubulin (Sigma-Aldrich, Saint Louis, MO). All other reagents were from Sigma-Aldrich.
Cell culture, starvation, and stimulation
HL-1 cardiomyocytes were provided by Dr. W. Claycomb (Louisiana State University, New Orleans, LA) and cultured in Claycomb medium (supplemented with 10% FCS, 0.1 mmol/l norepinephrine, 2 mmol/l l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) at 37°C and 5% CO2. Cells were starved for 16–18 h in depletion medium (DMEM low glucose, 2 mM glutamine, 100 μmol/l MEM nonessential amino acids, 2 mmol/l l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) and incubated for 20 min with or without 100 nM of insulin or 2 mM 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR).
Immunofluorescent microscopy
Cells grown on coverslips were fixed with ice-cold methanol (−20°C) for 20 min and blocked at room temperature for 1 h (PBS, 0.05% Tween 20, 1% BSA, 5% goat serum). Overnight incubation with primary antibodies was followed by 1 h incubation with Alexa Fluor 488- and/or 594-conjugated secondary antibodies, and the coverslips were mounted with fluorescent mounting medium (DakoCytomation; Carpinteria, CA). Confocal microscopy was performed using a 63× oil objective on a Zeiss LSM 510 laser-scanning confocal microscope, and images were processed using ImageJ software.
Constructs and DNA transfection
The CMV-10 plasmid encoding human AS160 with four Akt phosphorylation sites (Ser318, Ser588, Thr642, and Ser751) mutated to alanine (AS160 4P), and a triple FLAG-tag at the N terminus was provided by Dr. G. E. Lienhard (Dartmouth Medical School; Hanover, NH).
GFP-tagged wild-type (Wt) Rab8a (GFP-Rab8a Wt), constitutively active (CA) Rab8a [Q67L] (GFP-Rab8a CA), and dominant-negative (DN) Rab8a [T22N] (GFP-Rab8a DN) were provided by Dr. I. Mellman (Yale University School of Medicine; New Haven, CT). GFP-tagged Wt Rab10 (GFP-Rab10 Wt), CA Rab10 [Q68L] (GFP-Rab10 CA), DN Rab10 [T23N] (GFP-Rab10 DN), Wt Rab14 (GFP-Rab14 Wt), CA Rab14 [Q70L] (GFP-Rab14 CA), and DN Rab14 [S25N] (GFP-Rab14 DN) were provided by Dr. J. Brumell (The Hospital for Sick Children; Toronto, Ontario, Canada).
Confluent monolayers were transfected in antibiotics and norepinephrine-free Claycomb medium, using Lipofectamine 2000 (Invitrogen). Six hours later, the medium was changed to fully supplemented Claycomb medium. All assays were done 48 h after the transfection.
RNA interference (RNAi)-mediated silencing
AS160, Rab8a, Rab10, and Rab14 were successfully knocked down using the following small interfering RNA (siRNA) oligonucleotide sequences: AS160 siRNA (ID# sc-61655), Rab8a siRNA (ID#sc-41829), Rab10 siRNA (ID#sc-41833), and Rab14 siRNA (ID#sc-76313) (Santa Cruz, Inc.). Briefly, cells were transfected with the siRNA (100 nM) or irrelevant controls (siGLO control siRNA, Dharmacon) in antibiotic and norepinephrine-free culture medium, using Lipofectamine-RNAiMax (Invitrogen). Six hours later, the medium was changed to fully supplemented Claycomb medium. Assays were done 48 h after the transfection.
CD36 translocation assay
CD36 surface translocation was measured as previously described (28), with modifications. Briefly, cells seeded in 24-well plates until confluent were serum-starved and treated with different stimuli. They were fixed with 3% parformaldehyde and then incubated (1 h) with blocking buffer (PBS, 0.05% Tween 20, 1% BSA, 5% goat serum). Following incubation (30 min) with monoclonal rat anti-mouse CD36 antibody (Abd Serotec, MCA2748), the cells were washed (three times) with PBST (PBS, Tween 0.05%) and incubated (30 min) with secondary HRP-conjugated anti-rat antibody. This was followed with washing with PBST and incubation with 3,3′,5,5′-Tetramethylbenzidine Liquid Substrate System (TMB; Sigma). The reaction (room temperature) was stopped (30 min) by addition of 1 N NaOH, and absorbance was read at 450 nM. To adjust for well-to-well variability, cells were incubated (30 min) with Wheat Germ Agglutinin Alexa Fluor 680 (WGA; Invitrogen) and rinsed with PBS, and fluorescence was measured at 700 nM (Odyssey infrared imager, LI-COR). Background signals for TMB (primary antibody omitted) and for WGA Alexa-680 staining (WGA omitted) were subtracted from raw data.
SDS-PAGE and Western blotting
Cells were lysed (30 min) in ice-cold lysis buffer [20 mM Tris-HCL (pH 7.5), 150 mM NaCL, 1% Triton X-100, 60 mM octyl β-d-glucopyranoside (O-8001; Sigma-Aldrich), 200 μM sodium orthovanadate, 50 mM NaF, 1 mM PMSF, and 1.0 µg/ml protease inhibitor mix (Sigma-Aldrich)]. Lysates were cleared (at 10,000 g for 10 min), and protein content of supernatants was determined by the BCA assay (23225, Pierce Biotechnology; Rockford, IL) before SDS-PAGE and Western blotting.
