Abstract
We recently established that drugs used for the treatment and the prophylaxis of breast cancers, such as tamoxifen, were potent inhibitors of cholesterol-5,6-epoxide hydrolase (ChEH), which led to the accumulation of 5,6α-epoxy-cholesterol (5,6α-EC) and 5,6β-epoxy-cholesterol (5,6β-EC). This could be considered a paradox because epoxides are known as alkylating agents with putative carcinogenic properties. We report here that, as opposed to the carcinogen styrene-oxide, neither of the ECs reacted spontaneously with nucleophiles. Under catalytic conditions, 5,6β-EC remains unreactive whereas 5,6α-EC gives cholestan-3β,5α-diol-6β-substituted compounds. These data showed that 5,6-ECs are stable epoxides and unreactive toward nucleophiles in the absence of a catalyst, which contrasts with the well-known reactivity of aromatic and aliphatic epoxides. These data rule out 5,6-EC acting as spontaneous alkylating agents. In addition, these data support the existence of a stereoselective metabolism of 5,6α-EC.
Keywords: SN2, alkylation, ring opening, cholesterol oxides
Cholesterol-5,6-epoxide hydrolase (ChEH; EC 3.3.2.11) catalyzes the stereoselective hydration of cholesterol-5,6-epoxide diastereoisomers (5,6-EC: 5,6α-EC and 5,6β-EC) into cholestane-3β,5α,6β-triol (CT) (1). We recently reported the molecular identification of this enzyme and established that ChEH activity was carried out by the microsomal antiestrogen binding site (AEBS), which consists of two subunits: the 3β-hydroxysterol-Δ8,Δ7-isomerase (EC 5.3.3.5) and the 3β-hydroxysterol-Δ7-reductase (EC 1.3.1.21) that are both involved in postlanosterol cholesterol biosynthesis (2). We showed that ChEH was fully inhibited by therapeutic doses of AEBS ligands, including drugs belonging to different pharmacological classes and widely used nutritional compounds. These compounds include tamoxifen (tam), one of the main drugs used for the first-line and long-term hormone therapy of breast cancers; raloxifene, which has been approved for the prevention of osteoporosis (3); amiodarone, which is widely used for the prevention and the treatment of anti-arrhythmia (4); trifluoroperazine, an antipsychotic drug used for the treatment of schizophrenia (5); and the omega-3 fatty acid docosahexaenoic acid, which is widely proposed as dietary supplement with health benefits (2). Extensive knowledge of the mechanism of action of all these molecules is required to prevent toxicity to patients submitted to long-term treatment with such inhibitors of ChEH.
AEBS/ChEH ligands, such as tam and raloxifene, were recently shown to induce cell differentiation and death of breast cancer cells through a mechanism involving sterol accumulation and autoxidation (6–8) leading to the production of 5,6-EC, suggesting that 5,6-EC accumulation is involved in their anticancer and chemopreventitive activity. This is contrary to the initial hypothesis that the biological function of ChEH was to detoxify 5,6-EC. This hypothesis emerged because it was initially postulated that 5,6-EC could, like other chemicals bearing epoxide groups, be potent alkylating substances and, hence, carcinogenic (9). ChEH is ubiquitously expressed in healthy and tumoral mammalian tissues, and its inhibition by AEBS ligands could lead to the accumulation of 5,6-EC throughout patients’ bodies.
Epoxides are three-member strained rings leading in general or normally to electrophilic compounds with reactivity toward nucleophiles to give β-substituted alcohols in a regio- and stereoselective way in SN2 conditions (10–12). This reactivity makes chemicals bearing an epoxide group, such as benzo(a)pyrene diol epoxide or styrene oxide (SO), potent alkylating agents producing covalent adducts with proteins and nucleic acids, which contributes to their mutagenicity and carcinogenicity in mammals (13, 14).
5,6-EC exist as α- and β-diastereoisomers (5,6α-EC, 5,6β-EC), depending on the orientation of the oxirane group with regard to the plane defined by the sterol backbone (1). 5,6-EC are known as major autoxidation products of cholesterol (15), and 5,6α-EC has been reported to be produced stereoselectively by a cytochrome p450 in the bovine adrenal cortex (16). 5,6-EC diastereoisomers are rapidly metabolized in mammals through different routes. They are extensively transformed into CT by ChEH, and they can be esterified with fatty acids acyl ester by acyl-coA:cholesterol acyl transferases (ACAT1 and ACAT2) (17) into fatty acyl-cholesteryl esters, and with sulfate by cholesterol sulfotransferase (SULT2B1b) into the 3β-sulfate of 5,6-EC (18). The 3β-sulfate of 5,6α-EC has been shown to antagonize the liver X receptor (LXR) signaling pathway (19, 20), and recently 5,6α-EC was found to be a direct modulator of LXR, suggesting a physiological role for this 5,6-EC diastereoisomer (21). Interestingly 5,6α-EC has been shown to be a better substrate than 5,6β-EC for ACATs and SULT2B1b and the sole 5,6-EC substrate for glutathione transferase B (22, 23). The stereoselectivity of these enzymes and the receptors that are targets of 5,6α-EC suggest structural and possibly conformational differences in both diastereoisomers. Because of the presence of a putative reactive epoxide group, 5,6-EC were initially suspected to be carcinogenic and involved in the etiology of cutaneous cancers (24, 25), although 5,6-EC were shown to be nontumorigenic in rodents (26) and the reactivity of 5,6-EC has never been clearly addressed.
The objective of the present study was to evaluate the reactivity of 5,6-EC toward nucleophiles under SN2 conditions that could putatively give the compounds shown in Fig. 1. We showed that 5,6-EC were neither reactive at ambient room temperature nor at physiological temperature (37°C). We established the first structural analysis of 5,6-EC. We further established that 5,6α-EC was the only diastereoisomer that can undergo a ring opening reaction leading to the formation of cholestan-3β,5α-diol-6β-substituted compounds through a trans-diaxial mechanism under catalytic conditions.
Fig. 1.
