Abstract
Plant sterols such as sitosterol and campesterol are frequently administered as cholesterol-lowering supplements in food. Recently, it has been shown in mice that, in contrast to the structurally related cholesterol, circulating plant sterols can enter the brain. We questioned whether the accumulation of plant sterols in murine brain is reversible. After being fed a plant sterol ester-enriched diet for 6 weeks, C57BL/6NCrl mice displayed significantly increased concentrations of plant sterols in serum, liver, and brain by 2- to 3-fold. Blocking intestinal sterol uptake for the next 6 months while feeding the mice with a plant stanol ester-enriched diet resulted in strongly decreased plant sterol levels in serum and liver, without affecting brain plant sterol levels. Relative to plasma concentrations, brain levels of campesterol were higher than sitosterol, suggesting that campesterol traverses the blood-brain barrier more efficiently. In vitro experiments with brain endothelial cell cultures showed that campesterol crossed the blood-brain barrier more efficiently than sitosterol. We conclude that, over a 6-month period, plant sterol accumulation in murine brain is virtually irreversible.
Plant sterols differ structurally from cholesterol by an additional methyl or ethyl group at C24 and/or a double bond at C22 (Δ22). The most prevalent plant sterols are campesterol (methyl group at C24), sitosterol (ethyl group at C24), brassicasterol (methyl group at C24, Δ22), and stigmasterol (ethyl group at C24, Δ22) (1, 2). In contrast to cholesterol, these sterols are exclusively derived from the diet and cannot be synthesized endogenously in mammals. A high plant sterol intake (2–2.5 g/day) leads to reduced total and LDL-cholesterol (∼12%) in the circulation (3, 4). Therefore, plant sterols are frequently applied as functional, nonprescription food additives to prevent atherosclerosis and cardiovascular diseases (5). However, administration of high-dose plant sterols results in increased serum plant sterol concentrations (6). Hard end-point studies showing an effect on the number of cardiovascular events or on mortality after long-term intake of high-dose plant sterols are lacking (7), as are insights into the underlying molecular mechanisms (8). Because adverse events upon plant sterol administration in animal studies are increasingly being reported to cause adverse events (9), it is a current topic of debate (10).
In contrast to peripheral tissues, all cholesterol within the central nervous system is synthesized in situ because circulating cholesterol is not able to cross the blood-brain barrier (BBB) (11, 12). Recently, we reported that circulating plant sterols, in contrast to cholesterol, can enter the brains of ATP binding cassette g5 (Abcg5)-deficient mice, a model for phytosterolemia (13). ABCG5 and ABCG8 act as functional heterodimer transporters at the apical membranes of enterocytes and hepatocytes, where they excrete plant sterols into the intestinal lumen and bile, respectively (14). However, some plant sterols still end up in the serum, and small amounts reach the brain.
Accumulating evidence suggests a key role for disturbed brain cholesterol homeostasis in neurodegenerative diseases such as Alzheimer's disease (15–17). A reduction in total membrane cholesterol concentrations, particularly in lipid rafts, has been shown to reduce the production of amyloid-β by disrupting cleavage of its larger amyloid precursor protein (APP) by the γ-secretase complex (18, 19). Consequently, mild cholesterol depletion impairs APP trafficking to the membrane, making it less prone to cleavage within the lipid rafts or detergent-resistant membranes (DRMs) (18). Moreover, processing of APP into amyloid β requires cholesterol-rich DRM microdomains (20). Plant sterols are known to be organized in lipid rafts within plant cell membranes, but effects on the processing of APP remain to be established (21–23). In the present study, we first assessed if plant sterols that had accumulated in the brain are secreted into the circulation by depleting the blood of plant sterols by feeding mice a plant stanol-enriched, plant sterol-depleting diet. Subsequently, we investigated the redistribution of plant sterols across membranes of different brain cell types.
MATERIALS AND METHODS
Animals
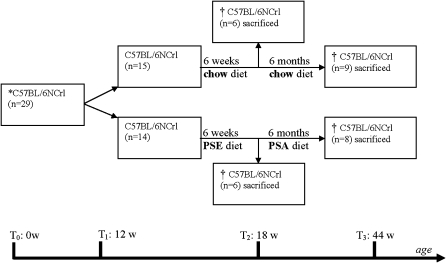
All animal experiments were approved by the local ethical committee for animal experiments of the Universitätsklinikum des Saarlandes according to institutional and governmental guidelines under project ID K 110/180-07 19/05. C57BL/6NCrl male mice were purchased from Charles River Laboratories GmbH (Sulzfeld, Germany). At 12 weeks (time point 1), mice (n = 29) were randomly divided into two groups (Fig. 1). One group was fed a 2% (w/w) plant sterol ester (PSE)-enriched diet (60% sitosterol, 30% campesterol, and 10% stigmasterol; n = 14), and the other group was fed a control diet (containing 0.015% (w/w) plant sterol esters: 65% sitosterol, 30% campesterol, and 5% stigmasterol). Both diets contained 0.0015% (w/w) plant stanol esters (55% sitostanol and 45% campestanol; n = 15). After 6 weeks of diet, six animals per group were euthanized (time point 2). Next, the remaining animals in the group previously fed a PSE-enriched diet (n = 8) were fed a 1.5% (w/w) plant stanol ester (PSA)-enriched diet (85% sitostanol, 15% campestanol). The PSA-enriched diet was administered to inhibit intestinal sterol uptake and to almost completely deplete the serum of plant sterols. The diet of the remaining animals in the control group (n = 9) remained unchanged for 6 months (time point 3; T3). All animals were euthanized at the end of the experiment with an overdose of ketamine (1 g/kg body weight) and xylazine (100 mg/kg/body weight). Liver and brain hemispheres were snap frozen in liquid nitrogen and stored at −80°C before further analysis. Blood was allowed to coagulate at room temperature for 1 h. Serum was obtained by centrifugation at 4°C for 10 min at 200 g. All dietary supplemented sterols and stanols (Raisio Research Laboratories, Finland) were mixed in standard rodent chow (Ssniff Spezialdiäten GmbH, Soest, Germany). Dietary sterol concentrations were verified by GC-MS.
Fig. 1.
Experimental scheme of C57BL/6NCrl feeding experiment. At 12 weeks (T1), C57BL/6NCrl (n = 29) were randomly subdivided into two groups fed a regular chow diet (n = 14) or a PSE-enriched diet (n = 15). After 6 weeks (T2), six animals per group were euthanized. The remaining animals on the regular chow diet (n = 9) continued to be fed the normal chow diet. The remaining mice from the PSE group (n = 8) were then fed a PSA-enriched diet. After 6 months of the second feeding term (T3), all animals were euthanized.
