Abstract
Although the evidence linking apoA-IV expression and triglyceride (TG)-rich lipoprotein assembly and secretion is compelling, the intracellular mechanisms by which apoA-IV could modulate these processes remain poorly understood. We therefore examined the functional impact of apoA-IV expression on endogenous apoB, TG, and VLDL secretion in stably transfected McA-RH7777 rat hepatoma cells. Expression of apoA-IV modified with the endoplasmic reticulum (ER) retention signal KDEL (apoA-IV-KDEL) dramatically decreased both the rate and efficiency of endogenous apoB secretion, suggesting a presecretory interaction between apoA-IV-KDEL and apoB or apoB-containing lipoproteins. Expression of native apoA-IV using either a constitutive or tetracycline-inducible promoter delayed the initial rate of apoB secretion and reduced the final secretion efficiency by ∼40%. However, whereas apoA-IV-KDEL reduced TG secretion by 75%, expression of native apoA-IV caused a 20–35% increase in TG secretion, accompanied by a ∼55% increase in VLDL-associated apoB, an increase in the TG:phospholipid ratio of secreted d < 1.006 lipoproteins, and a 10.1 nm increase in peak VLDL1 particle diameter. Native apoA-IV expression had a negligible impact on expression of the MTP gene. These data suggest that by interacting with apoB in the secretory pathway, apoA-IV alters the trafficking kinetics of apoB-containing TG-rich lipoproteins through cellular lipidation compartments, which in turn, enhances particle expansion and increases TG secretion.
Keywords: lipoprotein assembly, triglyceride, lipid trafficking, very low density lipoprotein, apolipoprotein B, apolipoprotein A-IV
The growth, reproduction, and survival of all multicellular organisms are dependent upon the efficient absorption, transport, and storage of exogenous lipids (1). The assembly and secretion of triglyceride (TG)-rich lipoproteins by the intestine and liver is central to these critical metabolic processes (2–4). TG-rich lipoprotein assembly is thought to occur in two sequential steps, in which the endoplasmic reticulum (ER)-localized cofactor, microsomal triglyceride transfer protein (MTP), plays an essential role (5, 6). In the first step, MTP directs the cotranslational lipidation of apolipoprotein (apo)B in the ER with phospholipid and TG to form precursor particles with diameters of ∼10–20 nm (7–10). In the second step, MTP promotes the movement of lipid from the cytosol into the ER to form luminal lipid droplets, which then fuse with apoB precursor particles in the ER and/or post-ER compartments (11–16). In the liver, this results in the secretion of VLDL particles with diameters ranging from 30 to 80 nm; however, in the intestine, apoB-containing lipoproteins expand into chylomicrons with diameters in excess of one micron (1, 4, 17). The ability of intestinal enterocytes to generate such large lipoproteins is unrelated to APOBEC1-mediated editing of apoB100 mRNA to yield apoB48 (18). Therefore, other intracellular factors must play a role in the expansion of chylomicron particles.
ApoA-IV may be one such factor. ApoA-IV, the largest member of the exchangeable apolipoprotein family (19), is synthesized by intestinal enterocytes during lipid absorption and secreted into mesenteric lymph on the surface of nascent chylomicrons (20, 21). Although a broad spectrum of physiologic functions has been proposed for apoA-IV in the three-and-a-half decades since its first description (22), a substantial body of evidence supports a role in chylomicron assembly: i) apoA-IV synthesis in intestinal enterocytes increases up to 5-fold during absorption of long-chain fatty acids (23, 24), which requires chylomicron assembly, but not during absorption of short-chain fatty acids, which does not (25); ii) intestinal apoA-IV synthesis is simultaneously inhibited when chylomicron secretion is blocked by the surfactant PL-81 (26); iii) within the enterocyte, apoA-IV colocalizes with apoB in pre-Golgi secretory transport vesicles (27); iv) the dynamic interfacial properties of apoA-IV are ideally suited to stabilizing expanding lipid interfaces (28, 29); v) plasma apoA-IV levels rise promptly after a fatty meal (30) and are depressed in intestinal disorders in which TG absorption is impaired (31); and vi) apoA-IV expression in porcine neonatal intestinal cells (IPEC-1) increases bulk TG transport by enabling formation and secretion of larger TG-rich lipoproteins (32, 33).
Although the evidence linking apoA-IV expression and chylomicron assembly and secretion is compelling, the intracellular mechanisms by which apoA-IV might impact these processes remain poorly understood. For apoA-IV to facilitate the expansion of TG-rich lipoproteins, it must interact either directly with apoB or with the surface of apoB-containing lipoproteins within the secretory pathway. In previous studies, we cotransfected apoA-IV, apoB, and MTP into COS cells and found that apoA-IV modified with the C-terminal ER retention signal KDEL (apoA-IV-KDEL) inhibited the secretion of apoB constructs larger than apoB25, suggesting the existence of a protein-protein interaction between apoA-IV and specific sequences in the N-terminal region of the apoB molecule in the early stages of TG-rich lipoprotein assembly (34). Herein, we have extended these investigations to examine the effect of apoA-IV on endogenous apoB-trafficking kinetics and the physical properties of secreted apoB-containing TG-rich lipoproteins. To do so, we stably transfected apoA-IV and apoA-IV-KDEL constructs into McA-RH7777 rat hepatoma cells, which unlike rodent liver, lack endogenous expression of apoA-IV and thus provide a null background (35).Our data suggest that apoA-IV alters the secretory trafficking of apoB and/or apoB-containing lipoproteins through cellular lipidation compartments, which in turn, enhances particle expansion and, ultimately, TG secretion.
EXPERIMENTAL PROCEDURES
Plasmids
Human apoA-IV and apoA-IV-KDEL expression plasmids were described previously (34). Plasmids containing the tetracycline regulator (pTet-On) and the tetracycline response element (pTRE2 hyg) were obtained from Clontech Laboratories, Inc. (Mountain View, CA). Human apoA-IV cDNA was generated by polymerase chain reaction using linearized apoA-IV plasmid DNA as template and 5′ and 3′ flanking primers containing engineered BglII and MluI restriction enzyme sites, respectively. Following BglII and MluI digestion, the cDNA was ligated to BamHI and MluI-digested pTRE2 hyg. The integrity of the plasmid was verified by sequence analysis.