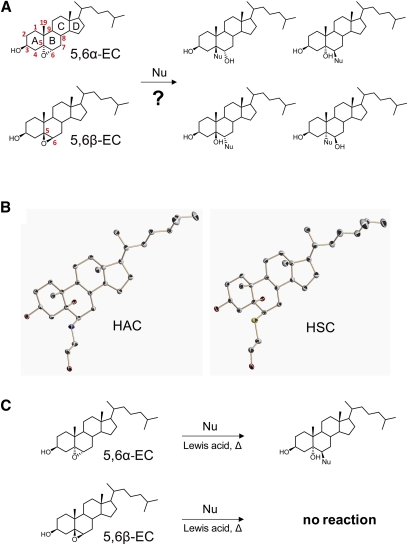
(A) Putative products of the addition of 5,6α-EC and 5,6β-EC to nucleophiles. The four rings of the steroid backbone are lettered A, B, C and D, and carbons are numbered on 5,6α-EC. Carbon 5 and 6 are numbered in red on 5,6β-EC. (B) Solid-state structure of HAC and HSC as determined by X-ray analysis. Oxygen atoms are in red, carbon atoms are in gray, and sulfur atoms are in yellow. (C) 5,6α-EC gives a single product of addition with nucleophiles only under catalytic conditions, whereas 5,6β-EC remains unreactive.
MATERIALS AND METHODS
Unless otherwise noted, all reagents were purchased from Sigma or Steraloids and checked for purity. [14C]5,6α-EC or [14C]5,6β-EC was synthesized exactly as previously described (2). All reactions were carried out under a positive pressure of argon. Reactions were analyzed by thin-layer chromatography on Merck Silica Gel 60-F254 250 μm precoated plates and visualized by warming after spraying either with 50% methanolic sulfuric acid or 0.2% ethanolic ninhydrin. Atmospheric pressure column chromatography on Merck-60 70-200 mesh silica was used for all purifications. 1H, 13C, COSY, HSBC, and HSQC NMR spectra were run at either 250 or 300 MHz using Bruker AC-250 or AC-300 spectrometers, and NOESY experiments were done using 500 MHz and mass spectrometry analysis on a quadrupolar Nermag R10-10 by either electrospray or chemical ionization. All RX data for all structures represented in this paper were collected at low temperatures using an oil-coated, shock-cooled crystal on a Bruker-AXS CCD 1000 diffractometer with MoKα radiation (λ = 0.71073 Å). The structures were solved by direct methods (27), and all nonhydrogen atoms were refined anisotropically using the least-squares method on F2 (SHELXL-97, Program for Crystal Structure Refinement, G. M. Sheldrick, University of Göttingen, 1997). Cambridge Crystallographic Data Centre (CCDC) 851178 (5,6α-EC • 1/2 methanol); CCDC 851179 (5,6β-EC • methanol); CCDC 851180 (HSC); and CCDC 851181 (HAC) contain the supplementary crystallographic data for this work. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; e-mail: deposit@ccdc.cam.ac.uk).
Procedure for comparing epoxide reactivity with 2-mercaptoethanol, 2-aminoethanol, and guanine
Styrene oxide, 5,6α-EC, or 5,6β-EC (0.1 mmol) were dissolved in ethanol (5 ml). Four equivalents of the test nucleophile [2-mercaptoethanol (ME), 2-aminoethanol (AE), or guanine (G)] were added and the mixture stirred at the appropriate temperature. After 48 h, the mixture was concentrated in vacuo, dissolved in EtOAc, washed with water, brined, dried over MgSO4, and the solvent was removed. The oil obtained was purified by column chromatography, and fractions containing adducts were pooled. For the reaction with G, the same experimental conditions were used except for the work-up; i.e., the concentrated mixture was diluted in butanol and extracted with 1 M aqueous HCl. Aqueous layers were pooled, neutralized, and extracted with butanol. The organic layer was dried over MgSO4, and the solvent was removed. The reaction was monitored by TLC (EtOAc) and revealed with concentrated sulfuric acid and heating at 180°C for 1 min for 5,6α-EC and 5,6β-EC and UV detection at 254 nm for SO. The disappearance of the spot corresponding to the 5,6-EC and SO was used to measure the occurrence of the reaction of addition of nucleophiles to epoxides to give more polar compounds that did not migrate under these conditions.
Procedure for comparing epoxide reactivity in cell culture medium
Cholestan-5,6-epoxy-3β-ol.
One microliter of an ethanolic solution of [14C]5,6α-EC or [14C]5,6β-EC was added to 1 ml of DMEM culture medium (CM) supplemented with 10% FCS to give a final concentration of 60 µM (1 µCi/condition). The mixture was stirred for 48 h at 37°C. At the indicated time (1 h, 48 h), a 30 µl aliquot was applied to silica gel 60, 20 × 20 cm. TLC plates (Fluka, Germany) that had previously been heated for 1 h at 100°C and were developed using EtOAc. Radioactive 5,6-EC were visualized using a Storm 840 apparatus (Molecular Dynamics) and quantified by densitometry with Imagequant software (Molecular Dynamics). Retention factors (Rf) were determined for each spot on the TLC plates as the ratio of the distance of migration of the eluate from the origin and the distance of the solvent from the origin. 5,6-EC have a Rf = 0.67 (2).
Styrene oxide.
Styrene oxide was added to 10 ml of DMEM culture medium supplemented with 10% FCS to a final concentration of 60 µM. The mixture was stirred either for 1 min or 48 h at 37°C. After the indicated time, total lipids were extracted using the Blight and Dyer methodology (28). The organics were concentrated to dryness in vacuo, dissolved in 50 µl ethanol, and submitted to TLC (elutant 1:1 hexane:diethyl ether) to visualize the disappearance of the SO spot after 48 h reaction (UV, 254 nm).
Procedure for quantifying cholesterol-5,6-epoxide reactivity with ME and AE
[14C]5,6α-EC or [14C]5,6β-EC (1 µmol) were dissolved in a solution of the considered nucleophile (4 equivalents) in ethanol (500 µl) containing LiClO4 (4 equivalents), if needed. The mixture was stirred at the appropriate temperature for 48 h, and a 25 µl aliquot was applied to silica gel 60 and analyzed as described above. The transformation rate was determined by quantification of the spot comigrating with nonlabeled HSC or HAC compared with the total radioactivity of the lane.
2-(2-Hydroxy-ethylsulfanyl)-2-phenyl-ethanol.
TLC Rf [EtOAc] = 0.38; NMR δH (300 MHz, MeOD) 7.42–7.24 (5H, m), 4.07–4.00 (2H, m), 3.73–3.69 (1H, m), 3.58 (2H, t, J 6.7 Hz), 2.59–2.54 (2H, m); NMR δC (75 MHz, MeOD) 143.72, 128.12, 128.02, 127.01, 65.55, 61.04, 52.01, 32.96; HRMS m/z = 198.0722 (calculated for C29H52SO3 198.0715).
2-(2-Hydroxy-ethylamino)-1-phenyl-ethanol.