Statistical analysis
All experiments presented were repeated at least three times, unless otherwise indicated. The data represent the mean ± SE. Statistical analysis was performed by one-way repeated-measure ANOVA with Student-Newman-Keuls test.
RESULTS
Insulin or AICAR stimulation induces CD36 surface translocation in HL-1 cardiomyocytes
The HL-1 cell line, derived from the AT-1 mouse atrial cardiomyocyte tumor lineage, maintains in culture a differentiated phenotype characteristic of adult cardiomyocytes (29) and has been used extensively in studies of cardiomyocyte physiology (as reviewed in Ref. 30). A recent study (28), using HL-1 cells, showed that CD36 and GLUT4 were recruited to the surface upon insulin stimulation or as the result of contraction. These findings are similar to those in rodent cardiomyocytes, where insulin stimulation (31) and AMPK activation by the cell-permeable adenosine analog AICAR (10) enhanced surface CD36 content and FA uptake.
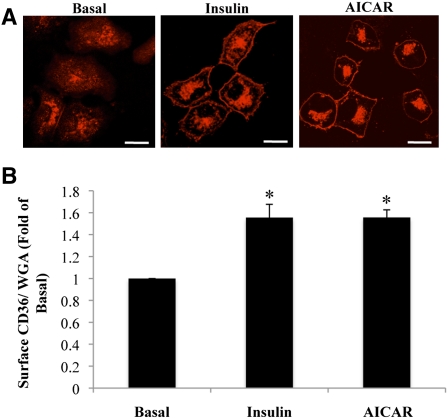
The effects of insulin or AICAR stimulation on intracellular distribution of CD36 were examined in HL-1 cells, using immunofluorescence (Fig. 1A) and a CD36 translocation assay (Fig. 1B) that quantified CD36 at the plasma membrane directly without cell fractionation. Upon serum starvation, CD36 was largely internalized into punctate structures, localized at the perinuclear region, with a small fraction of the protein still residing on the plasma membrane (Fig. 1A). Stimulation with insulin or AICAR triggered a pronounced redistribution of CD36 from intracellular compartments to the surface (Fig. 1A), which resulted in a 1.5-fold increase in average surface CD36 expression (Fig. 1B). These results are comparable to the effect of insulin or AICAR stimulation in primary cardiomyocytes, and therefore our translocation assay in HL-1 cells represents a valid and direct assessment of CD36 translocation.
Fig. 1.
CD36 is recruited to the surface of HL-1 cardiomyocytes by insulin or AICAR stimulation. HL-1 cells were serum starved, incubated with or without insulin or AICAR, and processed for immunofluorescence (A), as described in Materials and Methods. Images representative of three separate experiments are shown. Scale bar = 11 μM. B: CD36 translocation was assayed as described in Materials and Methods. Data were normalized by the WGA signal and converted to fold change relative to surface CD36 in untreated cells (basal). Bar graphs represent means ± SE from three independent experiments. * Significant as compared with untreated controls (P < 0.05).
RNAi-mediated knock-down of AS160 results in redistribution of CD36 to the surface
Both insulin and AMPK signaling converge to induce phosphorylation of the Rab GAP AS160, which results in GLUT4 membrane translocation. We examined whether AS160 might also mediate CD36 surface recruitment. This was first examined using RNAi-mediated depletion of AS160.
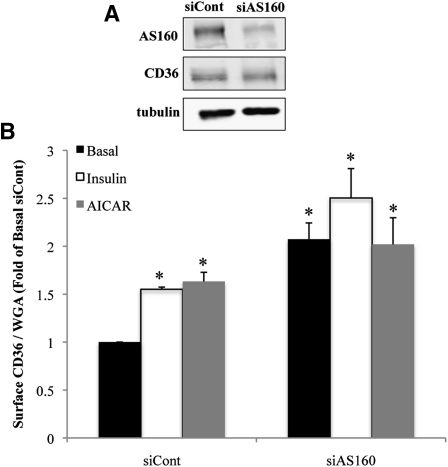
Transfection of HL-1 cells with siRNA directed against the mouse AS160 sequence resulted in a 70% decrease in expression of the endogenous AS160 protein (Fig. 2A). As a result of AS160 depletion, the level of total CD36 was not altered (Fig. 2A), but surface CD36 expression in basal (unstimulated) cells was increased 2.5-fold (Fig. 2B). Under these conditions, neither insulin nor AICAR stimulation had any additional effect on CD36 surface abundance. These results suggested that surface recruitment of CD36 in HL-1 cardiomyocytes is regulated by AS160 and that the protein mediates the effects of insulin and AMPK on CD36 translocation.
Fig. 2.
RNAi-mediated knockdown of AS160 induces surface recruitment of CD36. HL-1 cardiomyocytes were transfected with irrelevant siRNA (siCont) or with siRNA directed against AS160. A: Western blots (20 µg protein) representative of three separate experiments. B: Cells were serum starved and incubated with or without insulin or AICAR. Surface CD36 expression normalized to the WGA signal (see Materials and Methods) is expressed as fold change above basal levels of untreated siRNA controls. Bar graphs represent means ± SE of three independent experiments. * Significant as compared with untreated control siRNA (P < 0.05).