Chemical reagents
d6-Sitosterol/d6-campesterol (55–45%) (Sugaris GmbH, Münster, Germany), d6-cholesterol (99.0%) (CDN isotopes, Pointe-Claire, Quebec, Canada), and epi-coprostanol (99.9%) (Sigma-Aldrich Chemie, Steinheim, Germany) were quantified and tested for purity against 5α-cholestane (1 µg/µl) (Serva Electrophoresis GmbH, Heidelberg, Germany) using gas chromatography-flame ionization detection and GC-MS scan methods. Sterols were dissolved in 100% ethanol to obtain 10 mM stock solutions.
Cell culture experiments
Human astrocytoma CCF-STTG1 and human neuroblastoma SHSY5Y cell lines, purchased from the European Collection of Cell Cultures (CACC, Salisbury, UK), were cultured in 10% FCS (EU approved origin; Gibco, Invitrogen GmbH, Karlsruhe, Germany) containing 1:1 DMEM and Ham's F-12 Nutrient mixture (DMEM/F-12) (Gibco, Invitrogen GmbH, Karlsruhe, Germany) including 100 units penicillin and 100 µg streptomycin per milliliter (pen/strep from Gibco, Invitrogen GmbH, Karlsruhe, Germany). Immortalized human adult brain endothelial cells (hCMEC/D3 cells) were obtained from Weksler et al. (24). OLN-93 cells (oligodendroglioma cells derived from primary rat brain glial cultures) were kindly provided by Dr. Richter-Landsberg (25). Primary astrocytes were isolated from human brain as described (26) and applied in the experiments between passage 25 and 30.
In vitro BBB model
The human brain endothelial cell line (hCMEC/D3) was used as an in vitro BBB model (24). hCMEC/D3 cells display predominant features of BBB endothelial cells, including reduced paracellular permeability. hCMEC/D3 were seeded on collagen-coated Costar Transwell filters (pore size 0.4 µm; Corning Incorporated) in growth medium containing 2.5% FCS and were cultured for 4 days (200 µl medium in the apical compartment, 800 µl in the basolateral compartment) (24). Subsequently, 15 µM d6-sitosterol/d6-campesterol or d6-cholesterol was added to the apical compartment (blood side) of the Transwell filter setup and incubation was continued for 24 h. Cellular sterol uptake (%) was calculated as [(d6-sterols in endothelial cells) + (d6-sterols at brain side)]/[(d6-sterols at blood side) + (d6-sterols at brain side) + (d6-sterols in endothelial cells)] × 100%. Basolateral flux (%) was calculated as [d6-sterols at brain side]/[(d6-sterols at brain side) + (d6-sterols in endothelial cells)] × 100%. D6-sterols present in astrocytes were included in the “brain side” for calculations.
Astrocyte-conditioned medium has been shown to enhance maturation of endothelial cells, further strengthening endothelial tight junctions and restricting the permeability of the endothelial monolayer, thus improving the BBB model (27). Therefore, the trans-endothelial flow experiment was repeated with primary astrocytes cultured to confluency at the bottom of the basolateral compartment in the endothelial-Transwell setup. D6-sterols were measured after sterol extraction by applying GC-MS analysis.
To show that addition of sterols did not influence cellular permeability, permeability was measured as described previously (27). In short, after 23 h of culturing and 1 h before harvesting of the cells, FITC-coupled dextran (150 kDa, 1 µg/µl in culture medium; Sigma-Aldrich) was added to the apical compartment as described previously (28). One hour later, a 10 µl sample was collected from the apical and basolateral compartment for measurement of fluorescence intensity using a FLUOstar Galaxy microplate reader (BMG Labtechnologies; excitation 485 nm and emission 520 nm, at gain 64). The average 1 h flux of FITC-dextran through the endothelial monolayer was calculated, and extremes were excluded from further analyses based on Dixon's principles of exclusion for extreme values (29, 30).
Viability of the hCMEC/D3 cells, determined by water-soluble tetrazolium assay, was found to be unaltered after incubation with different concentrations of d6-sitosterol/d6-campesterol or d6-cholesterol (0, 5, 10, 15, 20, 25, and 30 µM) after different incubation times (6, 24, and 48 h) (data not shown).
Efflux studies
CCF-STTG1 (astrocytes), SHSY5Y (neurons), and OLN-93 (oligodendrocytes) cells were cultured in DMEM/F12 medium (Gibco, Invitrogen GmbH, Karlsruhe, Germany) supplemented with 10% FCS until 90% confluency. After washing cells with PBS, cells were incubated with medium containing 0.5% FCS and 15 µM d6-cholesterol or d6-sitosterol/d6-campesterol in the presence of 1 µM T0901317, a synthetic Liver X Receptor-agonist that is known to enhance cholesterol efflux from cells (31). After 24 h, the medium was discarded. Cells were washed thrice with PBS. Next, serum-free medium containing 25 µg/ml HDL (isolated as described previously[(32] from pooled serum of four healthy individuals) as a sterol-acceptor or vehicle control (0.2% BSA in PBS) was added, and cells were incubated 6 h in the presence of 1 µM T0901317. Medium and cells were collected separately and analyzed by GC-MS for d6-cholesterol and d6-sitosterol/d6-campesterol content, respectively.
DRM isolation from confluent cell lines
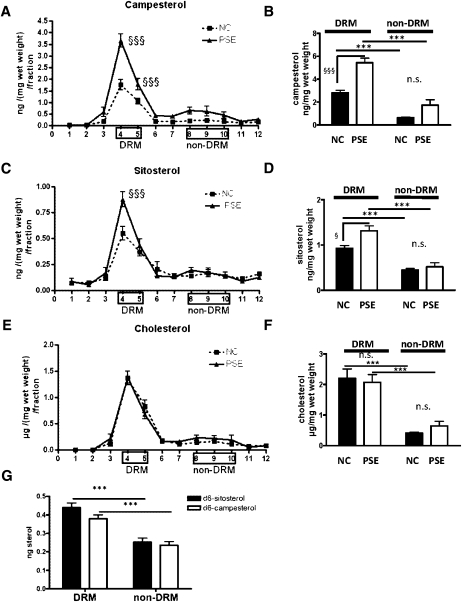
SHSY5Y cells were cultured in T-75 flasks until confluency in medium containing 10% FCS. Cells were then incubated in medium containing 0.5% FCS and 15 µM of d6-sitosterol/d6-campesterol, d6-cholesterol (10 mM stock), or vehicle (100% ethanol) for 24 h. Lipid rafts or DRMs were isolated as described previously (33). In brief, cells were washed twice with ice-cold PBS. Next, cells were scraped in 1.5 ml ice-cold TNE buffer (25 mM Tris, pH 7.4, 150 mM NaCl, and 2 mM EDTA) containing complete protease inhibitor mix (Roche Diagnostics GmbH, Mannheim, Germany) and disrupted by 15 strokes through a 25-G needle. Nuclei were precipitated by low-speed centrifugation (20 min, 1,000 g, 4°C) and discarded. Membranes were precipitated from the supernatant by high-speed centrifugation (90 min, 17,000 g, 4°C). The membrane pellet was dissolved in 1.5 ml 1% CHAPSO (Roche Diagnostics GmbH, Mannheim, Germany) containing complete protease inhibitor mix and incubated on ice for 30 min. The CHAPSO cell extracts were mixed with 2.5 ml TNE buffer containing 72% sucrose to yield a final concentration of 45% (w/v) sucrose and placed at the bottom of 12 ml Beckman ultracentrifuge tubes. TNE buffer (4 ml) containing 35% and 5% sucrose were layered on top of the CHAPSO cell extracts. The samples were spun at 4°C for 19 h at 40,000 rpm in a SW41 TI rotor (Beckman Coulter GmbH, Krefeld, Germany). Fractions (1 ml) were collected from top to bottom to yield a total of 12 fractions. The low buoyant DRM fraction was positioned at the interface between the 35% and 5% sucrose as amorphous white material visible to the naked eye. It was collected in fractions 4 and 5. DRMs (density: 1.09–1.13 g/cm3 34]) were confirmed by densitometry using a DMA48 density meter (Anton Paar GmbH, Graz, Austria) and by flotillin-1 expression on Western blotting, and cholesterol content was determined by GC/MS (see Fig. 4E, F). The nonDRM fractions were confirmed to be in fractions 8–10. All separate fractions were subjected to sterol extraction preceding GC-MS analysis.