TLC Rf [methanol] = 0.37; NMR δH (300 MHz, MeOD) 7.44–7.27 (5H, m), 4.85 (1H, t, J 6.6 Hz), 3.72 (1H, dd, J 5.2, 1.2 Hz), 3.35–3.33 (2H, m), 2.91–2.84 (4H, m); NMR δC (75 MHz, MeOD) 142.91, 128.11, 127.38, 125.71, 71.62, 59.62, 56.18, 50.55; HRMS m/z = 181.1098 (calculated for C29H52NO3 181.1103).
2-(7-Guanidyl)-1-phenyl-ethanol.
TLC Rf [28% aqueous NH3/methanol 9:1] = 0.71; NMR δH (300 MHz, MeOD) 8.38 (1H, s), 7.45–7.21 (5H, m), 4.91 (1H, t, J 7.3 Hz), 4.09 (2H, d, J 7.2 Hz); NMR δC (75 MHz, MeOD) 155.91, 153.77, 151.12, 143.24, 138.87, 128.01, 127.19, 124.71, 107.65, 74.62, 52.62; HRMS m/z = 271.1077 (calculated for C29H52NO3 271.1069).
Synthesis of HSC
To a stirred solution of 56α-EC (400 μmol) in ethanol (5 ml) were added 2 M sodium hydroxide (800 μmol, 2 equivalents) and then ME (1.6 mmol, 4 equivalents). After refluxing for 24 h, the mixture was cooled and diluted with ethyl acetate (10 ml) and washed sequentially with water and brine. The organic layer was dried over MgSO4, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (0–10% methanol in ethyl acetate) to obtain the product of interest as a white solid (90 μmol, 23%). To obtain crystals suitable for X-ray analysis, HSC was dissolved in hot acetone, and water was added drop-wise until supersaturation. The solution was then allowed to cool at room temperature overnight for optimal nucleation and crystallization. TLC Rf [EtOAc] = 0.25; NMR δH (300 MHz, DMSO-d6) 3.88–3.80 (1H, m), 3.43–3.40 (2H, m), 2.65–2.60 (1H, m), 2.40–2.35 (1H, m), 2.29 (1H, m), 1.03 (3H, s), 0.89 (3H, d, J 3.9 Hz), 0.86 (3H, d, J 1.5 Hz), 0.81 (3H, d, J 1.5 Hz), 0.63 (3H, s); NMR δC (75 MHz, DMSO-d6) 75.56, 66.43, 64.97, 60.86, 56.21, 56.12, 52.15, 45.45, 42.72, 42.51, 39.45, 38.46, 36.06, 35.68, 32.83, 31.36, 30.80, 30.43, 28.29, 27.81, 24.35, 23.64, 23.11, 22.83, 21.16, 18.95, 17.35, 12.47; HRMS m/z = 481.3701 (calculated for C29H52SO3 481.3710).
Synthesis of HAC
To a stirred solution of 5,6α-EC (400 μmol) in Ethanol (5 ml) were added LiClO4 (1.6 mmol, 4 equivalents) and then ethanolamine (1.6 mmol, 4 equivalents). The mixture was refluxed for 5 days, and after cooling was diluted with ethyl acetate (10 ml) and washed sequentially with water and brine. The organic layer was dried over MgSO4, and the solvent was removed under reduced pressure. The crude material was purified by column chromatography (0–10% methanol in ethyl acetate) to obtain the final product as a light-yellow solid (101 μmol, 25%). To obtain crystals suitable for X-ray analysis, HAC was dissolved in hot ethanol, and water was added drop-wise until supersaturation. The solution was then allowed to cool at room temperature overnight for optimal nucleation and crystallization. TLC Rf [EtOAc/methanol 3:1] = 0.42; NMR δH (300 MHz, DMSO-d6) 3.95–3.84 (1H, m), 3.78–3.67 (2H, m), 3.18–2.96 (3H, m), 1.03 (3H, s), 0.93 (3H, d, J 6.0 Hz), 0.87 (3H, d, J 1.2 Hz), 0.85 (3H, d, J 1.2 Hz), 0.68 (3H, s); NMR δC (75 MHz, DMSO-d6) 73.94, 65.77, 62.93, 56.16, 56.10, 55.90, 52.03, 50.43, 44.62, 42.94, 40.09, 38.01, 36.13, 35.73, 32.57, 31.24, 29.67, 28.32, 27.89, 27.33, 23.92, 23.73, 23.16, 22.89, 21.11, 19.92, 18.90, 16.34, 12.62; HRMS m/z = 464.4146 [M+H]+, calculated for C29H53NO3: 464.4104; [α]D = −4.6°/mol/dm.
Crystallographic data
To perform X-ray crystallographic analysis, commercial 5,6α-EC and 5,6β-EC were recrystallized in hot methanol.
5,6α-EC • 1/2 methanol.
C27.5H48O2.5, M = 418.66, monoclinic, P21, a = 12.406(1) Å, b = 6.043(1) Å, c = 35.224(2) Å, β = 94.042(3)°, V = 2634.1(2) Å3, Z = 4, T = 193(2) K. 20,471 reflections (7,379 independent, Rint = 0.0722) were collected. Largest electron density residue: 0.161 e Å-3, R1 [for I > 2σ(I)] = 0.0628 and wR2 = 0.1664 (all data) with R1 = Σ‖Fo|-|Fc‖/Σ|Fo| and wR2 = (Σw (Fo2-Fc2)2/Σw(Fo2)2)0.5.
5,6β-EC • methanol.
C28H50O3, M = 434.68, monoclinic, P21, a = 12.393(1) Å, b = 7.247(1) Å, c = 15.007(1) Å, β = 95.534(2)°, V = 1341.42(7) Å3, Z = 2, T = 193(2) K. 25587 reflections (5399 independent, Rint = 0.0583) were collected. Largest electron density residue: 0.293 e Å-3, R1 (for I>2σ(I)) = 0.0546 and wR2 = 0.1476 (all data).
HSC.
C29H52O3S, M = 480.77, orthorhombic, P212121, a = 7.688(1) Å, b = 11.080(1) Å, c = 33.401(1) Å, V = 2845.4(2) Å3, Z = 4, T = 193(2) K. 25,229 reflections (5,769 independent, Rint = 0.0301) were collected. Largest electron density residue: 0.270 e Å-3, R1 [for I> 2σ(I)] = 0.0372 and wR2 = 0.0955 (all data). The absolute structure parameter refined to 0.00(7) (29).
HAC.