CD36 colocalizes with AS160-targeted Rab GTPases
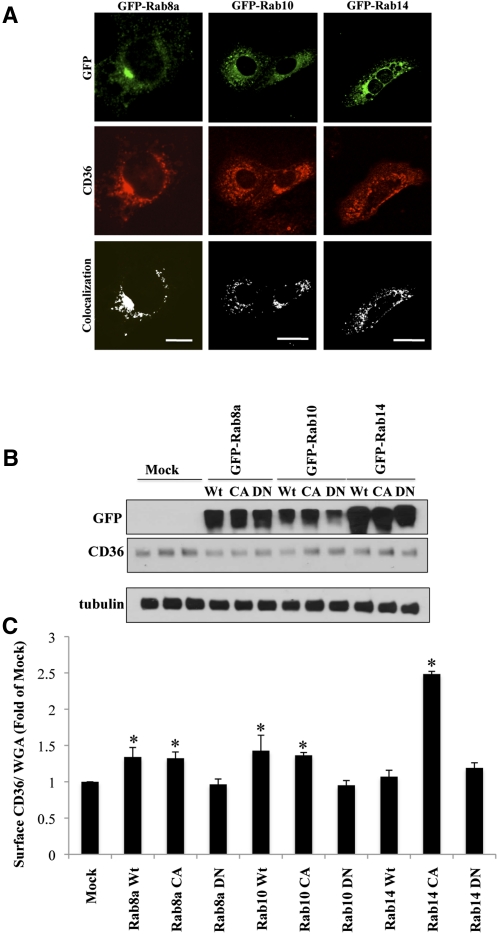
AS160 has a well-documented GAP activity toward Rab8a, Rab10, and Rab14. These Rabs were previously implicated in GLUT4 trafficking (25–27). To examine the possible role of these Rabs in CD36 trafficking, GFP-tagged constructs for the individual GTPases were transiently expressed in HL-1 cells (Fig. 3A) and their cellular distribution was analyzed by immunofluorescence. GFP-Rab8a and GFP-Rab14 constructs were preferentially localized to punctate structures in the perinuclear region, whereas GFP-Rab10 expression was more widely distributed throughout the perinuclear region and the cell periphery.
Fig. 3.
CD36 trafficking is regulated by AS160-targeted Rab-GTPases. A: HL-1 cardiomyocytes transfected with Wt GFP-Rab8a, GFP-Rab10, or GFP-Rab14 constructs were serum deprived, stained with anti-CD36 antibody, and processed for immunofluorescence. The images were analyzed using ImageJ software (National Institutes of Health), and colocalization was determined using the colocalization finder plug-in. Scale bar = 11 μM. B: HL-1 cells were lysed, and proteins (20 µg) were separated and analyzed by Western blotting. Data are representative of three experiments. C: CD36 translocation (assessed as in legends to Figs. 1, 2). Bar graphs represent mean ± SE of three independent experiments. * Significant as compared with mock-transfected untreated controls (P < 0.05).
Importantly, CD36-positive punctate staining could be shown to partially overlap with all three Rabs. These data indicated that exogenously expressed AS160-targeted Rabs are segregated into CD36-containing vesicular (endosomal) structures, implying that they could interact with CD36-containing vesicles to regulate CD36 trafficking (Fig. 3A).
Overexpression of AS160-targeted Rab GTPases increases surface abundance of CD36
The functional role of AS160-targeted Rabs in CD36 traffic was next studied by transient overexpression of Wt, CA, or DN GFP-tagged constructs of Rab8a, Rab10, or Rab14 (Fig. 3B, C).
Overexpression of each of these constructs did not alter total expression of CD36 (Fig. 3B); however, surface expression was significantly increased in cells expressing Wt or CA forms of all three Rabs (Fig. 3C). These results suggested that the AS160-targeted Rab GTPases promote translocation of CD36 to the surface. Interestingly, in the case of Rab8a and Rab10, overexpression of Wt or CA variants had similar effects on CD36 surface abundance. However, this pattern differed with Rab14, inasmuch as Wt Rab14 did not increase membrane CD36 significantly, whereas the CA form had a dramatic effect, increasing surface expression by about 2.5-fold. This could suggest that Rab14 is mostly maintained in the inactive form in basal HL-1 cells. Importantly, expression of the DN forms of each Rab did not promote surface translocation of CD36 (Fig. 3C), confirming that the effect of these GTPases on CD36 traffic specifically required the active form of each protein.
RNAi-mediated knock-down of Rab8a abrogates insulin/AICAR-induced redistribution of CD36 to the surface
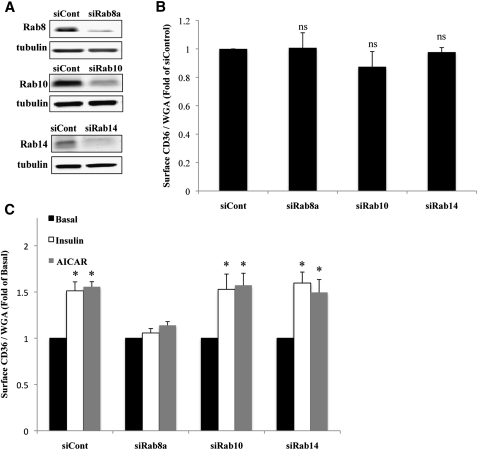
Expression of an exogenous Rab could have indirect effects on CD36 trafficking mediated by the sequestration of activators or inhibitors shared by another endogenous Rab. To address this, the role of the individual AS160-targeted Rabs in CD36 surface translocation was examined next using RNAi-mediated knock-down. We successfully depleted HL-1 cells of endogenous Rab8a, Rab10, and Rab14 by 60%, 70%, and 90%, respectively, using siRNA oligonucleotides directed against each of the proteins (Fig. 4A).