Fig. 4.
Plant sterol concentrations in homogenates and DRM fractions of murine brains with or without plant sterol or plant stanol administration. Twelve week old C57BL/6NCrl mice were fed NC (n = 15) or a PSE chow (n = 14). After 6 weeks, six animals per group were euthanized, and sitosterol (A), campesterol (B), and cholesterol (C) concentrations were determined in 12 × 1 ml (top-bottom) density fractions of the membranes (ng/fraction/mg wet weight). As depicted in the Materials and Methods section, membrane fractions were subdivided into DRMs (fractions 4 and 5) and nonDRMs (fractions 8, 9, and 10). Sitosterol (B), campesterol (D), and cholesterol (F) DRM and nonDRM fraction masses were added together. PSE administration leads to significantly elevated plant sterol concentrations in DRMs but not in nonDRM regions of the brain membranes. The increased accumulation of campesterol and sitosterol in DRMs compared with nonDRMs was validated by 24 h administration (n = 5) of a 10 µM d6-sitosterol/d6-campesterol (55–45%) mixture to the SHSY5Y neuroblastoma cell line (G). * DRM compared with nonDRM. § PSE compared with NC-fed C57BL/6/6NCrl mice. § P < 0.05. §§§,*** P < 0.001. n.s., not significant.
Preparation of total membrane fraction of mouse brain hemispheres
Snap frozen wet mouse brain hemispheres were weighed (mg) before homogenization as described previously (35), and 1.5 ml homogenization buffer (150 mM NaCl, 20 mM Na2HPO4, 2 mM NaH2PO4, 1 mM EDTA, and 20% (v/v) glycerol, pH 7.4) was added to each hemisphere (36). Two cycles of 30 s at 6,500 rpm in the Precellys24 homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) were run, separated by 5 min of cooling on ice. Total homogenate was used to measure protein concentrations (Biorad DC protein assay kit). After homogenization, the samples were diluted 1:4 in homogenization buffer and centrifuged at 5,000 g for 20 min. The pellet was discarded, and the supernatant was centrifuged for 90 min at 17,000 g. The resultant membrane pellet was resuspended in 1.5 ml TNE buffer containing 1% CHAPSO (Roche Diagnostics GmbH, Mannheim, Germany) and incubated on ice for 30 min. DRMs were isolated by density gradient centrifugation and characterized as described above for the confluent cell lines.
Sterol profile determination
Wet weights of hemispheres and protein concentrations of brain homogenates were determined to relate sterol content to wet weight or protein concentration. Sterol contents of fractions generated from brain homogenates, total brain homogenates, cell lysates, fractions generated by sucrose gradient ultracentrifugation, total medium, and cell lysates from the efflux experiment were determined by GC-MS as previously described (37, 38).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 4™. The sterol data in serum, liver, and brain were analyzed using one-way ANOVA with a post hoc Bonferroni's Multiple Comparison Test. Extreme values were excluded by means of Dixon's principles of exclusion of extreme values (29, 30). Membrane fraction sterol concentrations were compared by two-way ANOVA with fraction and treatment as variable factors (post hoc Bonferroni's Multiple Comparison Test). Significance levels were determined on #,§,* P < 0.05, ##,§§,** P < 0.01, or ###,§§§,*** P < 0.001.
RESULTS
Dietary administration of plant sterols stably increased brain plant sterol levels
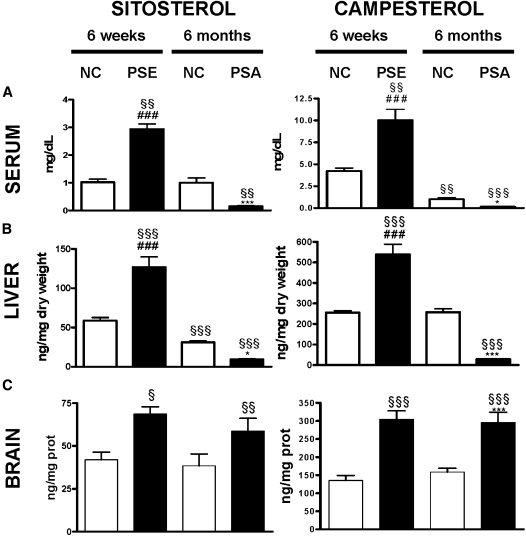
C57BL/6NCrl mice were fed a normal chow diet (NC) (n = 15) or were given a 2% PSE-enriched diet (n = 14) for 6 weeks (Fig. 1). In line with several previous reports (13, 39), the PSE-fed group displayed significantly increased sitosterol and campesterol concentrations in serum, liver, and brain in comparison to the NC group (Fig. 2A–C). When related to their serum concentrations, the enrichment of campesterol in the brain was 1.2-fold higher than sitosterol.
Fig. 2.
Plant sterol concentrations in serum, liver, and brain with or without plant sterol or plant stanol administration. Twelve week old C57BL/6NCrl mice were fed NC) (n = 15 or a PSE chow (n = 14). After 6 weeks, six animals per group were euthanized. The remaining animals on NC (n = 9) continued to be fed NC, and the animals previously fed PSE (n = 8) were given a PSA-enriched chow for 6 more months. Concentrations of sitosterol and campesterol in serum (mg/dl) (A), liver (ng/mg dry weight) (B), and brain (ng/mg protein) (C) are shown as mean ± SEM. PSE leads to elevated plant sterols in the serum (A), liver (B), and brain (C) after 6 weeks. PSA reduces serum and liver plant sterol, but not brain plant sterol, concentrations after 6 months. ANOVA, Bonferoni post hoc: § compared with 6 weeks NC fed C57BL/6/6NCrl mice. Student t-tests: # PSE versus PSA; * six months PSA versus 6 months NC. §,* P < 0.05. §§ P < 0.01. §§§, ###,*** P < 0.001.