C29H53NO3, M = 463.72, orthorhombic, P212121, a = 7.691(1) Å, b = 10.794(1) Å, c = 33.676(1) Å, V = 2795.7(2) Å3, Z = 4, T = 173(2) K. 28,335 reflections (4,710 independent, Rint = 0.0541) were collected. Largest electron density residue: 0.179 e Å-3, R1 [for I> 2σ(I)] = 0.0360 and wR2 = 0.0787 (all data).
Molecular modeling
The structure analysis and comparison between the three-dimensional (3D) structures obtained by X-ray analysis were done exactly as described previously with other compounds (30, 31). Superimposition of the structures of 5,6α-EC and 5,6β-EC was done by superimposing carbons of rings C and D of the steroid backbone (C8, C9, C14, and C17). Van der Waals volume analyses were done using the steroid core of 5,6-EC, excluding the isooctane side chain of superimposed 5,6-EC. The percentage corresponding to the difference in the van der Waals volumes of superimposed 5,6-EC was calculated by subtracting the van der Waals volumes of 5,6β-EC to 5,6α-EC and the ratio of this value with the van der Waals volume of 5,6α-EC. The total energy of 5,6α-EC, 5,6β-EC, and HAC was calculated using the structure obtained by X-ray analysis and the CVFF force field (InsightII, version 2000.1; Accelrys, San Diego, CA). The total energy of 5β-hydroxy-6α-[2-hydroxyethylamino]cholestan-3β-ol was calculated using the Discover calculation engine with the CVFF force field (InsightII). The Build module was used for the creation of the 5β-hydroxy-6α-[2-hydroxyethylamino]cholestan-3β-ol molecule, using the X-ray structure of 5,6β-EC as a starting point. The C5-O bond was deleted, and the O replaced by the N of the aminoethanol moiety. H6 was replaced by a hydroxyl group, and a new proton was added on C6 to complete the molecule. The resulting conformation was then minimized using 250 steps of the steepest descent method, followed by the conjugated gradient method until the root-mean-square gradient was less than 0.001 kcal/mol/Å. The CVFF force-field was used together with a distant-dependent dielectric term (ϵ = r) and a 20 Å nonbonded cut-off distance. The final energy of the system was compared with that of the solid-state structure of HAC. The conformations of the molecules were compared using the Search Compare module.
RESULTS
5,6α- and 5,6β-EC are unreactive toward nucleophiles in noncatalytic conditions, in contrast to SO
In the first set of experiments, we evaluated the reactivity of 5,6α- and 5,6β-EC toward nucleophiles and putative products of addition (Fig. 1A). AE, ME, G, and a complex cell CM were used as nucleophiles. AE contains a primary amine, ME contains a thiol group, and G contains two nucleophilic centers with an aromatic primary amine and a secondary aromatic amine. DMEM, the CM used for routine eukaryotic cell growth, contained about 13 mmol/l nucleophiles (mainly amino acids). The reactivity of the 5,6-EC was studied in protic solvents in which both reactants were soluble or directly without addition of solvent when testing CM. Thin layer chromatography was used to monitor the transformations of the 5,6-EC in conditions enabling their separation from putative transformation products. In the case of CM, we used [14C]5,6-EC and followed up their transformation by TLC and autoradiography. Table 1 shows that 5,6α-EC and 5,6β-EC did not react with nucleophiles after 48 h at room temperature or even at physiological temperature (37°C). Under the same conditions, the carcinogenic compound SO reacted with the tested nucleophiles. These data established that 5,6α-EC and 5,6β-EC are stable epoxide-bearing chemicals that are unreactive toward nucleophiles under our experimental conditions. This differs strongly from SO, which has alkylating properties that have been linked to carcinogenicity (32).
TABLE 1.
Evaluation of the alkylating properties 5,6α-EC, 5,6β-EC, and SO
| Compound | Solvent | Temperature | Nucleophile | Reaction |
| 5,6α-EC | Ethanol | RT | — | NR |
| Ethanol | RT | AE | NR | |
| Ethanol | RT | ME | NR | |
| Ethanol | RT | G | NR | |
| CM | RT | — | NR | |
| Ethanol | 37°C | — | NR | |
| Ethanol | 37°C | AE | NR | |
| Ethanol | 37°C | ME | NR | |
| Ethanol | 37°C | G | NR | |
| CM | 37°C | — | NR | |
| 5,6β-EC | Ethanol | RT | — | NR |
| Ethanol | RT | AE | NR | |
| Ethanol | RT | ME | NR | |
| Ethanol | RT | G | NR | |
| CM | RT | — | NR | |
| Ethanol | 37°C | — | NR | |
| Ethanol | 37°C | AE | NR | |
| Ethanol | 37°C | ME | NR | |
| Ethanol | 37°C | G | NR | |
| CM | 37°C | — | NR | |
| SO | Ethanol | RT | — | NR |
| Ethanol | RT | AE | + | |
| Ethanol | RT | ME | + | |
| Ethanol | RT | G | + | |
| C.M. | RT | — | + | |
| Ethanol | 37°C | — | NR | |
| Ethanol | 37°C | AE | + | |
| Ethanol | 37°C | ME | + | |
| Ethanol | 37°C | G | + | |
| CM | 37°C | — | + |
Compounds were incubated at a concentration of 8 mg/ml for 48 h in the absence or presence of AE, ME, or G at a concentration 0.32 mol/l or in complete growth CM for eukaryote cells at the indicated temperatures. The reaction was followed as described in Materials and Methods.
+, reaction; AE, 2-aminoethanol; CM, culture medium; G, guanine; ME, 2-mercaptoethanol; NR, no reaction, RT, room temperature; SO, styrene oxide.
5,6α-EC is the sole 5,6-EC diastereoisomer that reacts toward nucleophiles under catalytic conditions
Next, we tested whether [14C]5,6α-EC and [14C]5,6β-EC could react with AE and ME under catalytic conditions. When the reaction mixture was heated by refluxing in ethanol (79°C), 5,6α-EC reacted with AE or ME with a 2% and 7% yield, respectively, whereas no reaction was observed with 5,6β-EC under the same conditions (Table 2). The addition of a Lewis acid (LiClO4) did not catalyze the reaction at room temperature, but at 79°C there was a 25 and 53% yield of the products of transformation of 5,6α-EC with AE and ME respectively, showing a 12.5 and 7.5-fold increase in the reaction yield. Even in these conditions 5,6β-EC was unreactive (Table 2). These results highlight a difference in the reactivity of both diastereoisomers under catalytic conditions and established that both 5,6α-EC and 5,6β-EC are not reactive under noncatalytic conditions.
TABLE 2.