Fig. 4.
RNAi-mediated knock-down of Rab8a abrogates insulin/AICAR-induced surface recruitment of CD36. HL-1 cardiomyocytes were transfected with irrelevant siRNA (siCont) or with siRNA directed against Rab8a, Rab10, or Rab14. A: Cells were lysed and proteins (20 µg) were separated and analyzed by Western blotting. Data are representative of three experiments. B: Cells were serum starved, and surface CD36 was analyzed (as in legends to Figs. 1, 2). Bar graphs represent means ± SE of three independent experiments. ns, nonsignificant compared with siCont. C: Effect of insulin and AICAR. Cells were processed as in B. Bar graphs represent mean ± SE of three independent experiments. * Significant as compared with untreated controls within each group (P < 0.05).
The effect of RNAi-mediated knock-down on CD36 surface expression was examined under basal and insulin or AICAR-stimulated states (Fig. 4B, C). Depletion of each of the Rabs did not result in a significant change in basal CD36 surface expression (Fig. 4B). Transfection with irrelevant control siRNA, as well as with either Rab10 or Rab14 siRNA, did not interfere with insulin- or AICAR-induced CD36 recruitment to the surface (Fig. 4C). Strikingly, Rab8a depletion suppressed the insulin- or AICAR-induced CD36 translocation, implicating Rab8a specifically in the control of CD36 membrane recruitment in cardiomyocytes. These data indicated that although exogenous Rab8a, Rab10, and Rab14 similarly induced CD36 translocation, the effects of Rab10 and Rab14 might have been indirect.
UTP induces CD36 surface translocation in cardiomyocytes, and this effect requires Rab8a
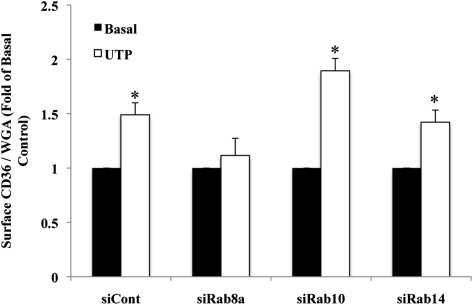
We next determined whether Rab8a might be involved in the effect of agonists other than insulin and AMPK that were documented to translocate CD36 to the membrane. UTP was shown to promote surface translocation of CD36 in Chinese hamster ovary (CHO) cells and macrophages (6). UTP is important to cardiovascular physiology because it is released during ischemia and has been shown to protect the myocardium from hypoxic stress (32). The protective effects of UTP are mediated by activation of membrane purinergic receptors (33). We determined whether UTP stimulation could translocate CD36 to the surface of HL-1 cardiomyocytes, and whether this translocation is Rab8a dependent.
HL-1 cells were transfected with scrambled siRNA or with siRNA directed against Rab8a, Rab10, or Rab14. UTP stimulation of cells transfected with scrambled siRNA resulted in a rapid 1.5-fold increase in surface CD36 expression (Fig. 5), suggesting that activation of purinergic receptors induces surface recruitment of CD36 in cardiomyocytes similar to that described in CHO cells and macrophages (6).
Fig. 5.
Rab8a is required for UTP-induced CD36 surface translocation. HL-1 cardiomyocytes were transfected as described in Fig. 4, serum depleted, and incubated with or without 100 µM UTP. The data were analyzed as described in Fig. 4C and compared with surface CD36 signal in control siRNA-transfected cells. Bar graphs represent mean ± SE of three independent experiments. * Significant as compared with untreated controls within each group (P < 0.05).
Importantly, RNAi-mediated knock-down of Rab8a, but not of Rab10 or Rab14, abolished the UTP-induced surface translocation of CD36. These results suggested that Rab8a is the major GTPase involved in CD36 membrane translocation, inasmuch as it mediated the effects of insulin, AMPK, and UTP, three physiological regulators of cardiomyocytes.
Overexpression of AS160 4P abrogates insulin/AICAR-induced translocation of CD36
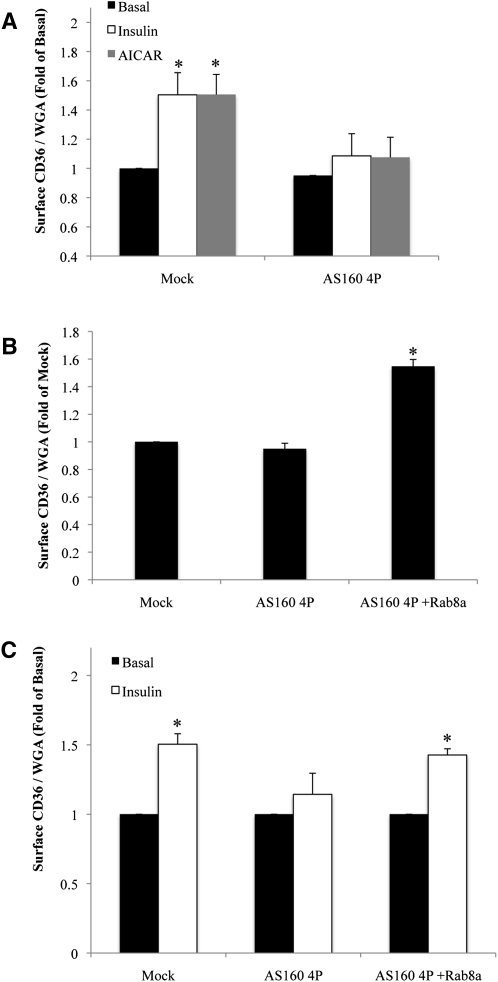
Insulin- or AMPK-mediated signaling inhibits the GAP activity of AS160 via its phosphorylation on several sites (18, 34). We examined the role of AS160 phosphorylation in membrane CD36 recruitment using a phosphorylation-deficient form of AS160 (AS160 4P) in which four of the phosphorylation sites (Thr642, Ser318, Ser588, and Ser751) were replaced by alanine (35). This mutant is unresponsive to metabolic stimuli and acts as a CA form of AS160, preventing GLUT4 translocation to the surface (35, 36).