Metabolic radiolabeling of transfected McA-RH7777 lines
Stable and inducible apoA-IV expressing McA-RH7777 cell lines were generated as described previously (36). Unless otherwise indicated, McA-RH7777 cells were grown in 100 mm dishes containing DMEM (Media Tech, Manassas, VA) with 10% FBS (growth medium). In some experiments with the inducible cells, 1 µg/ml doxycycline (Dox) was added to the media. At 2 h prior to experiments, media was replaced with growth medium supplemented with 0.8 mM oleic acid complexed to 1.5% fatty acid–free BSA (both from Sigma-Aldridge, St. Louis, MO). Transfected cells were metabolically radiolabeled by incubation for the indicated times with 100 µCi/ml [35S]Met and Cys (EasyTag Express Protein Labeling Mix; Perkin Elmer Life Sciences, Waltham, MA) in Met- and Cys-deficient DMEM (Gibco-Life Technologies), also supplemented with 10% FBS and 0.8 mM oleic acid. Pulse-chase studies were performed in 60 mm dishes. After a 10 min pulse with [35S]Met/Cys as described above, cells were washed and incubated with oleate-containing growth medium containing an additional 2.5 mM Met and 1 mM Cys for the indicated times. Cell lysate and media samples were prepared as described previously (34, 37) and subjected to immunoprecipitation with rabbit anti-apoA-IV or goat anti-human apoB (Academy Biomedical, Houston, TX). Immune complexes were fractionated by SDS-PAGE, and dried gels were exposed to BioMax MS film backed with a BioMax TransScreen-LE intensifying screen (Eastman Kodak, Rochester, NY) at −70°C; band intensities were quantified using a Molecular Dynamics 445 SI phosphorimager or a Fujifilm BAS5000 phosphorimager. These data were used to calculate apoB secretion efficiency, intracellular retention, and degradation.
Quantification of intracellular and secreted lipids from transfected McA-RH7777 cells
Transfected cells were plated in 150 mm dishes at 50% confluence. After 24 h, cells were incubated in growth medium supplemented with 0.8 mM oleate for 2 h. Media was then replaced with growth medium containing 0.8 mM oleate and 10 µCi [3H]oleate, and the cells were incubated for an additional 18 h. Media was then removed, concentrated in a Centricon YM-50 concentrator (Millipore, Billerica, MA), and subjected to Bligh-Dyer extraction (36, 38). Extracted lipids were fractioned by thin layer chromatography using heptane-ether-acetic acid [90:30:1]; bands were visualized by incubation in iodine vapor, and TG- and phospholipid (PL)-containing fractions were cut from the plate and quantified by liquid scintillation counting (36). Protein concentration of cell extracts was determined by the bicinchoninic acid method (39) using the Pierce BCA Protein Assay Reagent kit (Thermo Scientific, Rockford, IL).
Isolation and characterization of VLDL
Media from transfected cells was adjusted to a volume of 3 ml with phosphate buffered saline and centrifuged for 14 h at 100,000 rpm in a TLA100.3 rotor using a TL-100 tabletop ultracentrifuge (Beckman Coulter, Brea, CA). The d < 1.006 g/ml top (1 ml) and d > 1.006 g/ml bottom (2 ml) fractions were collected by tube slicing, and apoB was isolated by immunoprecipitation and analyzed by 4–20% SDS-PAGE and fluorography. Lipoproteins in the VLDL1 size range were isolated by cumulative rate density gradient ultracentrifugation, and the particle size distribution was analyzed using a Zetasizer Nano-S model ZEN1600 dynamic laser light-scattering instrument (Malvern Instruments, Worcestershire, UK) at 633 nm (36).
Gene expression in inducible McA-RH7777 cells
Inducible apoA-IV expressing McA-RH7777 cells were grown in 100 mm dishes containing DMEM with 10% FBS with and without 1 μg/ml Dox. After 48 h, cellular RNA was extracted using TRIzol (Invitrogen). Total RNA was converted into cDNA using Omniscript RT kits (Qiagen, Valencia, CA). Quantitative PCR (qPCR) was performed on a 7500 Fast Real Time PCR System (Applied Biosystems, Foster City, CA). A typical PCR reaction (20 μl) contained 10 μl 2 X Fast SYBR Green Master Mix (Applied Biosystems), 1 μl each of 5 μM forward and reverse primers, and a 1:10 dilution of cDNA. Copy numbers were normalized to GAPDH. The following primers were used: apoA-IV, forward TTC CTG AAG GCT GCG GTG CTG, reverse CTG CTG AGT GAC ATC CGT CTT CTG; MTP, forward CCT ACC AGG CCC AAC AAG AC, reverse CGC TCA ATT TTG CAT GTA TCC. Immunoblot analyses were performed with anti-mouse MTP monoclonal antibody (BD Biosciences) and rabbit anti-human apoA-IV, as described previously (34, 40).
RESULTS
Stable transfection of McA-RH7777 cells with apoA-IV and apoA-IV-KDEL alters endogenous apoB secretion
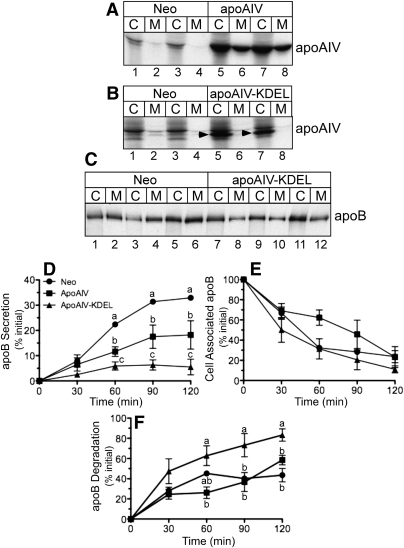
McA-RH7777 cells were stably transfected with apoA-IV, apoA-IV-KDEL, or a control neomycin (Neo) resistance plasmid, stimulated with BSA-oleate, and radiolabeled with [35S]Met and Cys for 4 h. Cell lysate and media fractions were subjected to immunoprecipitation with anti-apoA-IV or anti-apoB antibody and analyzed by SDS-PAGE and fluorography. As shown in Fig. 1A, in cells transfected with apoA-IV, both the cell (C) and media (M) fractions displayed a band with the expected electrophoretic mobility of ∼48 kDa (compare lanes 1–4 with lanes 5–8). In contrast, in cells transfected with apoA-IV-KDEL, the apoA-IV band was present in cell lysates (Fig. 1B, arrowhead) but not in the media fractions, demonstrating, as expected, that the KDEL ER retention-recycling signal dramatically reduced apoA-IV secretion. To assess the impact of apoA-IV-KDEL on endogenous apoB secretion, cell and media samples from Neo- and apoA-IV KDEL–transfected cells were examined for apoB100 content. As shown in Fig. 1C, apoA-IV-KDEL expression resulted in a ∼75% reduction in apoB in media compared with Neo cells (compare lanes 2, 4, and 6, with lanes 8, 10, and 12), suggesting an intracellular interaction between apoA-IV-KDEL and either apoB or apoB-containing lipoproteins (34).