Measurement of the reactivity of 5,6α-EC and 5,6β-EC toward nucleophiles in catalytic conditions
| Compound | Solvent | Temperature | Catalyst | Nucleophile | Reaction | Yield |
| 5,6α-EC | Ethanol | RT | — | EA | NR | — |
| RT | — | ME | NR | — | ||
| 5,6β-EC | Ethanol | RT | — | EA | NR | — |
| RT | — | ME | NR | — | ||
| 5,6α-EC | Ethanol | 79°C | — | EA | + | 2% |
| 79°C | — | ME | + | 7% | ||
| 5,6β-EC | Ethanol | 79°C | — | EA | NR | — |
| 79°C | — | ME | NR | — | ||
| 5,6α-EC | Ethanol | RT | LiCl04 | EA | NR | — |
| RT | LiCl04 | ME | NR | — | ||
| 5,6β-EC | Ethanol | RT | LiCl04 | EA | NR | — |
| RT | LiCl04 | ME | NR | — | ||
| 5,6α-EC | Ethanol | 79°C | LiCl04 | EA | + | 25% |
| 79°C | LiCl04 | ME | + | 53% | ||
| 5,6β-EC | Ethanol | 79°C | LiCl04 | EA | NR | — |
| 79°C | LiCl04 | ME | NR | — |
[14C]5,6-EC were incubated at a concentration of 0.8 mg/ml for 48 h in the absence or presence of AE or ME at room temperature, or refluxed in ethanol (79°C) in the absence or presence of LiClO4.
+, reaction; AE, 2-aminoethanol; ME, 2-mercaptoethanol; NR, no reaction, RT, room temperature.
5,6α-EC gives 6β-substituted-5α-cholestan-3β,5α-diols by reaction with AE or ME under catalytic conditions
We next analyzed the structure of the products obtained through the ring opening of the oxirane group of 5,6α-EC following addition of AE or ME under catalytic conditions. The 3D solid-state structures of the products of addition of 5,6α-EC with EA, 5α-hydroxy-6β-[2-hydroxyethylamino]cholestan-3β-ol (HAC), and with ME, 5α-hydroxy-6β-[2-hydroxyethylsulfanyl]cholestan-3β-ol (HSC) (Fig. 1B), were determined by X-ray diffraction analysis. Both HAC and HSC are 6β-substituted-5α-cholestan-3β,5α-diols, indicating a trans-diaxial opening of the epoxide ring with AE and ME, reflecting an SN2 mechanism resulting from the regioselective attack of the nucleophile at C6 (Fig. 1C). Both HAC and HSC had an extended conformation with the four rings of the steroid backbone in a chair conformation with trans junctions in the A, B, C, and D rings. Analyses of the structure of HSC by NMR showed that spatial H-H interactions were estimated between protons from C19 and protons from C28 (from the hydroxyethyl-sulfanyl moiety) and between protons from C19 and protons from C6. Only an interaction between protons from C19 and one proton from C28 could be observed, which is in accordance with the 5α-hydroxy-6β-substituted products (supplementary Fig. I).
Analysis of the structure of 5,6-EC diastereoisomers
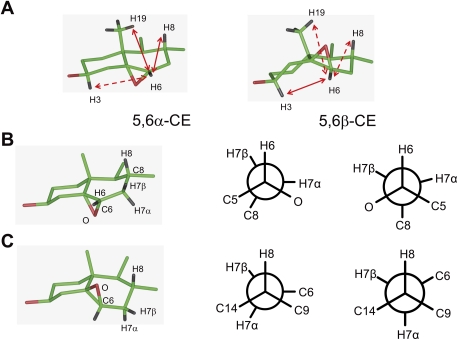
To determine whether the difference in the reactivity of 5,6-EC diastereoisomers was due to a difference in their conformation, we carried out structural analyses. Common two-dimensional (2D) representation of rings A and B of 5,6-EC epimers is presented in Fig. 2A (33, 34). 5,6-EC diastereoisomers were crystallized and their structures are shown in Fig. 2B. Rings A, C, and D are identical in both 5,6-EC and are in a chair conformation. Ring B of both 5,6-EC is twisted with a different distortion at the junction of rings A and B. In 5,6α-EC, the angle C1-C10-C9 is 110.91° and the angle C4-C5-C6 is 120.23°, whereas in 5,6β-EC these angles were 106.68° and 119.21°, respectively. The conformational differences are highlighted through the superposition of 5,6-EC (Fig. 2C). We measured a displacement of 0.68 Å of the oxygen from the hydroxyl in C3 and a displacement of 0.83 Å of the C19 methyl groups. The van der Waals volume of the steroid backbone of 5,6-EC was 241.84 Å3 for 5,6α-EC and 242.16 Å3 for 5,6β-EC. The difference in the van der Waals volume after superposition of 5,6-EC was 22.19 Å3, showing that 5,6α-EC and 5,6β-EC have 87% of their van der Waals volume in common; however, there was a 13% difference at the level of ring A of the methyl C19 and of the epoxide ring from 5,6α-EC (Fig. 2D). The calculation of the total energy of the 5,6-EC showed that 5,6β-EC is in a lower energy state and thus more stable than 5,6α-EC (ΔE = 15 kcal/mol). Measurement of dihedral angles at the junction of rings A and B showed different distortions around C7 and C10 (Table 3) between the 5,6-EC, but there were no significant differences in the length of C-O bonds and of the O-C6-C5 angles between the diastereoisomers. We then examined the conformations of 5,6-EC in solution through NOESY NMR experiments to study potential interactions between hydrogen atoms from rings A and B (supplementary Fig. I). In 5,6α-EC, the hydrogen H6 carried by the C6 and oriented toward the β-side of 5,6α-EC gave NOESY signals showing the interactions between the hydrogens from C8 and C19 (H8 and H19) (Fig. 3), which highlighted a similar conformation to that observed in the solid state (Fig. 2B). In 5,6β-EC, the hydrogen H6 is oriented toward the α-side of 5,6β-EC and interacts with the hydrogen H3 from C3 but not with H8 and H19, which is consistent with the bent conformation observed in the solid state (Fig. 2B). These data constitute the first experimental evidence of the existence of conformational differences between 5,6α-EC and 5,6β-EC. The calculation of the dihedral angles deduced from the measured coupling constants are given in Table 3. We observed less difference in the conformations of 5,6-EC in solution than that observed in the solid state, showing that the bending of rings at the A,B junction was also less in solution than in the solid state. Energy calculations on the solid state and the energy deduced from dihedral angle measurements in the liquid state showed that 5,6β-EC had a surprisingly lower energy state than 5,6α-EC. This results in a greater energy barrier for the transformation of 5,6β-EC under SN2 conditions (Fig. 3B, C). These results could explain a decrease, but not the lack of reactivity, of the 5,6β-EC toward nucleophiles if an attack occurred at C6: the optimal angle of attack for the opening of an epoxide is in the plane of the oxirane ring at 165° compared with the C-O bond to be opened (35). According to the rule of Furst and Plattner, the ring opening of an oxirane is mainly driven by the stability of the product of the reaction, which has to be in a trans-diaxial configuration (36). The calculated energy for 5β-Hydroxy-6α-[2-hydroxyethylamino]cholestan-3β-ol is 96 kcal/mol, establishing an increase of 11 kcal/mol compared with 5α-hydroxy-6β-[2-hydroxyethylamino]cholestan-3β-ol (85 kcal/mol). Taken together, the lower energy of 5,6β-EC combined with the higher energy of its product of aminolysis could explain the lack of reactivity of this diastereoisomer in our conditions.