Overexpression of a FLAG-tagged AS160 4P construct in HL-1 cardiomyocytes did not change basal surface abundance of CD36 (Fig. 6A), but it attenuated insulin- or AICAR-induced CD36 surface recruitment. This result suggested that regulation of AS160 phosphorylation is required for the action of insulin or AMPK to recruit CD36 to the membrane.
Fig. 6.
Phosphorylation of AS160 is required for insulin/AICAR-induced CD36 translocation. A: Cells were transfected with or without AS160 4P, serum starved, and incubated with or without insulin or AICAR. Surface CD36 content was analyzed and compared with that in untreated mock-transfected controls. * Significant as compared with untreated mock-transfected controls (P < 0.05). B: Cells were transfected with or without AS160 4P construct and with or without Wt GFP-Rab8a, serum starved, and processed for assay of surface CD36 translocation. * Significant as compared with mock-transfected controls (P < 0.05). C: Fold change relative to surface CD36 in mock-transfected controls. Bar graphs represent mean ± SE of three independent experiments. * Significant as compared with mock-transfected controls (P < 0.05).
Rab8a overexpression reverses the inhibition of CD36 translocation caused by AS160 4P
To further establish the specific mobilization of Rab8a downstream of AS160 in the control of CD36 membrane translocation, we tested the effect of Rab8a overexpression on CD36 translocation in cardiomyocytes expressing AS160 4P.
The AS160 4P construct was transiently expressed with or without GFP-Rab8a in HL-1 cardiomyocytes. As before, expression of AS160 4P did not significantly change the basal level of surface CD36 expression; however, introduction of GFP-Rab8a significantly raised basal CD36 cell surface abundance (Fig. 6B). Strikingly, expression of GFP-Rab8a, in cells expressing AS160 4P, relieved inhibition of insulin-induced CD36 translocation and restored CD36 surface recruitment to control levels (Fig. 6C).
These findings demonstrated that Rab8a functions downstream of AS160, because it was able to cancel the negative effects of AS160 4P mutant. This may potentially arise from GFP-Rab8a negating the effect of AS160 4P overexpression, allowing the endogenous AS160/Rab8a pathway to be activated by insulin signaling.
DISCUSSION
The molecular mechanisms governing CD36 trafficking remain little understood despite their documented importance to myocardial FA metabolism. This study showed that in cardiomyocytes the GTP-bound Rab8a protein under the control of the Rab GTPase-activating protein AS160 regulates trafficking of CD36 vesicles to the plasma membrane. Rab8a was shown to coincide with CD36-containing vesicles and to be involved in CD36 membrane recruitment after treatment with insulin, AICAR, or UTP. Thus, Rab8a might be a common regulator of CD36 vesicular traffic in cardiomyocytes.
AS160 and Rab8a regulate membrane recruitment of CD36
The AS160 Rab GAP protein appears to be a major downstream effector of insulin signaling in vivo. Its impaired insulin-induced phosphorylation was shown in muscle from type 2 diabetic subjects. This phosphorylation could be restored with endurance training, improving insulin sensitivity (37). Severe insulin resistance was recently documented in subjects carrying a premature stop mutation in one allele of AS160, resulting in a DN truncated variant and lower levels of AS160 protein expression (38). In mice, the knock-in of an AS160 phosphorylation mutant resulted in glucose intolerance, partially reflecting reduced insulin sensitivity and altered GLUT4 trafficking in muscle (39).
AS160 was shown to regulate GLUT4 translocation in adipocytes and myocytes (as reviewed in Ref. 18). Under basal conditions, AS160, through its GAP activity, retains GLUT4 vesicles intracellularly. Following insulin treatment or AMPK activation, AS160 is rapidly phosphorylated, which reduces its GAP activity. In myocytes, this allows activation of AS160-controlled Rab8a, which then promotes membrane translocation of GLUT4 vesicles, enhancing glucose transport (25, 40). Using a variety of approaches, we showed that CD36 surface recruitment in cardiomyocytes is mediated by AS160 through its control of Rab8a. Knock-down of AS160 resulted in redistribution of CD36 to the surface, which was not further induced by insulin or AICAR stimulation (Fig. 2). In contrast, overexpression of a CA, phosphorylation-deficient AS160 4P variant inhibited the stimulated membrane translocation of CD36. Among the AS160-regulated Rabs, knock-down of Rab8a, but not that of Rab10 or Rab14, resulted in suppression of insulin- or AICAR-induced CD36 surface recruitment (Fig. 4). Furthermore, inhibition of CD36 translocation by the CA AS160 4P was reversed by overexpression of Rab8a (Fig. 6C).