Fig. 1.
Effect of apoA-IV and apoA-IV-KDEL on endogenous apoB secretion in McA-RH7777 cells. A, B: Duplicate dishes of McA-RH7777 cells stably transfected with apoA-IV (A, lanes 5–8), apoA-IV-KDEL (B, lanes 5–8), or control neomycin (Neo) vector (A and B, lanes 1–4) were radiolabeled with [35S]Met and Cys for 4 h, and equal aliquots of cell lysates (C) and media (M) were immunoprecipitated with anti-apoA-IV antibody followed by SDS-PAGE and fluorography. Arrowheads (B) show position of the apoA-IV-KDEL band. C: Neo and apoA-IV-KDEL cells were labeled as described above, and cell lysate and media samples were immunoprecipitated with anti-apoB antibody and analyzed as above. D, E, F: Neo, apoA-IV, and apoA-IV-KDEL cells were pulse-radiolabeled for 10 min with [35S]Met and Cys and then chased for the indicated times with media containing cold amino acids. At the end of each chase period, labeled apoB100 protein in cell lysates and media was quantified by immunoprecipitation, SDS-PAGE, and phosphorimager analysis to determine the percentage of newly synthesized apoB that was secreted into the media (D), retained within the cells (E), and degraded (F). Data are mean ± SE; n = 3. Where error bars are not visible, the SE is less than the size of the symbol. Time points with different letters are (D) P = 0.001 at 60 min, P = 0.001 at 90 min, and P = 0.003 at 120 min; (F): P = 0.044 at 60 min, P = 0.055 at 90 min, and P = 0.014 at 120 min by ANOVA.
To quantitatively assess the impact of apoA-IV and apoA-IV-KDEL expression on apoB secretion kinetics, cells were subjected to a 10 min pulse with [35S]Met and Cys, followed by 0–120 min chase with media containing cold amino acids. As anticipated, apoA-IV-KDEL expression reduced both the initial rate of apoB secretion into the culture cell media and the final secretion efficiency relative to Neo controls by ∼80%. Interestingly, native apoA-IV also reduced the initial rate and final efficiency of apoB secretion to a level intermediate between the Neo- and apoA-IV-KDEL–transfected cells (Fig. 1D). Recovery of apoB from the cell lysates indicated that, in the apoA-IV–transfected cells, there was a trend toward greater intracellular apoB retention at 60 min (P = 0.07); however, by 120 min, very little of the initial pulse-labeled apoB remained within the cells under all three transfection conditions (Fig. 1E). As expected, apoB underwent rapid degradation at all times in the apoA-IV-KDEL–transfected cells, whereas apoB degradation was slower but similar in native apoA-IV cells and Neo-transfected cells (Fig. 1F). These data establish that apoA-IV expression alters the rate and efficiency of endogenous apoB secretion.
Stable transfection of McA-RH7777 cells with apoA-IV and apoA-IV-KDEL alters lipid secretion
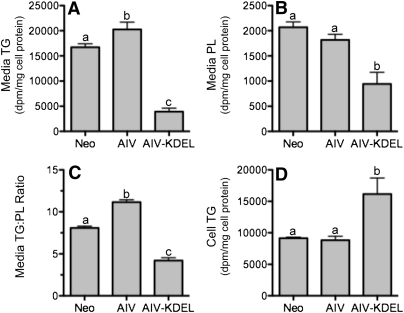
We next examined the effect of apoA-IV and apoA-IV-KDEL on lipid secretion. In accord with its strong inhibitory effect on apoB secretion, apoA-IV-KDEL expression caused a dramatic decrease in the secretion of TG (Fig. 2A) and PL (Fig. 2B), associated with a reciprocal increase in cell-associated TG (Fig. 2D). In contrast, apoA-IV increased TG secretion (Fig. 2A), despite the decrease in apoB secretion (Fig. 1C, D), but with little change in intracellular TG (Fig. 2D). The constant intracellular TG levels could be a consequence of a homeostatic balance between secretion and cellular TG synthesis. ApoA-IV–induced stimulation in TG secretion was also accompanied by a ∼40% increase in the media TG:PL ratio relative to Neo controls (Fig. 2C), consistent with an increase in the size of secreted apoB-containing lipoproteins.
Fig. 2.
Lipid secretion is altered by expression of apoA-IV and apoA-IV-KDEL in McA-RH7777 cells. McA-RH7777 cells stably transfected with control neomycin vector (Neo), apoA-IV (AIV), or apoA-IV-KDEL (AIV-KDEL) were incubated with media containing 10% serum, 0.8 mM oleate, and 10 µCi [3H]oleate for 18 h. Lipids were isolated from media and cell lysates by solvent extraction, separated by TLC, and quantitated by liquid scintillation counting. Lipid data are normalized to cell protein. A: Media TG. B: Media PL. C: Media TG:PL ratio. D: Cell TG. Data are mean ± SD (n = 3). Bars labeled with different letters are P < 0.05 by ANOVA.