In the PSE-fed group, feeding the mice a 1.5% PSA-enriched diet for 6 months significantly reduced sitosterol and campesterol concentrations in serum (sitosterol: P < 0.001; campesterol: P < 0.001 [Fig. 2A]) and liver (sitosterol: P < 0.001; campesterol: P < 0.001 [Fig. 2B]). However, brain plant sterol concentrations remained unchanged despite almost complete depletion of circulating plant sterols (Fig. 2C). Brain cholesterol and sitostanol were not affected by the different diets, whereas brain campestanol increased 2-fold after administration of the PSA diet, albeit remaining 300-fold lower in concentration compared with campesterol (data not shown). Also relative to the cerebral cholesterol content, the amount of cerebral plant sterols did not decrease in the PSE-fed group after administration of the PSA diet for 6 months {[campesterol (ng)/cholesterol (µg)]: 2.36 ± 0.18 (PSE) vs. 2.65 ± 0.31 (PSA), n.s. and [sitosterol (ng)/cholesterol (µg)]: 0.58 ± 0.03 (PSE) vs. 0.53 ± 0.11 (PSA), n.s.}. Animals fed a NC diet displayed a modest increase in the campesterol to cholesterol ratio and a comparable sitosterol to cholesterol ratio over 6 months {[campesterol (ng)/cholesterol (µg)]: 1.10 ± 0.05 (NC 6 weeks) vs. 1.30 ± 0.05 (NC 6 months), P < 0.05 and [sitosterol (ng)/cholesterol (µg)]: 0.34 ± 0.01 (NC 6 weeks) vs. 0.31 ± 0.05 (NC 6 months), n.s.}.
Together, these results strongly suggest that plant sterols enter the brain depending on the concentration in the serum and accumulate stably in the brain despite the fact that plant sterol concentrations in serum were strongly reduced during the 6 month PSA diet.
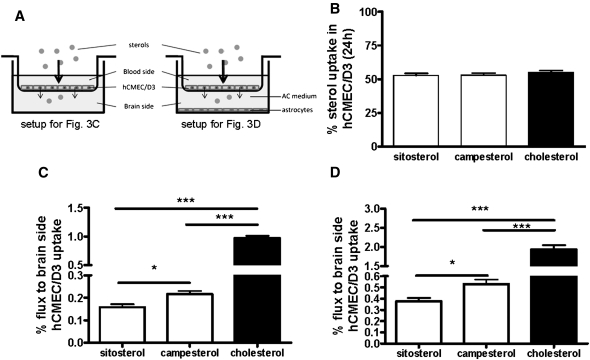
Campesterol is more efficient than sitosterol in traversing a brain endothelial monolayer
To examine whether sitosterol, campesterol, and/or cholesterol traverse the endothelial cell layer of the BBB or accumulate within these cells, d6-sitosterol/d6-campesterol or d6-cholesterol were added to the apical side of a polarized confluent human brain endothelial hCMEC/D3 monolayer in an in vitro Transwell® setup (Fig. 3A). After 24 h, the internalization of all sterols was comparable (Fig. 3B). However, the amount of d6-cholesterol in the basolateral compartment (0.97 ± 0.10% of uptake) was approximately five times higher than d6-sitosterol and d6-campesterol (Fig. 3C). Despite a comparable apical uptake of d6-sitosterol and d6-campesterol, d6-campesterol was 1.4-fold higher in the basolateral compartment compared with d6-sitosterol (0.22 ± 0.04% vs. 0.15 ± 0.03%; P = 0.016; Fig. 3B). Using endothelial monolayers cultured in the presence of astrocytes, we found that the absolute amount of d6-sterols was increased about 2-fold in the basolateral compartment (Fig. 3D). One third of the d6-sterols in the lower compartment was incorporated by the astrocytes (d6-cholesterol: 40 ± 7%; d6-campesterol: 34 ± 6%; and d6-sitosterol: 29 ± 6%). These data suggest that passage of the sterols across the endothelial monolayer depends on the molecular complexity of their side chain.
Fig. 3.
Apical uptake and subsequent basolateral efflux of plant sterols and cholesterol across a human endothelial cell monolayer. hCMEC/D3 endothelial cells were grown to confluency on permeable Transwell membranes (A) in the absence (C) or presence (D) of a confluent layer of primary astrocytes (AC) at the bottom of the lower chamber. d6-Sitosterol/d6-campesterol (15 µM) and d6-cholesterol (15 µM) were added to the upper compartment. After 24 h, the apical uptake rate (B) and consequent basolateral flux (C and D) were quantified. Uptake (%) was calculated as [(d6-sterols in endothelial cells) + (d6-sterols at brain side)]/[(d6-sterols at blood side) + (d6-sterols at brain side) + (d6-sterols in endothelial cells)] × 100%) (B). Basolateral flux (%) was calculated as [(d6-sterols at brain side)] [(d6-sterols at brain side) + (d6-sterols in endothelial cells)] × 100%) (C and D). D6-sterols present in astrocytes were included in the “brain side” for calculations (D). Flux was significantly higher for d6-cholesterol than for the plant sterols. d6-Campesterol flux was significantly higher than d6-sitosterol. Values represent the mean (%) of six independent experiments ± SD. Please note different scales. ** P < 0.01 and *** P < 0.001.
Dietary administration of plant sterols increased brain plant sterol concentrations predominantly in DRMs
Considering the functional importance of cholesterol in neuronal membranes, the distribution of plant sterols within the membranes was determined. Membranes were isolated from brain homogenates of mice fed for 6 weeks with a PSE-enriched or a NC diet. Higher levels of plant sterols in DRMs than in the nonDRM fractions (sitosterol NC: DRM vs. nonDRM, P < 0.001; sitosterol PSE: DRM vs. nonDRM, P < 0.001; campesterol NC: DRM vs. nonDRM, P < 0.001; campesterol PSE: DRM vs. nonDRM; P < 0.001) (Fig. 4A–D) were observed irrespective of the diet (NC or PSE). Moreover, the PSE diet induced a significantly higher accumulation in the DRM fractions, but not in the nonDRM fractions, in comparison with the NC diet (sitosterol DRM: NC vs. PSE, P < 0.05; campesterol DRM: NC vs. PSE, P < 0.001) (Fig. 4A–D). In contrast, the amount of cholesterol and the distribution over DRM and nonDRM fractions were not affected by the diet (Fig. 4E, F). Therefore, these data show that plant sterols accumulate within the DRM fractions of the brain cell membranes dependent on the plant sterol concentrations in the brain.
Plant sterols accumulate in DRMs in cultured brain cells
Plant sterol and cholesterol uptake and efflux to HDL were determined using different human brain-derived cell lines. Neuronal cells incorporated significantly less plant sterols and cholesterol than glial cells (oligodendrocytes and astrocytes) after 6 and 24 h of incubation (Table 1). Slightly, but not significantly, more cholesterol than plant sterols was taken up by all cell lines. In contrast to glial cells, sterol efflux in the neuronal cell line could not be enhanced by adding external HDL as acceptor. The efflux of plant sterols was slightly higher than of cholesterol. However, when the percentages were corrected for absolute uptake values, the absolute amounts of cholesterol and plant sterols were comparable.