Overall, insulin- or AMPK-induced CD36 exocytosis in cardiomyocytes requires phosphorylation of AS160 and subsequent activation of Rab8a, thus following the same trafficking pattern previously described for GLUT4 in myocytes. Therefore, the AS160/Rab8a axis integrates physiological and metabolic cues to couple membrane recruitment of CD36 and GLUT4 vesicles in cardiomyocytes. Interestingly, the AS160/Rab8a pathway was also recently shown to be involved in surface recruitment of GLUT1 in human embryonic kidney cells in response to the protein kinase WNK1 (41). Together the findings would suggest that the AS160/Rab8a pathway is not specific to GLUT4 recruitment in muscle and may be shared by a number of other transporters.
Uncoupling of GLUT4 and CD36 membrane recruitment in the insulin-resistant myocardium
The AS160/Rab8a pathway coordinates insulin- or AMPK-stimulated membrane recruitment of CD36 and GLUT4 in healthy cardiomyocytes. However, this coordination is disrupted in the insulin-resistant state.
Several factors could potentially contribute to uncoupling of GLUT4 and CD36 trafficking. We observed that AS160 depletion of cardiomyocytes resulted in membrane redistribution of CD36 in the basal state, while completely suppressing insulin/AICAR-stimulated CD36 translocation. In contrast, AS160 depletion in 3T3-L1 adipocytes (42) and L6 myotubes (40) increased membrane GLUT4 content, but it did not abolish insulin stimulation of GLUT4 translocation. This suggests that reduction of AS160 activity (level or phosphorylation) would contribute to persistent plasma membrane expression of CD36 and simultaneously impair its regulated recruitment. In contrast, the stimulated recruitment of GLUT4 would not be initially impaired and its dysfunction would occur later as a result of abnormal FA metabolism. This interpretation is consistent with earlier findings documenting that the aberrant CD36 trafficking in an insulin-resistant state develops before the dysregulation of GLUT4 trafficking (5, 12–14).
Another important consideration is that AS160 has multiple phosphorylation sites and is targeted by various upstream kinases other than Akt and AMPK (41, 43, 44). As a result, AS160 functions in the surface translocation of a variety of membrane transporters as diverse as Na+,K+-ATPase, aquaporin, or GLUT1 (41, 45, 46), and its regulation integrates multiple signaling influences with differential effects on various transport pathways. It is possible that as we gain a better understanding of the functional relevance of the various phosphorylation sites on AS160, sites and conditions that differentially regulate surface recruitment of CD36 versus GLUT4 will be uncovered. The role of Rabs other than Rab8a would also need to be examined within such a context. For example, Rab14 involvement in GLUT4 traffic was described in myocytes (25), whereas our results clearly demonstrate, that although overexpressed Rab14 segregated to CD36-positive vesicles and increased CD36 surface expression, its depletion did not suppress the insulin/AICAR/UTP-induced translocation of CD36. This implies that Rab14 does not mediate insulin/AICAR/UTP-induced CD36 membrane translocation, but it may be relevant to other conditions involved in regulating CD36 trafficking that will need to be determined.
It is worth noting that a close homolog of AS160, Rab GAP TBC1D1, was reported to function in GLUT4 translocation in adipocytes (47) and muscle cells (48). This homolog has substrate specificity similar to that of AS160 (49) and could also be involved in CD36 trafficking. Furthermore, the DENND4 subfamily of Rab10 exchange factors was recently implicated in insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes (50). A possible role of Rab10 and the DENND4 subfamily of exchange factors in CD36 trafficking will need to be investigated in the future.
Alternatively, the uncoupling of CD36 and GLUT4 membrane recruitment could occur at sites downstream of AS160. In line with this, the involvement of different isoforms of vesicle-associated membrane proteins in the control of CD36 and GLUT4 membrane insertion has been recently reported (28). Moreover, additional Rab8a effectors, such as Rab8a GEFs, could be differentially regulated by various physiological conditions, thus allowing further discrimination between CD36 and GLUT4 cell surface recruitment.
In conclusion, the trafficking of CD36, a facilitator of myocardial FA uptake is regulated by AS160, a protein that is a major effector of Akt signaling that is also targeted by a number of other physiological regulators. The ability of AS160 to regulate membrane recruitment of CD36 as well as that of GLUT4 supports its key position in mediating physiological control of cardiomyocyte metabolism. Previous work (1, 2) documented the importance of CD36 trafficking in metabolic regulation and its potential contribution to the etiology of insulin resistance. The present findings further our understanding of the molecular regulation of membrane CD36 and provide a framework that could help future investigation of the organelles and vesicular proteins likely to be involved in regulating recruitment of CD36 to the sarcolemma.
Acknowledgments
The authors thank Dr. G. E. Lienhard, Dr. I. Mellman, and Dr. J. Brumell for the generous provision of constructs, and Dr. A. Klip for her assistance with acquiring the constructs. The authors thank Dr. W. Claycomb for kindly providing the HL-1 cardiomyocytes.