Dox-induced apoA-IV expression in McA-RH7777 cells alters apoB and lipid secretion
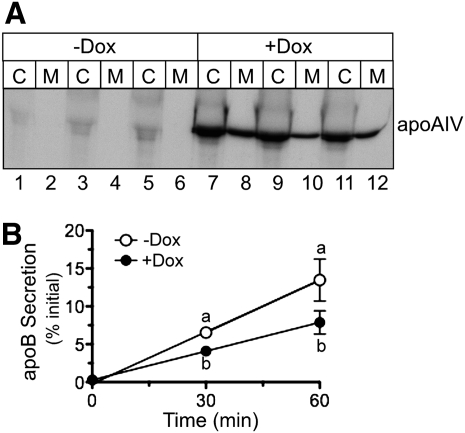
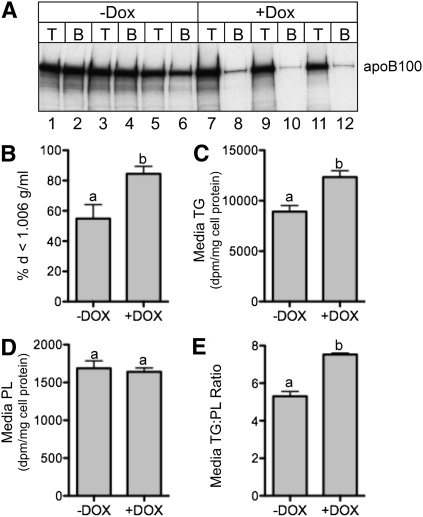
To ensure that the observed differences in apoB and lipid secretion were not caused by clonal variation among stably transfected cell lines, a cell line was created that expressed apoA-IV under the control of a tetracycline (Tet)-responsive promoter. Upon incubation in media containing Dox, these cells displayed a dramatic increase in apoA-IV abundance in cell lysates and media (Fig. 3A, compare lanes 1–6 with lanes 7–12). To assess how apoA-IV expression affects the initial kinetics of apoB secretion, pulse-chase experiments were performed in cells induced for 48 h either without or with Dox in the tissue culture media. As shown in Fig. 3B, Dox-induced apoA-IV expression reduced the initial apoB secretion rate by 44%, relative to the Dox (−) control, in good agreement with data obtained using the constitutive cell lines (Fig. 1D). To assess the impact of apoA-IV expression on apoB particle characteristics, cells were radiolabeled with [35S]Met and Cys for 4 h in the absence and presence of Dox, and media were subjected to density gradient centrifugation at d = 1.006 g/ml. The percentage of apoB in the d < 1.006 top fraction increased from 55% to 84% with Dox-induced apoA-IV expression (Fig. 4A, B). As was also seen in constitutive apoA-IV expression experiments (Fig. 2), Dox-induced apoA-IV expression increased media TG (Fig. 4C) and the media TG:PL ratio (panel E), again suggesting that apoA-IV expression facilitated the secretion of larger, more TG-rich lipoproteins.
Fig. 3.
Inducible expression of apoA-IV decreases the rate of endogenous apoB100 secretion. A: Triplicate dishes of McA-RH7777 cells stably transfected with Tet-inducible apoA-IV were incubated without (−) or with (+) 1 µg/ml doxycycline (Dox) for 48 h. Cells were then radiolabeled with [35S]Met and Cys for 4 h, followed by immunoprecipitation of cell lysates (C) and media (M) with anti-apoA-IV antibody, SDS-PAGE, and fluorography. B: Triplicate dishes of McA-RH7777 cells were incubated without or with Dox as indicated, and then subjected to a 10 min pulse with [35S]Met and Cys followed by 0, 30, and 60 min chase with media containing cold amino acids. Data are mean ± SD (n = 3). Where error bars are not visible, the SD is less than the size of the symbol. Time points with different letters are P = 0.013 at 30 min and P = 0.034 at 60 min by ANOVA.
Fig. 4.
Expression of apoA-IV in McA-RH7777 cells increases the percentage of apoB secreted as VLDL and increases bulk TG secretion. A: Triplicate dishes of Tet-inducible apoA-IV McA-RH7777 cells were incubated without (−) or with (+) 1 µg/ml doxycycline (Dox) for 48 h. Cells were then radiolabeled with [35S]Met and Cys for 6 h, and the media was subjected to density gradient centrifugation to obtain VLDL d < 1.006 g/ml top (T) and d > 1.006 g/ml bottom (B) fractions. ApoB was immunoprecipitated and analyzed by SDS-PAGE and fluorography. B: Band intensities were quantified by phosphorimager analysis and the percentage of total apoB in the top d < 1.25 g/ml was plotted. C–E: Cells treated with and without Dox were incubated with [3H]oleate for 18 h, and radiolabeled lipids were analyzed as described in Fig. 2. Data are mean ± SD (n = 3). Bars labeled with different letters are P < 0.05 by ANOVA.
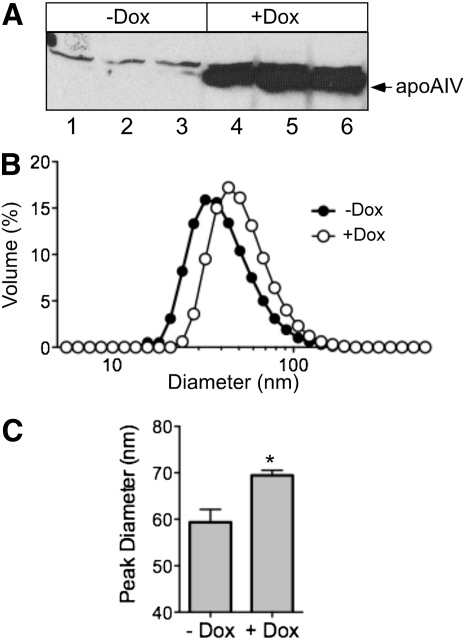
Dox-induced apoA-IV expression in McA-RH7777 cells increases VLDL particle size
As the previous results demonstrated that apoA-IV expression increased TG secretion in association with an increased percentage of apoB that forms VLDL, we directly measured the effect of apoA-IV expression on VLDL particle diameter. When an inducible apoA-IV cell line was incubated with Dox for 48 h, intracellular apoA-IV increased dramatically (Fig. 5A). Media from these Dox-treated cells were then subjected to density gradient centrifugation to obtain the VLDL1 fraction, and its particle size distribution was determined by dynamic light scattering (36). Addition of Dox caused a rightward shift in VLDL1 particle distribution (Fig. 5B), caused by an increase in peak diameter from 59.4 to 69.5 nm (Fig. 5C), and an increase in the intensity-weighted mean (“Z-averaged”) diameter from 49.35 to 59.18 nm. Together these data suggest that Dox-induced apoA-IV expression in McA-RH7777 cells facilitates second-step particle lipidation, thereby resulting in the secretion of larger, more TG-enriched lipoproteins.