Fig. 2.
(A) Commonly used 2D representation of rings A and B of 5,6α-EC and 5,6β-EC. (B) Solid-state structures of 5,6α-EC and 5,6β-EC as established by X-ray analysis and 2D representation of rings A and B of both diastereoisomers. (C) Superimposition of 5,6α-EC (orange) and 5,6β-EC (blue). Superposition was done using the Search Compare module of Insight II (Accelrys Inc., CA). (D) The van der Waals volume of 5,6α-EC and 5,6β-EC (excluding the isodecane side chain) are 241.84 Å3 and 242.16 Å3, respectively. The difference between the van der Waals volumes of 5,6-EC (depicted in yellow) is 22.19 Å3, showing that 5,6α-EC and 5,6β-EC have 87% of their van der Waals volume in common and 13% difference.
TABLE 3.
Comparison of torsion angles of 5,6-EC diastereoisomers in the solid state and in solution
| Compound | Torsion Angle | Measured Angle | 3JH-H | Calculated Angle |
| Solid state | Hz | Solution | ||
| 5,6α-EC | C3-C4-C5-C6 | −143 | ||
| C4-C5-C6-C7 | −158.32 | |||
| C2-C1-C10-C9 | 172.76 | |||
| C1-C10-C9-C8 | −171 | |||
| H6-C6-C7-H7α | 88 | 0 | 90 | |
| H6-C6-C7-H7β | −28 | 4.5 | −41 | |
| H7α-C7-C8-H8 | −149 | 9.45 | −148 | |
| H7β-C7-C8-H8 | −32 | 6.0 | −42 | |
| 5,6β-EC | C3-C4-C5-C6 | −108 | ||
| C4-C5-C6-C7 | −159.10 | |||
| C2-C1-C10-C9 | 171.95 | |||
| C1-C10-C9-C8 | −142 | |||
| H6-C6-C7-H7α | 61 | 0.6 | 78 | |
| H6-C6-C7-H7β | −57 | 3.45 | −63 | |
| H7α-C7-C8-H8 | −176 | 10.95 | −159 | |
| H7β-C7-C8-H8 | −58 | 3.45 | −56 |
For solid-state data, angles were measured using the measure tool from InsightII software (Accelrys, Inc., CA). 3JH-H were determined from 1H-NMR analysis of the molecule in MeOD, and angles were calculated using the online interactive data table. When 3JH-H corresponded to two or more possible angles, the closest one (between −90 and +90 degrees from the solid-state angle) was chosen.
Fig. 3.
Determination of 5,6α-EC and 5,6β-EC conformation in solution using 1D and 2D-NMR. (A) NOE interactions of proton on carbon 6 (H6) for 5,6α-EC (left) and 5,6β-EC (right). Plain arrows represent spatial interactions effectively found, and dashed arrows represent interactions not found. (B) 3D (left) and Newman's representations (center and right) of ring B of 5,6α-EC. Newman representations are made along the C6-C7 (center) or C8-C7 (right) bond. They show that the carbons of the B ring are in a synperiplanar conformation. (C) 3D (left) and Newman's representations (center and right) of the B ring of 5,6β-EC. Newman representations are made along the C6-C7 (center) or C8-C7 (right) bond.
Conclusions
In this work, we report that under normal SN2 conditions, 5,6-EC were not reactive toward nucleophiles. This is in contrast to SO, which was reactive in the tested conditions and which is well-known as a weak, directly mutagenic and carcinogenic substance. The reactivity of SO is due to its alkylating properties on nucleophiles found in biomolecules (37). We demonstrated that the oxirane group present on the C5-C6 position of each diastereoisomer of 5,6-EC is exceptionally stable toward nucleophiles, including guanine, showing that 5,6-EC are not spontaneous alkylating substances. This ruled out a direct mutagenic and carcinogenicity potency of 5,6-EC in mammals. We next established that 5,6α-EC can react at C6 with nucleophiles only under catalytic conditions and through a trans-diaxial ring opening, leading to 6β-substituted-5α-cholestan-3β,5α-diols. In that case the hydroxyl at C5 is in an anti position with regard to the C19 methyl. In the case of 5,6β-EC, no reaction occurred because i) the product of trans-diaxial opening at C6 would have given an energetically unfavorable constrained structure with the hydroxyl at C5 in the syn position with regard to the C19 methyl, and ii) no attack occurred at C5 for the same reason reported above for 5,6α-EC. Structure analyses of the 5,6-EC diasteroisomers showed a less pronounced distortion than expected and usually depicted between rings A and B in 5,6β-EC, in which the A-B ring junction is cis (33, 34).
The main difference in the structure of the rigid steroid backbone of 5,6-EC diastereoisomers occurred at the level of the ring A-B junction and showed differences in the positioning of the O of the hydroxyl on C3 and of the C19 methyl, in addition to differences due to the presence of the oxirane ring of the α and the β sides of the 5,6-EC. These elements could represent structural motifs responsible for discrimination by enzymes and receptor targets of the 5,6-EC.
Although cholesterol was shown to have antioxidant properties because of the sensitivity of its double bond to oxidation (38), we showed here that the oxidation product at Δ5, such as in 5,6-epoxides, is also a possible route for stereoselective transformations. We show that 5,6-EC have a restricted possibility of transformation from one diastereoisomer to obtain one product through a SN2 mechanism, allowing a stereoselectivity in the reactants as well as in the products of the reaction as only one (5,6α-EC) of the two 5,6-EC diastereoisomers gave a reaction and only one out four possible theoretical products was obtained through a SN2 mechanism.