TABLE 1.
Uptake and efflux of d6-sitosterol, d6-campesterol and d6-cholesterol for a neuroblastoma, an astrocytoma, and an oligodendroglioma cell line
| d6-sitosterol | d6-campesterol | d6-cholesterol | ||||
| 6 h | 24 h | 6 h | 24 h | 6 h | 24 h | |
| Uptake (%) | ||||||
| Neuronal cells | 4.2 (0.6) | 15.5 (1.0) | 4.4 (0.6) | 15.5 (0.9) | 7.9 (1.5) | 20.4 (1.9) |
| Astrocytes | 11.1 (0.6) | 42.1 (5.7) | 10.2 (0.6) | 44.1 (3.5) | 10.1 (0.5) | 44.5 (3.6) |
| Oligodendrocytes. | 9.8 (0.8) | 34.4 (3.0) | 10.4 (0.7) | 34.2 (2.7) | 15.7 (1.9) | 37.5 (6.5) |
| Efflux (%) | BSA | HDL | BSA | HDL | BSA | HDL |
| Neuronal cells | 16.7 (0.4) | 16.8 (2.3) | 15.9 (0.4) | 16.7 (2.1) | 5.5 (0.4) | 6.81 (0.7) |
| Aastrocytes | 13.2 (4.1) | 37.3 (2.9)* | 12.7 (3.4) | 34.7 (2.9)* | 5.4 (1.4) | 26.5 (0.6)* |
| Oligodendrocytes | 4.2 (0.7) | 5.7 (1.4)* | 4.1 (0.7) | 5.6 (1.4)* | 2.6 (0.2) | 5.3 (0.5)** |
Uptake is shown after 6 and 24 h of incubation. Efflux is shown after 24 h of incubation and a subsequent efflux period of 6 h, during which either HDL was added as an acceptor or 0.2% BSA as a vehicle. Values are displayed in percentage (±SD) of the amount added to the cells for uptake and percentage (±SD) of the amount that was internalized by the cells after 24 h incubation for efflux. * P < 0.05; ** P < 0.01 Mann-Whitney U test HDL vs. BSA. Data represent the mean of three independent experiments.
After administration of 10 µM d6-sitosterol/d6-campesterol (55% to 45%) mixture to SHSY5Y cells, d6-sitosterol (P < 0.001) and d6-campesterol (P < 0.001) were found to accumulate preferentially in the cholesterol-rich DRMs rather than in the phospholipid-enriched nonDRM fractions (Fig. 4G). The ratio of both plant sterols of the administered mixture was maintained within each fraction measured and the accumulation paralleled the accumulation of administered d6-cholesterol (data not shown).
In conclusion, these data show that two different plant sterols and cholesterol are handled similarly by the individual cerebral cell lines and subsequently accumulate within the DRMs.
DISCUSSION
We demonstrate for the first time that increased dietary intake of plant sterols results in a concentration-dependent, almost irreversible accumulation of plant sterols within the murine brain. In addition, we showed, in vitro and ex vivo, that plant sterols preferentially accumulate within DRMs of brain cells.
Previously, we reported that dietary-derived plant sterols can enter the brain (13, 39). Our present data confirm that cerebral plant sterol concentrations significantly increase after 6 weeks of dietary plant sterol supplementation, with a selective advantage for campesterol. More importantly, 6 months after plant stanol ester supplementation, plasma and liver were almost depleted of plant sterols, whereas plant sterol concentrations in the brain remained unaffected, indicating an almost irreversible accumulation of the plant sterols. Plant stanol treatment was used because of its very low intestinal absorbability compared with the pharmaceutical sterol uptake blocker ezetimibe (40). Direct influences of ezetimibe on sterol transport at the BBB cannot be excluded. Although we did not run a time-course experiment, it is possible to estimate the rate of sterol accumulation in the brain from our previous studies. We showed that 15 week old Abcg5−/− mice have plasma campesterol concentrations of 10 mg/dl, resulting in 6.43 mmol campesterol/mol cholesterol in the brain (13). In the current experiment, C57BL/6NCrl mice were fed a 2% enriched plant sterol diet for 6 weeks, resulting in comparable plasma campesterol concentrations of 10 mg/dl and brain campesterol concentrations of 2.28 mmol campesterol/mol cholesterol. Three month old Abcg5−/− mice were exposed 2.5 times longer to the same 10 mg/dl plasma campesterol concentrations, corresponding to a parallel 2.8-fold higher cerebral campesterol concentration. Although this is only a theoretical calculation, it supports a linear time-dependent uptake of plant sterols in the brain. Importantly, the Abcg5 transporter is not detectable within the brain and therefore is unlikely to influence plant sterol transport across the BBB (13). Given the assumptions that residual plasma volume is 10 μl per gram of brain tissue and that human brain contains about 100 mg protein per gram wet weight, together with the data shown in Fig. 2C, it would seem that less than 5% of sitosterol and campesterol in the brain is contained within the residual serum of PSE-fed mice.