Footnotes
Abbreviations:
- AICAR
- 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside
- AMPK
- AMP-dependent protein kinase
- AS160
- Akt substrate 160
- CA
- constitutively active
- CD36
- fatty acid translocase cluster of differentiation 36
- CHO
- Chinese hamster ovary
- DN
- dominant-negative
- GAP
- GTPase-activating protein
- GEF
- guanine exchange factor
- GFP
- green fluorescent protein
- RNAi
- RNA interference
- siRNA
- small interfering RNA
- UTP
- uridine-5 ′ -triphosphate
- Wt
- wild-type
This work was supported in part by National Institutes of Health Grants P01 HL-057278, R01 DK-33301, DK-56351, and GM-086566 and by American Heart Association Scientist Development Grant 835140N (X.S.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Glatz J. F., Luiken J. J., Bonen A. 2010. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90: 367–417 [DOI] [PubMed] [Google Scholar]
- 2.Steinbusch L. K., Schwenk R. W., Ouwens D. M., Diamant M., Glatz J. F., Luiken J. J. 2011. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell. Mol. Life Sci. 68: 2525–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong F., Black P. N., Coleman R. A., DiRusso C. C. 2006. Fatty acid transport by vectorial acylation in mammals: roles played by different isoforms of rat long-chain acyl-CoA synthetases. Arch. Biochem. Biophys. 447: 46–52 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg I. J., Eckel R. H., Abumrad N. A. 2009. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 50 (Suppl.): 86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coort S. L., Hasselbaink D. M., Koonen D. P., Willems J., Coumans W. A., Chabowski A., van der Vusse G. J., Bonen A., Glatz J. F., Luiken J. J. 2004. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes. 53: 1655–1663 [DOI] [PubMed] [Google Scholar]
- 6.Kuda O., Jenkins C. M., Skinner J. R., Moon S. H., Su X., Gross R. W., Abumrad N. A. 2011. CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J. Biol. Chem. 286: 17785–17795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran T. T., Poirier H., Clement L., Nassir F., Pelsers M. M., Petit V., Degrace P., Monnot M. C., Glatz J. F., Abumrad N. A., et al. 2011. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J. Biol. Chem. 286: 25201–25210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith J., Su X., El-Maghrabi R., Stahl P. D., Abumrad N. A. 2008. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J. Biol. Chem. 283: 13578–13585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su X., Abumrad N. A. 2009. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metab. 20: 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luiken J. J., Coort S. L., Willems J., Coumans W. A., Bonen A., van der Vusse G. J., Glatz J. F. 2003. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 52: 1627–1634 [DOI] [PubMed] [Google Scholar]
- 11.Jeppesen J., Albers P. H., Rose A. J., Birk J. B., Schjerling P., Dzamko N., Steinberg G. R., Kiens B. 2011. Contraction-induced skeletal muscle FAT/CD36 trafficking and FA uptake is AMPK independent. J. Lipid Res. 52: 699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouwens D. M., Diamant M., Fodor M., Habets D. D., Pelsers M. M., El Hasnaoui M., Dang Z. C., van den Brom C. E., Vlasblom R., Rietdijk A., et al. 2007. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia. 50: 1938–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguer C., Mercier J., Man C. Y., Metz L., Bordenave S., Lambert K., Jean E., Lantier L., Bounoua L., Brun J. F., et al. 2010. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia. 53: 1151–1163 [DOI] [PubMed] [Google Scholar]
- 14.van den Brom C. E., Huisman M. C., Vlasblom R., Boontje N. M., Duijst S., Lubberink M., Molthoff C. F., Lammertsma A. A., van der Velden J., Boer C., et al. 2009. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc. Diabetol. 8: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller H., Deckers K., Eckel J. 2002. The fatty acid translocase (FAT)/CD36 and the glucose transporter GLUT4 are localized in different cellular compartments in rat cardiac muscle. Biochem. Biophys. Res. Commun. 293: 665–669 [DOI] [PubMed] [Google Scholar]
- 16.Barr F., Lambright D. G. 2010. Rab GEFs and GAPs. Curr. Opin. Cell Biol. 22: 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda M. 2011. TBC proteins: GAPs for mammalian small GTPase Rab? Biosci. Rep. 31: 159–168 [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto K., Holman G. D. 2008. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 295: E29–E37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane S., Sano H., Liu S. C., Asara J. M., Lane W. S., Garner C. C., Lienhard G. E. 2002. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 277: 22115–22118 [DOI] [PubMed] [Google Scholar]
- 20.Deshmukh A., Coffey V. G., Zhong Z., Chibalin A. V., Hawley J. A., Zierath J. R. 2006. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes. 55: 1776–1782 [DOI] [PubMed] [Google Scholar]
- 21.Kramer H. F., Witczak C. A., Taylor E. B., Fujii N., Hirshman M. F., Goodyear L. J. 2006. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J. Biol. Chem. 281: 31478–31485 [DOI] [PubMed] [Google Scholar]
- 22.Kramer H. F., Witczak C. A., Fujii N., Jessen N., Taylor E. B., Arnolds D. E., Sakamoto K., Hirshman M. F., Goodyear L. J. 2006. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 55: 2067–2076 [DOI] [PubMed] [Google Scholar]
- 23.Larance M., Ramm G., Stockli J., van Dam E. M., Winata S., Wasinger V., Simpson F., Graham M., Junutula J. R., Guilhaus M., et al. 2005. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 280: 37803–37813 [DOI] [PubMed] [Google Scholar]
- 24.Mîinea C. P., Sano H., Kane S., Sano E., Fukuda M., Peranen J., Lane W. S., Lienhard G. E. 2005. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 391: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikura S., Bilan P. J., Klip A. 2007. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem. Biophys. Res. Commun. 353: 1074–1079 [DOI] [PubMed] [Google Scholar]