Fig. 5.
Effect of apoA-IV expression on VLDL size distribution. Tet-inducible apoA-IV McA-RH7777 cells were incubated without (−) or with (+) 1 µg/ml doxycycline (Dox) for 48 h and then treated with DMEM containing 20% FBS and 0.4 mM oleate complexed to 0.75% BSA for 4 h. A: Cell lysates (50 µg) were analyzed by 12.5% SDS-PAGE, followed by immunoblot analysis with anti-apoA-IV antibody. B: Media were harvested and subjected to cumulative rate flotation ultracentrifugation, as described in Refs. 12 and 36. The size distributions in the VLDL1 fractions were determined using dynamic laser light scattering. C: Peak VLDL1 particle diameters (mean ± SE; n = 3; *P < 0.001 by unpaired t-test).
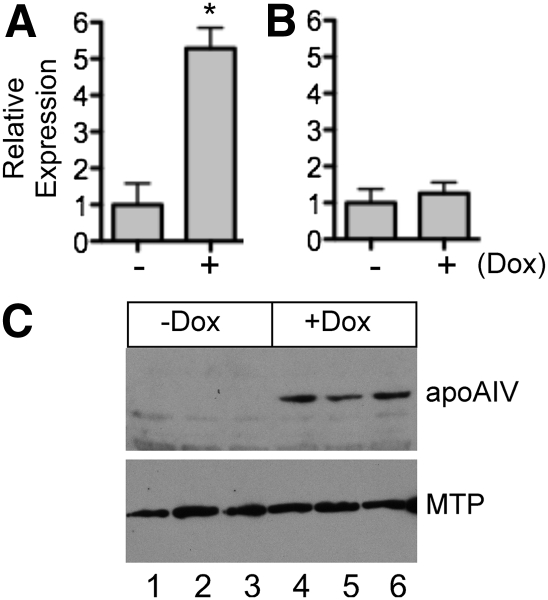
Dox-induced apoA-IV expression in McA-RH7777 cells does not alter MTP gene expression
As it was previously reported that the effects of apoA-IV on TG transport in IPEC-1 cells was associated with an increase in endogenous MTP expression (41), we assessed whether the changes in lipid and lipoprotein secretion and particle characteristics observed in our Dox-inducible McA-RH7777 cells could also be attributable in whole or part to a similar upregulation of MTP. To assess the effect of apoA-IV expression on MTP, cells were incubated for 48 h either without or with Dox, and apoA-IV and MTP gene expression was measured by qPCR. As shown in Fig. 6A, as expected, incubation with Dox increased apoA-IV gene expression ∼5-fold; however, in the same cells, MTP gene expression displayed a nonsignificant increase of 25% (Fig. 6B). To confirm this outcome, extracts from both −Dox- and +Dox-treated cells were subjected to immunoblot analysis. While apoA-IV was upregulated by Dox, no detectable change in MTP protein abundance was observed (Fig. 4C).
Fig. 6.
Impact of Dox-induced expression of apoA-IV on MTP mRNA and protein abundance. Tet-inducible apoA-IV McA-RH7777 cells were incubated in the absence (−) or presence (+) of 1 µg/ml doxycycline (Dox) for 48 h and apoA-IV (A) and MTP (B) gene expression were measured by qPCR, normalized to GAPDH, and shown as the fold increase in the presence of Dox. Data are mean ± SD; n = 3; *P < 0.001 by unpaired t-test. C: Immunoblot analysis of transfected human apoA-IV and endogenous rat MTP.
DISCUSSION
Previous studies in IPEC-1 cells have demonstrated a direct impact of apoA-IV on the biogenesis of TG-rich lipoproteins (32, 33), specifically, that expression of native and C-terminal–modified human apoA-IV constructs increases transcellular TG transport by enabling secretion of larger TG-rich lipoproteins, while decreasing secretion of apoB. As our previous study in COS cells found evidence for a direct presecretory protein-protein interaction between apoA-IV and apoB (34), we asked whether apoA-IV could facilitate lipoprotein expansion by altering the trafficking kinetics of apoB. To examine this issue, we used McA-RH7777 cells as a model, because they possesses the requisite cellular machinery (i.e., constitutive expression of apoB and MTP) to assemble and secrete TG-rich lipoproteins in the VLDL size range (42). Furthermore, whereas rodent hepatocytes express endogenous apoA-IV and its expression is strongly induced by increased liver fat content (43–45), McA-RH7777 cells lack apoA-IV expression and thus provide an essentially null background (35). Using this system, we observed that although transfection of apoA-IV-KDEL and native apoA-IV both reduced the secretory efficiency of apoB relative to control cells, apoA-IV-KDEL markedly inhibited cellular TG secretion, whereas native apoA-IV increased TG secretion into media, the association of apoB with d < 1.006 g/ml lipoproteins, and the diameter of VLDL1 particles. Considered together, these data suggest that apoA-IV may act as a chaperone that modulates the trafficking of apoB through the secretory pathway, thereby prolonging the residence time of nascent apoB-containing particles in cellular compartments where lipidation occurs (13, 14, 16, 46), enhancing TG loading and, ultimately, increasing TG secretion.
Although these data corroborate the previous studies of Lu et al., who found that constitutive and Dox-induced overexpression of apoA-IV in IPEC-1 cells facilitates transcellular TG transport by increasing lipoprotein size (32, 33), they are seemingly at odds with work of Yao et al. (35). These investigators observed that constitutive expression of rat apoA-IV under control of the constitutive CMV promoter in McA-RH7777 cells had no obvious effect on the size of d < 1.006 g/ml lipoprotein particles as assessed by electron microscopy (35). However, these studies used serum-free media and a much lower concentration of oleate (0.1 mM), which may have resulted in suboptimal stimulation of apoB and VLDL secretion, thereby obscuring an effect of apoA-IV. Another important caveat in comparing these studies is that rat apoA-IV is much more hydrophobic than human apoA-IV (29) and it lacks one of four EQQQ repeats that are a unique feature of the human apoA-IV C terminus (47). As discussed below, this deletion occurs in a region that may mediate the interaction between apoA-IV and apoB.