In vivo, based on plasma concentrations, campesterol accumulated to a relatively higher amount (1.2-fold) in the brain compared with sitosterol, and in vitro campesterol transfer across a hCMEC/d3 monolayer was 1.4 times more efficient than for sitosterol. The 2-fold increase in the flow of sterols across the endothelial cell monolayer in the presence of primary astrocytes at the bottom of the lower chamber might involve the secretion of sterol-accepting lipoprotein like particles by the astrocytes. Therefore, endothelial cells and astrocytes may be involved in plant sterol transport across the BBB. Compared with plant sterols, we found a 5-fold higher secretion of the apically internalized cholesterol at the basolateral side after 24 h incubation. Considering the large pool of endogenous cholesterol within the cell, the total amount of cholesterol released at the basolateral side is probably even higher. Thus, transendothelial flux efficiency is determined by the molecular complexity of the sterol side-chain. A possible explanation for this may be a difference in the esterification rate within the endothelial cells. Like acyl-CoA:cholesterol O-acyltransferase (ACAT)2, which is mainly expressed in enterocytes, it has been shown that ACAT1, which is ubiquitously expressed, esterifies cholesterol more efficiently than sitosterol, independent of ACAT1 protein expression (2, 41, 42). However, the ratio of free/total cholesterol in hCMEC/D3 cells was >99%, making it difficult to reliably detect differences in esterification between cholesterol and plant sterols. It is generally assumed that the brain synthesizes all of the required cholesterol in situ and is therefore independent of peripheral cholesterol supply (11, 43). However, several in vitro and in vivo studies demonstrated a small but significant transport of cholesterol across the BBB. Administration of 4-C14-cholesterol to terminally ill patients revealed an average cerebral cholesterol accumulation of 3.2% of the serum-specific activity levels 1 to 244 days after injection (44). However, a defective BBB could not be excluded in these terminally ill patients. Administration of d6-cholesterol in an adult guinea pig resulted in a 1.23% and 0.93% accumulation in cerebrum and cerebellum, respectively (45). In addition, feeding of 0.5% d6-cholesterol to mice and rats for 10 days resulted in cerebral accumulation of less than 1% in either species (46). An in vitro porcine brain endothelial cell monolayer Transwell setup revealed an approximately 2% apical flux of cholesterol after basolateral administration (47). In our experiments, the basolateral uptake of plant sterols and cholesterol over 24 h was very limited (campesterol: 0.26%, sitosterol: 0.21%, and cholesterol: 3.68% compared with the common 52% uptake at the apical side; data not shown). The subsequent apical amount was a small percentage of the absorbed sterols (campesterol: 4.74%, sitosterol: 1.83%, and cholesterol: 2.32%; data not shown), making the absolute flux more efficient from the apical to the basolateral side than the reverse transport. Together, these data support a small but significant flux of cholesterol across the BBB. Due to the high cerebral cholesterol content and tightly regulated cholesterol turnover, the amount of cholesterol crossing the BBB is expected to be negligible and to be balanced by cerebral cholesterol homeostatic mechanisms. Under nondiseased conditions, little cholesterol from the circulation enters the brain. However, this may be different under diseased conditions with impaired BBB. In the human brain, cholesterol is metabolized into brain-specific 24(S)-hydroxycholesterol to facilitate cerebral cholesterol efflux, accounting for 60% of cholesterol release (48–52). Although plant sterols cross the endothelial monolayer less efficiently than cholesterol, they cannot be metabolized into 24(S)-hydroxysterols. 24-hydroxylation of plant sterols by the highly specific cholesterol-24-hydroxylase, encoded by CYP46A1, is prevented because of steric hindrance by the alkyl group at C24 (53). Furthermore, hydroxylation of plant sterols at position 7α to metabolize 24-alkyl plant sterols is unlikely because 24(S)-hydroxycholesterol, in contrast to 27-hydroxycholesterol and 25-hydroxycholesterol, is not 7α hydroxylated by cultured astrocytes, Schwann cells, or neurons (51, 54). The inability to convert plant sterols into more polar hydroxysterols explains why plant sterols, once having crossed the BBB, cannot easily leave the brain, leading to their persistent accumulation as described in this study. However, a very small but nonsignificant reduction of cerebral sitosterol (20%; P = 0.15) and campesterol (3%; P = 0.81) was observed after a 6 month PSA-enriched diet. Because the brain sterol turnover is known to be extremely slow, a low rate of plant sterol efflux cannot be excluded over longer time intervals.
Our previous studies indicated that plant sterol accumulation in the brain of Abcg5-deficient mice affected cerebral cholesterol homeostasis because levels of desmosterol and 24(S)-hydroxycholesterol in the hippocampus were decreased and lanosterol levels in the cortex were increased. This was not associated with alterations in learning and memory functions of the Abcg5-deficient mice (55). However, plant sterol accumulation may have consequences for brain function in neuropathological diseases.
Our in vitro endothelial monolayer Transwell system shows that plant sterols are transported rather slowly through endothelial cells of the vessel walls. Plant sterol accumulation in endothelial cells may impair endothelium-dependent vasorelaxation and increase cerebral lesion size after middle cerebral artery occlusion followed by reperfusion (9, 56). The mechanisms by which plant sterols are taken up by cerebral endothelial cells have not been revealed to date. Lipoprotein receptors, like the LDL receptor and scavenger receptor BI (SRBI), are expressed by cerebral endothelial cells (57, 58). Comparative studies with LDLR knockout and wild-type mice revealed that LDLR is not involved in cerebral plant sterol uptake (unpublished observations). Plasma plant sterols are mainly transported in HDL particles. Similar to Abcg5 knockout mice, ApoE knockout mice display high plasma plant sterol concentrations. However, in contrast to Abcg5 knockout mice, ApoE knockout mice display low HDL-related plant sterol concentrations, resulting in relatively strongly reduced cerebral plant sterol concentrations (13). Therefore, a HDL receptor such as SRBI might be a suitable candidate for plant sterol uptake by endothelial cells in the brain. Our observation that plant sterol levels are increased to the same extent in Abcg5 knockout mice and in Abcg5 knockout mice that also lack the SRBI suggests that the SRBI is not involved in the transport of plant sterols across the BBB (unpublished observations).
We demonstrated that plant sterols reside in lipid rafts within the brain cell membranes. Moreover, plant sterols reduce molecular order and fluidity of membranes by interacting less efficiently with saturated phospholipids than with cholesterol as is required in the formation of compact, liquid-ordered lipid rafts (59, 60). It was demonstrated that the magnitude of the effect on membrane fluidity depends on the geometry of the sterol molecule that is determined by the structure of its side chain (cholesterol >> campesterol > sitosterol > stigmasterol) (59). Mild reductions in membrane cholesterol affect the cleavage of APP upstream of γ-secretase and is mediated by changes in APP trafficking and partitioning into lipid rafts (18). Therefore, incorporation of plant sterols in lipid rafts potentially influences γ-secretase-mediated cleavage of APP in lipid rafts. The influence of plant sterol accumulation on Alzheimer's disease pathology remains to be determined.
In conclusion, these data show that long-term consumption of large amounts of plant sterols results in virtually irreversible accumulation of plant sterols in murine brains. Together with our in vitro results, these data indicate the need for further studies into the influence of long-term plant sterol intake on normal and pathological brain function.
Acknowledgments
The authors thank Prof. Dr. J. Dietschy, Prof. Dr. I. Björkhem, Dr. P. Delhanty, and Dr. A. J. Verhoeven for critical remarks and advice.
Footnotes
Abbreviations:
- ACAT
- acyl-CoA:cholesterol O-acyltransferase
- APP
- amyloid precursor protein
- Abcg5
- ATP binding cassette g5
- BBB
- blood-brain barrier
- DRM
- detergent-resistant membrane
- hCMEC/D3
- human brain endothelial cell line
- NC
- normal chow
- PSA
- plant stanol ester
- PSE
- plant sterol ester
- SRBI
- scavenger receptor BI
This work was supported by funding from the EU FP7 project LipiDiDiet (FP7 ⁄ 2007-2013; grant agreement no. 211696) and Marie Curie Early Stage Training Fellowships (grant agreement no. MEST-CT-2005-02058).