- 26.Sano H., Eguez L., Teruel M. N., Fukuda M., Chuang T. D., Chavez J. A., Lienhard G. E., McGraw T. E. 2007. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 5: 293–303 [DOI] [PubMed] [Google Scholar]
- 27.Sano H., Roach W. G., Peck G. R., Fukuda M., Lienhard G. E. 2008. Rab10 in insulin-stimulated GLUT4 translocation. Biochem. J. 411: 89–95 [DOI] [PubMed] [Google Scholar]
- 28.Schwenk R. W., Dirkx E., Coumans W. A., Bonen A., Klip A., Glatz J. F., Luiken J. J. 2010. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia. 53: 2209–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claycomb W. C., Lanson N. A., Jr, Stallworth B. S., Egeland D. B., Delcarpio J. B., Bahinski A., Izzo N. J., Jr 1998. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA. 95: 2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White S. M., Constantin P. E., Claycomb W. C. 2004. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am. J. Physiol. Heart Circ. Physiol. 286: H823–H829 [DOI] [PubMed] [Google Scholar]
- 31.Luiken J. J., Koonen D. P., Willems J., Zorzano A., Becker C., Fischer Y., Tandon N. N., Van Der Vusse G. J., Bonen A., Glatz J. F. 2002. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 51: 3113–3119 [DOI] [PubMed] [Google Scholar]
- 32.Shainberg A., Yitzhaki S., Golan O., Jacobson K. A., Hochhauser E. 2009. Involvement of UTP in protection of cardiomyocytes from hypoxic stress. Can. J. Physiol. Pharmacol. 87: 287–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huwiler A., Rolz W., Dorsch S., Ren S., Pfeilschifter J. 2002. Extracellular ATP and UTP activate the protein kinase B/Akt cascade via the P2Y(2) purinoceptor in renal mesangial cells. Br. J. Pharmacol. 136: 520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S., Murphy J., Toth R., Campbell D. G., Morrice N. A., Mackintosh C. 2008. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem. J. 409: 449–459 [DOI] [PubMed] [Google Scholar]
- 35.Sano H., Kane S., Sano E., Miinea C. P., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. 2003. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 278: 14599–14602 [DOI] [PubMed] [Google Scholar]
- 36.Zeigerer A., McBrayer M. K., McGraw T. E. 2004. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell. 15: 4406–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vind B. F., Pehmoller C., Treebak J. T., Birk J. B., Hey-Mogensen M., Beck-Nielsen H., Zierath J. R., Wojtaszewski J. F., Hojlund K. 2011. Impaired insulin-induced site-specific phosphorylation of TBC1 domain family, member 4 (TBC1D4) in skeletal muscle of type 2 diabetes patients is restored by endurance exercise-training. Diabetologia. 54: 157–167 [DOI] [PubMed] [Google Scholar]
- 38.Dash S., Sano H., Rochford J. J., Semple R. K., Yeo G., Hyden C. S., Soos M. A., Clark J., Rodin A., Langenberg C., et al. 2009. A truncation mutation in TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc. Natl. Acad. Sci. USA. 106: 9350–9355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S., Wasserman D. H., MacKintosh C., Sakamoto K. 2011. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 13: 68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikura S., Klip A. 2008. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am. J. Physiol. Cell Physiol. 295: C1016–C1025 [DOI] [PubMed] [Google Scholar]
- 41.Mendes A. I., Matos P., Moniz S., Jordan P. 2010. Protein kinase WNK1 promotes cell surface expression of glucose transporter GLUT1 by regulating a Tre-2/USP6-BUB2-Cdc16 domain family member 4 (TBC1D4)-Rab8A complex. J. Biol. Chem. 285: 39117–39126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eguez L., Lee A., Chavez J. A., Miinea C. P., Kane S., Lienhard G. E., McGraw T. E. 2005. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2: 263–272 [DOI] [PubMed] [Google Scholar]
- 43.Geraghty K. M., Chen S., Harthill J. E., Ibrahim A. F., Toth R., Morrice N. A., Vandermoere F., Moorhead G. B., Hardie D. G., MacKintosh C. 2007. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem. J. 407: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang X., Butterworth M. B., Peters K. W., Frizzell R. A. 2010. AS160 modulates aldosterone-stimulated epithelial sodium channel forward trafficking. Mol. Biol. Cell. 21: 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alves D. S., Farr G. A., Seo-Mayer P., Caplan M. J. 2010. AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression. Mol. Biol. Cell. 21: 4400–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H. Y., Choi H. J., Lim J. S., Park E. J., Jung H. J., Lee Y. J., Kim S. Y., Kwon T. H. 2011. Emerging role of Akt substrate protein AS160 in the regulation of AQP2 translocation. Am. J. Physiol. Renal Physiol. 301: F151–F161 [DOI] [PubMed] [Google Scholar]
- 47.Peck G. R., Chavez J. A., Roach W. G., Budnik B. A., Lane W. S., Karlsson H. K., Zierath J. R., Lienhard G. E. 2009. Insulin-stimulated phosphorylation of the Rab GTPase-activating protein TBC1D1 regulates GLUT4 translocation. J. Biol. Chem. 284: 30016–30023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chavez J. A., Roach W. G., Keller S. R., Lane W. S., Lienhard G. E. 2008. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J. Biol. Chem. 283: 9187–9195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roach W. G., Chavez J. A., Miinea C. P., Lienhard G. E. 2007. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem. J. 403: 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sano H., Peck G. R., Kettenbach A. N., Gerber S. A., Lienhard G. E. 2011. Insulin-stimulated GLUT4 protein translocation in adipocytes requires the Rab10 guanine nucleotide exchange factor Dennd4C. J. Biol. Chem. 286: 16541–16545 [DOI] [PMC free article] [PubMed] [Google Scholar]