Given the critical role of MTP in the assembly and secretion of TG-rich lipoproteins (5) it is pertinent to consider whether the impact of apoA-IV on TG secretion observed in the present studies was mediated by an effect on MTP gene expression. Using the IPEC-1 intestinal cell model, Yao et al. (40) observed that Dox-induced expression of swine apoA-IV increased MTP gene expression by ∼80%; in a previous study, this was associated with a 2-fold increase in TG secretion (33). However, Dox-induced expression of a truncated “pig-like” human apoA-IV (lacking the distinctive C-terminal repeated EQQQ motif) increased MTP gene expression by ∼50%, but in the previous study, this was associated with a 25-fold increase in TG secretion (33). In the present study, we observed that Dox-induced expression of human apoA-IV caused only a 25% increase in MTP mRNA abundance and no detectable change in protein levels (Fig. 6), with an associated 1.4-fold increase in TG secretion. Differences in the cell models and the structures of the transfected apoA-IV proteins preclude a direct comparison among these findings. Nonetheless, we believe that the nonsignificant induction of MTP gene expression may not be the sole factor determining the impact of apoA-IV on TG-rich lipoprotein assembly in our hepatocyte model.
We previously noted that the inhibitory effect of apoA-IV-KDEL on apoB secretion in COS cells appears between apoB21–25 and is independent of apoB lipidation (34). Furthermore, IPEC-1 cell transfection studies found that deletion of residues 345 to 357 in the apoA-IV C terminus increases transcellular TG transport 25-fold but that additional truncation by only 11 residues completely ablates the effect (33). These data are consistent with a protein-protein interaction between specific domains in apoA-IV and apoB, and suggest that apoA-IV may act as a secretory pathway chaperone for apoB. In this regard, apoA-IV displays slower secretion kinetics than apoB (34, 35), and thus, its association with apoB could retard export of apoB-containing TG-rich particles from the ER and/or slow their passage through post-ER lipidation compartments. ApoA-IV could also serve as a bridge between apoB and other ER chaperone proteins that modulate apoB secretion, such as ERp72, GRP94, calreticulin, and BiP (48, 49).
While these data elucidate how apoA-IV could modulate lipoprotein assembly and lipid absorption at the cellular level, the puzzling issue has remained that no obvious phenotypes were observed in either apoA-IV knockout (50) or human apoA-IV transgenic mice (51). One possibility is that in these studies lipid absorption was determined by plasma TG appearance curves after a single lipid bolus. Because the intestine possesses a large reserve capacity for lipid absorption (52, 53), it is possible that these animals were not given a big enough lipid challenge to discern an effect of apoA-IV deletion or overexpression on dietary fat absorption efficiency. In this regard, we recently reported that although fat balance and lymph duct cannulation studies in wild-type and apoA-IV knockout mice found no effect of apoA-IV on total dietary fat absorption, lipid transport studies using everted gut sacs revealed that apoA-IV facilitates TG transport in the proximal gut (54). These data establish that the ability of apoA-IV to facilitate TG absorption at the cellular level is in fact discernible at the suborgan level but is masked in the intact animal by the functional redundancy of the small bowel. Thus, the impact of apoA-IV gene expression on intestinal lipid absorption in vivo may be manifest only under circumstances where intestinal lipid transport capacity is impaired, such as the neonatal period (55). For example, estrogen-related receptor-α knockout mice display decreased intestinal apoA-IV levels and impaired fat absorption in pups but not in adults (56).
In summary, these data establish that both constitutive and regulated expression of human apoA-IV in McA-RH7777 rat hepatoma cells alters the secretory trafficking of apoB and facilitates TG transport by increasing the size and core TG content of lipoproteins. When considered in the light of previous work on the structure and function of apoA-IV and apoB, we propose that by functioning as a secretory chaperone for apoB, apoA-IV increases the residence time of nascent apoB-containing lipoproteins in intracellular compartments where second-step lipidation occurs, thereby allowing more time for them to undergo core expansion before secretion. In the liver, increased apoA-IV expression induced by hepatic fat accumulation could play a role in mediating more efficient TG export (57). In the intestine, such intracellular actions of apoA-IV, under certain specific physiological conditions, could facilitate more efficient absorption of dietary lipids.
Footnotes
Abbreviations:
- Dox
- doxycycline, ER, endoplasmic reticulum
- IPEC-1
- porcine neonatal intestinal cell line
- MTP
- microsomal triglyceride transfer protein
- Neo
- neomycin
- PL
- phospholipid
- qPCR
- quantitative PCR
- Tet
- tetracycline
- TG
- triglyceride
This work was supported by National Institutes of Health Grants HL-49373 (G.S.S.) and HL-30897 (R.B.W.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. J.W.G. was supported by a predoctoral fellowship from the American Heart Association, Mid-Atlantic Affiliate.