REFERENCES
- 1.Pollak O. J., Kritchevsky D. 1981. Sitosterol. Monogr. Atheroscler. 10: 1–219 [PubMed] [Google Scholar]
- 2.Salen G., Ahrens E. H., Jr, Grundy S. M. 1970. Metabolism of beta-sitosterol in man. J. Clin. Invest. 49: 952–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law M. 2000. Plant sterol and stanol margarines and health. BMJ. 320: 861–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees A. M., Mok H. Y., Lees R. S., McCluskey M. A., Grundy S. M. 1977. Plant sterols as cholesterol-lowering agents: clinical trials in patients with hypercholesterolemia and studies of sterol balance. Atherosclerosis. 28: 325–338 [DOI] [PubMed] [Google Scholar]
- 5.Thompson G. R., Grundy S. M. 2005. History and development of plant sterol and stanol esters for cholesterol-lowering purposes. Am. J. Cardiol. 96: 3D–9D [DOI] [PubMed] [Google Scholar]
- 6.Weststrate J. A., Meijer G. W. 1998. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 52: 334–343 [DOI] [PubMed] [Google Scholar]
- 7.Clifton P. 2009. Lowering cholesterol: a review on the role of plant sterols. Aust. Fam. Physician. 38: 218–221 [PubMed] [Google Scholar]
- 8.Calpe-Berdiel L., Escola-Gil J. C., Blanco-Vaca F. 2009. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 203: 18–31 [DOI] [PubMed] [Google Scholar]
- 9.Weingärtner O., Lutjohann D., Ji S., Weisshoff N., List F., Sudhop T., von Bergmann K., Gertz K., Konig J., Schafers H. J., et al. 2008. Vascular effects of diet supplementation with plant sterols. J. Am. Coll. Cardiol. 51: 1553–1561 [DOI] [PubMed] [Google Scholar]
- 10.Calpe-Berdiel L., Mendez-Gonzalez J., Blanco-Vaca F., Carles Escola-Gil J. 2009. Increased plasma levels of plant sterols and atherosclerosis: a controversial issue. Curr. Atheroscler. Rep. 11: 391–398 [DOI] [PubMed] [Google Scholar]
- 11.Turley S. D., Burns D. K., Rosenfeld C. R., Dietschy J. M. 1996. Brain does not utilize low density lipoprotein-cholesterol during fetal and neonatal development in the sheep. J. Lipid Res. 37: 1953–1961 [PubMed] [Google Scholar]
- 12.Dietschy J. M., Turley S. D. 2001. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12: 105–112 [DOI] [PubMed] [Google Scholar]
- 13.Jansen P. J., Lutjohann D., Abildayeva K., Vanmierlo T., Plosch T., Plat J., von Bergmann K., Groen A. K., Ramaekers F. C. S., Kuipers F., et al. 2006. Dietary plant sterols accumulate in the brain. Biochim. Biophys. Acta. 1761: 445–453 [DOI] [PubMed] [Google Scholar]
- 14.Yu L., Hammer R. E., Li-Hawkins J., Von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. 2002. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA. 99: 16237–16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulder M. 2009. Sterols in the central nervous system. Curr. Opin. Clin. Nutr. Metab. Care. 12: 152–158 [DOI] [PubMed] [Google Scholar]
- 16.Shobab L. A., Hsiung G. Y., Feldman H. H. 2005. Cholesterol in Alzheimer's disease. Lancet Neurol. 4: 841–852 [DOI] [PubMed] [Google Scholar]
- 17.Stefani M., Liguri G. 2009. Cholesterol in Alzheimer's disease: unresolved questions. Curr. Alzheimer Res. 6: 15–29 [DOI] [PubMed] [Google Scholar]
- 18.Guardia-Laguarta C., Coma M., Pera M., Clarimon J., Sereno L., Agullo J. M., Molina-Porcel L., Gallardo E., Deng A., Berezovska O., et al. 2009. Mild cholesterol depletion reduces amyloid-beta production by impairing APP trafficking to the cell surface. J. Neurochem. 110: 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledesma M. D., Dotti C. G. 2006. Amyloid excess in Alzheimer's disease: what is cholesterol to be blamed for? FEBS Lett. 580: 5525–5532 [DOI] [PubMed] [Google Scholar]
- 20.Ehehalt R., Keller P., Haass C., Thiele C., Simons K. 2003. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 160: 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin S. W., Glover B. J., Davies J. M. 2005. Lipid microdomains–plant membranes get organized. Trends Plant Sci. 10: 263–265 [DOI] [PubMed] [Google Scholar]
- 22.Mongrand S., Morel J., Laroche J., Claverol S., Carde J. P., Hartmann M. A., Bonneu M., Simon-Plas F., Lessire R., Bessoule J. J. 2004. Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J. Biol. Chem. 279: 36277–36286 [DOI] [PubMed] [Google Scholar]
- 23.Rebolj K., Ulrih N. P., Macek P., Sepcic K. 2006. Steroid structural requirements for interaction of ostreolysin, a lipid-raft binding cytolysin, with lipid monolayers and bilayers. Biochim. Biophys. Acta. 1758: 1662–1670 [DOI] [PubMed] [Google Scholar]
- 24.Weksler B. B., Subileau E. A., Perriere N., Charneau P., Holloway K., Leveque M., Tricoire-Leignel H., Nicotra A., Bourdoulous S., Turowski P., et al. 2005. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19: 1872–1874 [DOI] [PubMed] [Google Scholar]
- 25.Richter-Landsberg C., Heinrich M. 1996. OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J. Neurosci. Res. 45: 161–173 [DOI] [PubMed] [Google Scholar]
- 26.Van Doorn R., Van Horssen J., Verzijl D., Witte M., Ronken E., Van Het Hof B., Lakeman K., Dijkstra C. D., Van Der Valk P., Reijerkerk A., et al. 2010. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 58: 1465–1476 [DOI] [PubMed] [Google Scholar]
- 27.Prat A., Biernacki K., Wosik K., Antel J. P. 2001. Glial cell influence on the human blood-brain barrier. Glia. 36: 145–155 [DOI] [PubMed] [Google Scholar]
- 28.Schreibelt G., Kooij G., Reijerkerk A., van Doorn R., Gringhuis S. I., van der Pol S., Weksler B. B., Romero I. A., Couraud P. O., Piontek J., et al. 2007. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 21: 3666–3676 [DOI] [PubMed] [Google Scholar]
- 29.Dixon W. J. 1959. Analyses of extreme values. Ann. Math. Stat. 21: 488–506 [Google Scholar]
- 30.Dixon W. J. 1959. Ratios involving extreme values. Ann. Math. Stat. 22: 68–78 [Google Scholar]
- 31.Maxwell K. N., Soccio R. E., Duncan E. M., Sehayek E., Breslow J. L. 2003. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J. Lipid Res. 44: 2109–2119 [DOI] [PubMed] [Google Scholar]
- 32.Redgrave T. G., Roberts D. C., West C. E. 1975. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal. Biochem. 65: 42–49 [DOI] [PubMed] [Google Scholar]
- 33.Yoon I. S., Chen E., Busse T., Repetto E., Lakshmana M. K., Koo E. H., Kang D. E. 2007. Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J. 21: 2742–2752 [DOI] [PubMed] [Google Scholar]
- 34.Nebl T., Pestonjamasp K. N., Leszyk J. D., Crowley J. L., Oh S. W., Luna E. J. 2002. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem. 277: 43399–43409 [DOI] [PubMed] [Google Scholar]