This delineates an attractive mechanism for the stereoselective transformation of 5,6-EC. These data suggest that if an enzyme could catalyze such stereoselective biotransformations, it would give 6β-substituted-5α-cholestan-3β,5α-diol. This hypothesis is supported by the fact that 5,6α-EC was shown to be selectively transformed by glutathione to give 3β,5α-dihydroxycholestan-6β-yl-S-glutathione (22, 23) and to produce 5α-hydroxy-6β-[2-(1H-imidazol-4-yl)ethylamino] cholestan-3β-ol (dendrogenin A) in mammalian tissues (de Medina et al., unpublished data). In a previous study, we reported that the products of the catalytic aminolysis of 5,6α-EC with polyamines such as spermidine and spermine had potent neurodifferentiation properties (39), showing that 5,6α-EC could control the production of other putative metabolites and thus justifying the existence of a metabolic pathway centered on 5,6α-EC. The fact that the Δ5 double bond of cholesterol drives a stereospecific process of transformation supports previous studies underlining the importance of the Δ5 double bond of cholesterol. These studies established that the Δ5 was required for proliferation (40), whereas genetic studies have shown that the null mutation on the gene encoding lathosterol oxidase, which catalyses the formation of Δ5 to give cholesterol from lathosterol, led to a malformation syndrome known as lathosterolosis associated with pre- or postnatal death (41).
Supplementary Material
Acknowledgments
The authors thank the members of the European Network on Oxysterol Research (ENOR) for informative discussion.
Footnotes
Abbreviations:
- 2/3D
- two/three dimensional
- 5,6α-EC
- 5,6α-epoxy-cholesterol (common name) or 5,6α-epoxy-5α-cholestan-3β-ol (systematic name)
- 5,6β-EC
- 5,6β-epoxy-cholesterol (common name) or 5,6β-epoxy-5β-cholestan-3β-ol (systematic name)
- AE
- 2-aminoethanol
- AEBS
- microsomal antiestrogen binding site
- ChEH
- cholesterol-5,6-epoxide hydrolase
- CM
- culture medium
- CT
- cholestane-3β,5α,6β-triol
- G
- guanine
- HAC
- 5α-hydroxy-6β-[2-hydroxyethylamino]-cholestan-3β-ol
- HSC
- 5α-hydroxy-6β-[2-hydroxyethylsulfanyl]-cholestan-3β-ol
- LXR
- liver X receptor
- ME
- 2-mercaptoethanol
- SN2
- second-order nucleophilic substitution
- SO
- styrene oxide
- SULT2B1b
- cholesterol sulfotransferase
This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Conseil Regional Midi-Pyrénées, and the Ministère Français de la Recherche et de l'Enseignement Supérieur through the GenHomme. S.S.-P. is in charge of research at the Centre National de la Recherche Scientifique.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
REFERENCES
- 1.Newman J. W., Morisseau C., Hammock B. D. 2005. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog. Lipid Res. 44: 1–51 [DOI] [PubMed] [Google Scholar]
- 2.de Medina P., Paillasse M. R., Segala G., Poirot M., Silvente-Poirot S. 2010. Identification and pharmacological characterization of cholesterol-5,6-epoxide hydrolase as a target for tamoxifen and AEBS ligands. Proc. Natl. Acad. Sci. USA. 107: 13520–13525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan V. C. 2007. SERMs: meeting the promise of multifunctional medicines. J. Natl. Cancer Inst. 99: 350–356 [DOI] [PubMed] [Google Scholar]
- 4.Van Herendael H., Dorian P. 2010. Amiodarone for the treatment and prevention of ventricular fibrillation and ventricular tachycardia. Vasc. Health Risk Manag. 6: 465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marques L. O., Lima M. S., Soares B. G. 2004. Trifluoperazine for schizophrenia. Cochrane Database Syst. Rev. CD003545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Medina P., Silvente-Poirot S., Poirot M. 2009. Tamoxifen and AEBS ligands induced apoptosis and autophagy in breast cancer cells through the stimulation of sterol accumulation. Autophagy. 5: 1066–1067 [DOI] [PubMed] [Google Scholar]
- 7.de Medina P., Payre B., Boubekeur N., Bertrand-Michel J., Terce F., Silvente-Poirot S., Poirot M. 2009. Ligands of the antiestrogen-binding site induce active cell death and autophagy in human breast cancer cells through the modulation of cholesterol metabolism. Cell Death Differ. 16: 1372–1384 [DOI] [PubMed] [Google Scholar]
- 8.Payré B., de Medina P., Boubekeur N., Mhamdi L., Bertrand-Michel J., Terce F., Fourquaux I., Goudouneche D., Record M., Poirot M., et al. 2008. Microsomal antiestrogen-binding site ligands induce growth control and differentiation of human breast cancer cells through the modulation of cholesterol metabolism. Mol. Cancer Ther. 7: 3707–3718 [DOI] [PubMed] [Google Scholar]
- 9.Martinez G. R., Loureiro A. P., Marques S. A., Miyamoto S., Yamaguchi L. F., Onuki J., Almeida E. A., Garcia C. C., Barbosa L. F., Medeiros M. H., et al. 2003. Oxidative and alkylating damage in DNA. Mutat. Res. 544: 115–127 [DOI] [PubMed] [Google Scholar]
- 10.Nielsen L. P. C., Jacobsen E. N.2006. Catalytic asymmetric epoxide ring-opening chemistry. In Aziridines and Epoxide in Organic Chemistry. A. K. Yudin, editor. Wiley-VCH Verlag, Weinheim. 229–269.