REFERENCES
- 1.Black D. D. 2007. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G519–G524 [DOI] [PubMed] [Google Scholar]
- 2.Shelness G. S., Ledford A. S. 2005. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr. Opin. Lipidol. 16: 325–332 [DOI] [PubMed] [Google Scholar]
- 3.Hussain M. M., Shi J., Dreizen P. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44: 22–32 [DOI] [PubMed] [Google Scholar]
- 4.Iqbal J., Hussain M. M. 2009. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296: E1183–E1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain M. M., Rava P., Pan X., Dai K., Dougan S. K., Iqbal J., Lazare F., Khatun I. 2008. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr. Opin. Lipidol. 19: 277–284 [DOI] [PubMed] [Google Scholar]
- 6.Shoulders C. C., Shelness G. S. 2005. Current biology of MTP: implications for selective inhibition. Curr. Top. Med. Chem. 5: 283–300 [DOI] [PubMed] [Google Scholar]
- 7.Olofsson S. O., Asp L., Borén J. 1999. The assembly and secretion of apolipoprotein B-containing lipoproteins. Curr. Opin. Lipidol. 10: 341–346 [DOI] [PubMed] [Google Scholar]
- 8.Alexander C. A., Hamilton R. L., Havel R. J. 1976. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J. Cell Biol. 69: 241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shelness G. S., Sellers J. A. 2001. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 12: 151–157 [DOI] [PubMed] [Google Scholar]
- 10.Valyi-Nagy K., Harris C., Swift L. L. 2002. The assembly of hepatic very low density lipoproteins: evidence of a role for the Golgi apparatus. Lipids. 37: 879–884 [DOI] [PubMed] [Google Scholar]
- 11.Kulinski A., Rustaeus S., Vance J. E. 2002. Microsomal triglyceride transfer protein is required for lumenal accretion of triacylglycerol not associated with apo B, as well as for apoB lipidation. J. Biol. Chem. 277: 31516–31525 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Tran K., Yao Z. 1999. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within the microsomes in McA-RH7777 cells. J. Biol. Chem. 274: 27793–27800 [DOI] [PubMed] [Google Scholar]
- 13.Tran K., Thorne-Tjomsland G., DeLong C. J., Cui S., Shan J., Burton L., Jamieson J. C., Yao Z. 2002. Intracellular assembly of very low density lipoproteins containing apoB100 in rat hepatoma McA-RH7777 cells. J. Biol. Chem. 277: 31187–31200 [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi J., Gamble M. V., Conlon D., Liang J. S., Ginsberg H. N. 2003. The conversion of apoB100 low density lipoprotein/high density lipoprotein particles to apoB100 very low density lipoproteins in response to oleic acid occurs in the endoplasmic reticulum and not in the Golgi in McA RH7777 cells. J. Biol. Chem. 278: 42643–42651 [DOI] [PubMed] [Google Scholar]
- 15.Hamilton R. L., Wong J. S., Cham C. M., Nielsen L. B., Young S. G. 1998. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J. Lipid Res. 39: 1543–1557 [PubMed] [Google Scholar]
- 16.Rusiñol A., Verkade H., Vance J. E. 1993. Assembly of rat hepatic very low density lipoproteins in the endoplasmic reticulum. J. Biol. Chem. 268: 3555–3562 [PubMed] [Google Scholar]
- 17.Tso P., Balint J. A. 1986. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am. J. Physiol. 250: G715–G726 [DOI] [PubMed] [Google Scholar]
- 18.Farese R. V., Jr, Véniant M. M., Cham C. M., Flynn L. M., Pierotti V., Loring J. F., Traber M., Ruland S., Stokowski R. S., Huszar D., et al. 1996. Phenotypic analysis of mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. Proc. Natl. Acad. Sci. USA. 93: 6393–6398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo C. C., Li W. H., Moore M. N., Chan L. 1986. Structure and evolution of the apolipoprotein multigene family. J. Mol. Biol. 187: 325–340 [DOI] [PubMed] [Google Scholar]
- 20.Green P. H., Glickman R. M., Saudek C. D., Blum C. B., Tall A. R. 1979. Human intestinal lipoproteins. Studies in chyluric subjects. J. Clin. Invest. 64: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi H., Nutting D. F., Fujimoto K., Cardelli J. A., Black D., Tso P. 1990. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J. Lipid Res. 31: 1613–1625 [PubMed] [Google Scholar]
- 22.Stan S., Delvin E., Lambert M., Seidman E., Levy E. 2003. Apo A-IV: an update on regulation and physiologic functions. Biochim. Biophys. Acta. 1631: 177–187 [DOI] [PubMed] [Google Scholar]
- 23.Kalogeris T. J., Fukagawa K., Tso P. 1994. Synthesis and lymphatic transport of intestinal apolipoprotein A-IV in response to graded doses of triglyceride. J. Lipid Res. 35: 1141–1151 [PubMed] [Google Scholar]
- 24.Kalogeris T. J., Rodriguez M-D., Tso P. 1997. Control of synthesis and secretion of intestinal apolipoprotein A-IV by lipid. J. Nutr. 127: 537S–543S [DOI] [PubMed] [Google Scholar]
- 25.Kalogeris T. J., Monroe F., Demichele S. J., Tso P. 1996. Intestinal synthesis and lymphatic secretion of apolipoprotein A-IV vary with chain length of intestinally infused fatty acids in rats. J. Nutr. 126: 2720–2729 [DOI] [PubMed] [Google Scholar]
- 26.Tso P., Balint J. A., Bishop M. B., Rodgers J. B. 1981. Acute inhibition of intestinal lipid transport by Pluronic L-81 in the rat. Am. J. Physiol. 241: G487–G497 [DOI] [PubMed] [Google Scholar]
- 27.Kumar N. S., Mansbach C. M., 2nd 1999. Prechylomicron transport vesicle: isolation and partial characterization. Am. J. Physiol. 276: G378–G386 [DOI] [PubMed] [Google Scholar]
- 28.Weinberg R. B., Anderson R. A., Cook V. R., Emmanuel F., Denefle P., Hermann M., Steinmetz A. 2000. Structure and interfacial properties of chicken apolipoprotein A-IV. J. Lipid Res. 41: 1410–1418 [PubMed] [Google Scholar]
- 29.Weinberg R. B., Cook V. R., DeLozier J. A., Shelness G. S. 2000. Dynamic interfacial properties of human apolipoprotein A-IV and B17 at the air/water and oil/water interface. J. Lipid Res. 41: 1419–1427 [PubMed] [Google Scholar]
- 30.Green P. H., Glickman R. M., Riley J. W., Quinet E. 1980. Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J. Clin. Invest. 65: 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koga S., Miyata Y., Funakoshi A., Ibayashi H. 1985. Plasma apolipoprotein A-IV levels decrease in patients with chronic pancreatitis and malabsorption syndrome. Digestion. 32: 19–24 [DOI] [PubMed] [Google Scholar]