- 35.Parkin E. T., Hussain I., Karran E. H., Turner A. J., Hooper N. M. 1999. Characterization of detergent-insoluble complexes containing the familial Alzheimer's disease-associated presenilins. J. Neurochem. 72: 1534–1543 [DOI] [PubMed] [Google Scholar]
- 36.Parkin E. T., Turner A. J., Hooper N. M. 1999. Amyloid precursor protein, although partially detergent-insoluble in mouse cerebral cortex, behaves as an atypical lipid raft protein. Biochem. J. 344: 23–30 [PMC free article] [PubMed] [Google Scholar]
- 37.Lütjohann D., Brzezinka A., Barth E., Abramowski D., Staufenbiel M., von Bergmann K., Beyreuther K., Multhaup G., Bayer T. A. 2002. Profile of cholesterol-related sterols in aged amyloid precursor protein transgenic mouse brain. J. Lipid Res. 43: 1078–1085 [DOI] [PubMed] [Google Scholar]
- 38.Thelen K. M., Rentsch K. M., Gutteck U., Heverin M., Olin M., Andersson U., von Eckardstein A., Bjorkhem I., Lutjohann D. 2006. Brain cholesterol synthesis in mice is affected by high dose of simvastatin but not of pravastatin. J. Pharmacol. Exp. Ther. 316: 1146–1152 [DOI] [PubMed] [Google Scholar]
- 39.Fricke C. B., Schroder M., Poulsen M., von Bergmann K., Wester I., Knudsen I., Mortensen A., Lutjohann D. 2007. Increased plant sterol and stanol levels in brain of Watanabe rabbits fed rapeseed oil derived plant sterol or stanol esters. Br. J. Nutr. 98: 890–899 [DOI] [PubMed] [Google Scholar]
- 40.Kosoglou T., Statkevich P., Johnson-Levonas A. O., Paolini J. F., Bergman A. J., Alton K. B. 2005. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin. Pharmacokinet. 44: 467–494 [DOI] [PubMed] [Google Scholar]
- 41.Liu J., Chang C. C., Westover E. J., Covey D. F., Chang T. Y. 2005. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem. J. 391: 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Temel R. E., Gebre A. K., Parks J. S., Rudel L. L. 2003. Compared with Acyl-CoA:cholesterol O-acyltransferase (ACAT) 1 and lecithin:cholesterol acyltransferase, ACAT2 displays the greatest capacity to differentiate cholesterol from sitosterol. J. Biol. Chem. 278: 47594–47601 [DOI] [PubMed] [Google Scholar]
- 43.Dietschy J. M., Turley S. D. 2002. Control of cholesterol turnover in the mouse. J. Biol. Chem. 277: 3801–3804 [DOI] [PubMed] [Google Scholar]
- 44.Chobanian A. V., Hollander W. 1962. Body cholesterol metabolism in man. I. The equilibration of serum and tissue cholesterol. J. Clin. Invest. 41: 1732–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lütjohann D., Stroick M., Bertsch T., Kuhl S., Lindenthal B., Thelen K., Andersson U., Bjorkhem I., Bergmann Kv K., Fassbender K. 2004. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids. 69: 431–438 [DOI] [PubMed] [Google Scholar]
- 46.Meaney S., Lutjohann D., Diczfalusy U., Bjorkhem I. 2000. Formation of oxysterols from different pools of cholesterol as studied by stable isotope technique: cerebral origin of most circulating 24S-hydroxycholesterol in rats, but not in mice. Biochim. Biophys. Acta. 1486: 293–298 [DOI] [PubMed] [Google Scholar]
- 47.Panzenboeck U., Balazs Z., Sovic A., Hrzenjak A., Levak-Frank S., Wintersperger A., Malle E., Sattler W. 2002. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 277: 42781–42789 [DOI] [PubMed] [Google Scholar]
- 48.Xie C., Lund E. G., Turley S. D., Russell D. W., Dietschy J. M. 2003. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J. Lipid Res. 44: 1780–1789 [DOI] [PubMed] [Google Scholar]
- 49.Björkhem I., Lutjohann D., Diczfalusy U., Stahle L., Ahlborg G., Wahren J. 1998. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J. Lipid Res. 39: 1594–1600 [PubMed] [Google Scholar]
- 50.Lund E. G., Xie C., Kotti T., Turley S. D., Dietschy J. M., Russell D. W. 2003. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 278: 22980–22988 [DOI] [PubMed] [Google Scholar]
- 51.Björkhem I., Lutjohann D., Breuer O., Sakinis A., Wennmalm A. 1997. Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J. Biol. Chem. 272: 30178–30184 [DOI] [PubMed] [Google Scholar]
- 52.Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Siden A., Diczfalusy U., Bjorkhem I. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 93: 9799–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mast N., Norcross R., Andersson U., Shou M., Nakayama K., Bjorkhem I., Pikuleva I. A. 2003. Broad substrate specificity of human cytochrome P450 46A1 which initiates cholesterol degradation in the brain. Biochemistry (Mosc.). 42: 14284–14292 [DOI] [PubMed] [Google Scholar]
- 54.Zhang J., Akwa Y., el-Etr M., Baulieu E. E., Sjovall J. 1997. Metabolism of 27-, 25- and 24-hydroxycholesterol in rat glial cells and neurons. Biochem. J. 322: 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanmierlo T., Rutten K., van Vark-van der Zee L. C., Friedrichs S., Bloks V. W., Blokland A., Ramaekers F. C., Sijbrands E., Steinbusch H., Prickaerts J., et al. 2011. Cerebral accumulation of dietary derivable plant sterols does not interfere with memory and anxiety related behavior in Abcg5−/− mice. Plant Foods Hum. Nutr. 66: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodzic A., Rappolt M., Amenitsch H., Laggner P., Pabst G. 2008. Differential modulation of membrane structure and fluctuations by plant sterols and cholesterol. Biophys. J. 94: 3935–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dehouck B., Fenart L., Dehouck M. P., Pierce A., Torpier G., Cecchelli R. 1997. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 138: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goti D., Hrzenjak A., Levak-Frank S., Frank S., van der Westhuyzen D. R., Malle E., Sattler W. 2001. Scavenger receptor class B, type I is expressed in porcine brain capillary endothelial cells and contributes to selective uptake of HDL-associated vitamin E. J. Neurochem. 76: 498–508 [DOI] [PubMed] [Google Scholar]
- 59.Hac-Wydro K., Wydro P., Dynarowicz-Latka P., Paluch M. 2009. Cholesterol and phytosterols effect on sphingomyelin/phosphatidylcholine model membranes: thermodynamic analysis of the interactions in ternary monolayers. J. Colloid Interface Sci. 329: 265–272 [DOI] [PubMed] [Google Scholar]
- 60.Xu X., Bittman R., Duportail G., Heissler D., Vilcheze C., London E. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 276: 33540–33546 [DOI] [PubMed] [Google Scholar]