- 11.Kolodiazhnyi O. I. 2003. Multiple stereoselectivity and its application in organic synthesis. Tetrahedron. 59: 5953–6018 [Google Scholar]
- 12.Parker R. E., Isaacs N. S. 1959. Mechanisms of epoxide reactions. Chem. Rev. 59: 737–799 [Google Scholar]
- 13.Marnett L. J., Riggins J. N., West J. D. 2003. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Invest. 111: 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koskinen M., Plná K. 2000. Specific DNA adducts induced by some mono-substitued epoxides in vitro and in vivo. Chem. Biol. Interact. 129: 209–229 [DOI] [PubMed] [Google Scholar]
- 15.Schroepfer G. J., Jr 2000. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 80: 361–554 [DOI] [PubMed] [Google Scholar]
- 16.Watabe T., Sawahata T. 1979. Biotransformation of cholesterol to cholestane-3beta,5alpha,6beta-triol via cholesterol alpha-epoxide (5alpha,6alpha-epoxycholestan-3beta-ol) in bovine adrenal cortex. J. Biol. Chem. 254: 3854–3860 [PubMed] [Google Scholar]
- 17.Zhang Y., Yu C., Liu J., Spencer T. A., Chang C. C., Chang T. Y. 2003. Cholesterol is superior to 7-ketocholesterol or 7 alpha-hydroxycholesterol as an allosteric activator for acyl-coenzyme A:cholesterol acyltransferase 1. J. Biol. Chem. 278: 11642–11647 [DOI] [PubMed] [Google Scholar]
- 18.Fuda H., Javitt N. B., Mitamura K., Ikegawa S., Strott C. A. 2007. Oxysterols are substrates for cholesterol sulfotransferase. J. Lipid Res. 48: 1343–1352 [DOI] [PubMed] [Google Scholar]
- 19.Argmann C. A., Edwards J. Y., Sawyez C. G., O'Neil C. H., Hegele R. A., Pickering J. G., Huff M. W. 2005. Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: a role for RhoA in ABCA1-mediated cholesterol efflux. J. Biol. Chem. 280: 22212–22221 [DOI] [PubMed] [Google Scholar]
- 20.Song C., Hiipakka R. A., Liao S. 2001. Auto-oxidized cholesterol sulfates are antagonistic ligands of liver X receptors: implications for the development and treatment of atherosclerosis. Steroids. 66: 473–479 [DOI] [PubMed] [Google Scholar]
- 21.Berrodin T. J., Shen Q., Quinet E. M., Yudt M. R., Freedman L. P., Nagpal S. 2010. Identification of 5alpha, 6alpha-epoxycholesterol as a novel modulator of liver X receptor activity. Mol. Pharmacol. 78: 1046–1058 [DOI] [PubMed] [Google Scholar]
- 22.Watabe T., Sawahata T., Horie J. 1979. Evidence for the formation of a steroid S-glutathione conjugate from an epoxysteroid precursor. Biochem. Biophys. Res. Commun. 87: 469–475 [DOI] [PubMed] [Google Scholar]
- 23.Meyer D. J., Ketterer B. 1982. 5 alpha,6 alpha-Epoxy-cholestan-3 beta-ol (cholesterol alpha-oxide): a specific substrate for rat liver glutathione transferase B. FEBS Lett. 150: 499–502 [DOI] [PubMed] [Google Scholar]
- 24.Chan J. T., Black H. S. 1974. Skin carcinogenesis: cholesterol-5alpha,6alpha-epoxide hydrase activity in mouse skin irradiated with ultraviolet light. Science. 186: 1216–1217 [DOI] [PubMed] [Google Scholar]
- 25.Lo W., Black H. S. 1973. Inhibition of carcinogen formation in skin irradiated with ultraviolet light. Nature. 246: 489–491 [DOI] [PubMed] [Google Scholar]
- 26.el-Bayoumy K., Ji B. Y., Upadhyaya P., Chae Y. H., Kurtzke C., Rivenson A., Reddy B. S., Amin S., Hecht S. S. 1996. Lack of tumorigenicity of cholesterol epoxides and estrone-3,4-quinone in the rat mammary gland. Cancer Res. 56: 1970–1973 [PubMed] [Google Scholar]
- 27.Sheldrick G. M. 1990. Phase annealing in SHELX-90: direct methods for larger structures. Acta Crystallogr. A. 46: 467–473 [Google Scholar]
- 28.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 29.Flack H. D. 1983. On enantiomorph-polarity estimation. Acta Crystallogr. A. 39: 876–881 [Google Scholar]
- 30.de Medina P., Genovese S., Paillasse M. R., Mazaheri M., Caze-Subra S., Bystricky K., Curini M., Silvente-Poirot S., Epifano F., Poirot M. 2010. Auraptene is an inhibitor of cholesterol esterification and a modulator of estrogen receptors. Mol. Pharmacol. 78: 827–836 [DOI] [PubMed] [Google Scholar]
- 31.de Medina P., Boubekeur N., Balaguer P., Favre G., Silvente-Poirot S., Poirot M. 2006. The prototypical inhibitor of cholesterol esterification, Sah 58–035 [3-[decyldimethylsilyl]-n-[2-(4-methylphenyl)-1-phenylethyl]propanamide], is an agonist of estrogen receptors. J. Pharmacol. Exp. Ther. 319: 139–149 [DOI] [PubMed] [Google Scholar]
- 32.Bond J. A. 1989. Review of the toxicology of styrene. Crit. Rev. Toxicol. 19: 227–249 [DOI] [PubMed] [Google Scholar]
- 33.Murphy R. C., Johnson K. M. 2008. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. J. Biol. Chem. 283: 15521–15525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massey J. B., Pownall H. J. 2006. Structures of biologically active oxysterols determine their differential effects on phospholipid membranes. Biochemistry. 45: 10747–10758 [DOI] [PubMed] [Google Scholar]
- 35.Benedetti F., Berti F., Fabrissin S., Gianferrara T. 1994. Intramolecular ring opening of epoxides by bis-activated carbanions. The influence of ring size on reactivity and selectivity. J. Org. Chem. 59: 1518–1524 [Google Scholar]
- 36.Furst A., Plattner P. A. 1949. Uber steroide und sexual hormone. Helv. Chim. Acta. 32: 275–283 [DOI] [PubMed] [Google Scholar]
- 37.Jágr M., Mraz J., Linhart I., Stransky V., Pospisil M. 2007. Synthesis and characterization of styrene oxide adducts with cysteine, histidine, and lysine in human globin. Chem. Res. Toxicol. 20: 1442–1452 [DOI] [PubMed] [Google Scholar]
- 38.Smith L. L. 1991. Another cholesterol hypothesis: cholesterol as antioxidant. Free Radic. Biol. Med. 11: 47–61 [DOI] [PubMed] [Google Scholar]
- 39.de Medina P., Paillasse M. R., Payre B., Silvente-Poirot S., Poirot M. 2009. Synthesis of new alkylaminooxysterols with potent cell differentiating activities: identification of leads for the treatment of cancer and neurodegenerative diseases. J. Med. Chem. 52: 7765–7777 [DOI] [PubMed] [Google Scholar]
- 40.Xu F., Rychnovsky S. D., Belani J. D., Hobbs H. H., Cohen J. C., Rawson R. B. 2005. Dual roles for cholesterol in mammalian cells. Proc. Natl. Acad. Sci. USA. 102: 14551–14556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krakowiak P. A., Wassif C. A., Kratz L., Cozma D., Kovarova M., Harris G., Grinberg A., Yang Y., Hunter A. G., Tsokos M., et al. 2003. Lathosterolosis: an inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency. Hum. Mol. Genet. 12: 1631–1641 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.