- 32.Lu S., Yao Y., Meng S., Cheng D., Black D. D. 2002. Overexpression of apolipoprotein A-IV enhances lipid transport in newborn swine epithelia cells. J. Biol. Chem. 277: 31929–31937 [DOI] [PubMed] [Google Scholar]
- 33.Lu S., Yao Y., Cheng X. Y., Mitchell S., Leng S. Y., Meng S. M., Gallagher J. W., Shelness G. S., Morris G. S., Mahan J., et al. 2006. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J. Biol. Chem. 281: 3473–3483 [DOI] [PubMed] [Google Scholar]
- 34.Gallagher J. W., Weinberg R. B., Shelness G. S. 2004. ApoA-IV tagged with the ER retention signal KDEL perturbs the intracellular trafficking and secretion of apoB. J. Lipid Res. 45: 1826–1834 [DOI] [PubMed] [Google Scholar]
- 35.Yao Z., Lauer S. J., Sanan D. A., Fazio S. 1993. ApoA-IV is secreted on discrete HDL particles by the rat hepatoma cell line McA-RH7777 transfected with ApoA-IV cDNA. Arterioscler. Thromb. 13: 1476–1486 [DOI] [PubMed] [Google Scholar]
- 36.Blade A. M., Fabritius M. A., Hou L., Weinberg R. B., Shelness G. S. 2011. Biogenesis of apolipoprotein A-V and impact its on VLDL triglyceride secretion. J. Lipid Res. 52: 237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sellers J. A., Shelness G. S. 2001. Lipoprotein assembly capacity of the mammary tumor-derived cell line C127 is due to the expression of functional microsomal triglyceride transfer protein. J. Lipid Res. 42: 1897–1904 [PubMed] [Google Scholar]
- 38.Sellers J. A., Hou L., Athar H., Hussain M. M., Shelness G. S. 2003. A Drosophila microsomal triglyceride transfer protein homolog promotes the assembly and secretion of human apolipoprotein B - implications for human and insect lipid transport and metabolism. J. Biol. Chem. 278: 20367–20373 [DOI] [PubMed] [Google Scholar]
- 39.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. 1985. Protein assay using bicinchoninic acid. Anal. Biochem. 150: 76–85 [DOI] [PubMed] [Google Scholar]
- 40.Cheng D., MacArthur P. S., Rong S., Parks J. S., Shelness G. S. 2010. Alternative splicing attenuates transgenic expression directed by the ApoE promoter-enhancer based expression vector, pLIV11. J. Lipid Res. 51: 849–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Y., Lu S., Huang Y., Beeman-Black C. C., Lu R., Pan X., Hussain M. M., Black D. D. 2011. Regulation of microsomal triglyceride transfer protein by apolipoprotein A-IV in newborn swine intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 300: G357–G363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borén J., Rustaeus S., Olofsson S-O. 1994. Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem. 269: 25879–25888 [PubMed] [Google Scholar]
- 43.Langner C. A., Birkenmeier E. H., Ben-Zeev O., Schotz M. C., Sweet H. O., Davisson M. T., Gordon J. I. 1989. The fatty liver dystrophy (fld) mutation. A new mutant mouse with a developmental abnormality in triglyceride metabolism and associated tissue-specific defects in lipoprotein lipase and hepatic lipase activities. J. Biol. Chem. 264: 7994–8003 [PubMed] [Google Scholar]
- 44.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inui Y., Keno Y., Fukuda K., Igura T., Makamura T., Tokunaga K., Kawata S., Matsuzawa Y. 1997. Modulation of apolipoprotein gene expression in fatty liver of obese rats: enhanced APOA-IV, but no APOB expression by a high sucrose diet. Int. J. Obes. Relat. Metab. Disord. 21: 231–238 [DOI] [PubMed] [Google Scholar]
- 46.Gusarova V., Seo J., Sullivan M. L., Watkins S. C., Brodsky J. L., Fisher E. A. 2007. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J. Biol. Chem. 282: 19453–19462 [DOI] [PubMed] [Google Scholar]
- 47.Weinberg R. B. 1994. Identification of functional domains in the plasma apolipoproteins by analysis of inter-species sequence variability. J. Lipid Res. 35: 2212–2222 [PubMed] [Google Scholar]
- 48.Zhang J., Herscovitz H. 2003. Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B. J. Biol. Chem. 278: 7459–7468 [DOI] [PubMed] [Google Scholar]
- 49.Linnik K. M., Herscovitz H. 1998. Multiple molecular chaperones interact with apolipoprotein B during its maturation. J. Biol. Chem. 273: 21368–21373 [DOI] [PubMed] [Google Scholar]
- 50.Weinstock P. H., Bisgaier C. L., Hayek T., Aalto-Setala K., Sehayek E., Wu L., Sheiffele P., Merkel M., Essenburg A. D., Breslow J. L. 1997. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J. Lipid Res. 38: 1782–1794 [PubMed] [Google Scholar]
- 51.Aalto-Setälä K., Bisgaier C. L., Ho A., Kieft K. A., Traber M. G., Kayden H. J., Ramakrishnan R., Walsh A., Essenburg A. D., Breslow J. L. 1994. Intestinal expression of human apolipoprotein A-IV in transgenic mice fails to influence dietary lipid absorption or feeding behavior. J. Clin. Invest. 93: 1776–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carey M. C., Small D. M., Bliss C. M. 1983. Lipid digestion and absorption. Annu. Rev. Physiol. 45: 651–677 [DOI] [PubMed] [Google Scholar]
- 53.Field F. J., Mathur S. N. 1995. Intestinal lipoprotein synthesis and secretion. Prog. Lipid Res. 34: 185–198 [DOI] [PubMed] [Google Scholar]
- 54.Simon T., Cook V. R., Rao A., Weinberg R. B. 2011. Impact of murine intestinal apolipoprotein A-IV expression on regional lipid absorption, gene expression, and growth. J. Lipid Res. 52: 1984–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy E., Ménard D. 2000. Developmental aspects of lipid and lipoprotein synthesis and secretion in human gut. Microsc. Res. Tech. 49: 363–373 [DOI] [PubMed] [Google Scholar]
- 56.Carrier J. C., Deblois G. V., Champigny C., Levy E., Giguère V. 2004. Estrogen-related receptor alpha (ERRalpha) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. J. Biol. Chem. 279: 52052–52058 [DOI] [PubMed] [Google Scholar]
- 57.Horton J. D., Shimano H., Hamilton R. L., Brown M. S., Goldstein J. L. 1999. Disruption of LDL receptor gene in transgenic SREBP-1a mice unmasks hyperlipidemia resulting from production of lipid-rich VLDL. J. Clin. Invest. 103: 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]