The common peroneal nerve and the deep peroneal nerve are the most commonly injured nerves in the lower limb. Injury, such as trauma or peripheral nerve lesions, can lead to permanent weakness and paresis of toe and ankle dorsiflexors. Although nonoperative, orthotic treatment options exist, they are associated with a significantly lower quality of life in several domains. Others, such as decompression at the fibular head and tendon transfers, often result in poor functional outcomes, especially when compared with similar reconstructive procedures performed in major nerves of the upper extremity. To date, however, nerve transfers using expendable donor nerves in the lower limb have been regarded as experimental and have yet to be thoroughly investigated in the lower limb. Accordingly, this anatomical study aimed to identify potential and expendable donors of motor axons from the tibial nerve to reanimate damaged common peroneal nerves.
Keywords: Foot drop, Lower extremity reinnervation, Lower leg reanimation, Never transfer, Peroneal nerve
Abstract
BACKGROUND:
The common peroneal nerve is the most commonly injured nerve in the lower limb. Nerve transfer using expendable donor nerves is emerging in the literature as an alternative surgical procedure to traditional treatments.
OBJECTIVE:
To identify potential donors of motor axons from the tibial nerve that can be transferred to the common peroneal nerve branches.
METHODS:
Using 10 human cadaveric lower extremities, all motor nerve branches of the tibial nerve were identified and biopsied. These were compared with the motor branches to tibialis anterior and extensor hallucis longus (branches of the deep peroneal nerve).
RESULTS:
The most suitable donor nerves with respect to cross-sectional area to tibialis anterior (cross sectional area [mean ± SD] 0.255±0.111 mm) was the motor branch to lateral gastrocnemius (0.256±0.105 mm). When comparing the total number of axons, the branch to the tibialis anterior had a mean of 3363±1997 axons. The branch to the popliteus was most similar, with 3317±1467 axons. The most suitable donor nerves for the motor branch to extensor hallucis longus (cross sectional area 0.197±0.302 mm) with respect to cross-sectional area was the motor branch to flexor hallucis longus (0.234±0.147 mm). When comparing the total number of axons, the branch to the extensor hallucis longus had an average of 2062±2314 axons. The branch to the lateral gastrocnemius was most similar with 2352±1249 axons and was a suitable donor.
CONCLUSION:
Nerve transfers should be included in the armamentarium for lower extremity reinnervation, as it is in the upper limb.
Abstract
HISTORIQUE :
Le nerf péronier commun est le nerf des membres inférieurs qui subit le plus de blessures. Le transfert nerveux au moyen de nerfs sacrifiables de donneurs émerge dans les publications comme une intervention chirurgicale qui remplace les traitements classiques.
OBJECTIF :
Déterminer les donneurs potentiels d’axones moteurs du nerf tibial qui peuvent être transférés aux branches du nerf péronier commun.
MÉTHODOLOGIE :
Au moyen de dix membres inférieurs cadavériques humains, les chercheurs ont repéré toutes les branches nerveuses motrices du nerf tibial et en ont fait la biopsie. Ils les ont comparées avec les branches motrices du muscle tibial antérieur et du muscle long extenseur de l’hallux (branches du nerf péronier profond).
RÉSULTATS :
Les nerfs de donneurs qui convenaient le mieux à l’égard de la région transversale du muscle tibial antérieur (région transversale [moyenne±ÉT] de 0,255±0,111 mm) étaient la branche motrice du muscle gastrocnémien latéral (0,256±0,105 mm). Par rapport au nombre total d’axones, la branche du muscle tibial antérieur présentait une moyenne de 3 363±1 997 axones. La branche du muscle poplité était la plus similaire, avec 3 317±1 467 axones. Les nerfs de donneurs qui convenaient le mieux à la branche motrice du muscle long extenseur de l’hallux (région transversale de 0,197±0,302 mm) à l’égard de la région transversale étaient la branche motrice du muscle long fléchisseur de l’hallux (0,234±0,147 mm). Par rapport au nombre total d’axones, la branche du muscle long extenseur de l’hallux avait une moyenne de 2 062±2 314 axones. La branche du muscle gastrocnémien latéral était la plus similaire, avec 2 352±1 249 axones, et constituait un donneur convenable.
CONCLUSION :
Les transferts nerveux devraient faire partie de l’armada de réinnervation des membres inférieurs, comme ils le sont dans les membres supérieurs.
The common peroneal nerve (CPN) is the most commonly injured nerve in the lower limb. Injury to the CPN and, more specifically, the deep peroneal nerve (DPN), can lead to permanent weakness or paresis of toe and ankle dorsiflexors. Causes include trauma, dislocation, iatrogenic injury and peripheral nerve lesions (1). Patients experience difficulties with the swing phase of gait. To compensate, patients adopt a high stepping gait to lift the foot higher off the ground. These injuries can be treated nonoperatively using an ankle-foot orthotic. It is known that an individual using an ankle-foot orthotic has a significantly lower quality of life in the domains of physical functioning, mental health, vitality, bodily pain and general health perception when compared with a patient without an impairment necessitating the device (2).
Current surgical treatments include decompression at the fibular head for compressive palsies (3,4). Injuries that do not show signs of recovery require early nerve grafting to reconstruct the injured nerve segment with or without tendon transfers (5,6). Tendon transfers alone may also be performed depending on the duration of time since injury (7). However, for both of these procedures, functional outcomes are often poor compared with similar reconstructive procedures for other major nerves in the upper extremity.
The current literature characterizes nerve transfers using expendable donor nerves in the lower limb as experimental or alternative surgical procedures, rather than as an accepted surgical option. The authors suggest that this may be a preferred treatment plan – over other traditional options – for several reasons. Nerve transfers minimize dissection and potential injury to surrounding tissues. They facilitate faster recovery because they can lead to more rapid reinnervation than nerve grafts due to the fact that the transferred axons are closer to the targeted motor end plates. In addition, nerve transfers avoid the problem of ‘missed zone of injury’ when using nerve grafts. This is of particular concern for the common peroneal nerve, which can suffer significant and widespread stretch in lateral knee injuries.
Nerve transfers are common and effective treatment for the timely restoration of motor and sensory function for nerve injuries in the upper limb and facial palsy (8,9). Nerve transfers, however, have yet to be thoroughly investigated in the lower limb.
Case reports and case series describing the surgical technique and the results for lower limb nerve transfer have been published. This includes the introduction and novel description of the procedure for CPN palsy (10). In this particular case series, the authors performed a nerve transfer of the anterior tibial nerve with branches from the soleus and lateral gastrocnemius. The case series consisted of eight children and one adult. The adult patient and six of the children, all of whom had nerve palsies of less than eight months duration, experienced total resolution of the foot drop within one year of the surgery. The two other children described in the study experienced a nerve palsy of 14 and 16 months duration at the time of surgery, and their foot drop did not resolve after nerve transfer.
Another article published in 2008 (11) described foot reanimation with nerve transfer of functional fascicles from either the superficial peroneal nerve or the tibial nerve. The article reported that 11 of 14 patients had successful restoration of British motor grade 3+ to 4/5 ankle dorsiflexion, and one patient had restoration of grade 3 ankle dorsiflexion.
It has been suggested that one important factor in the selection of ideal nerve donors is a high and comparable axon count. Mackinnon et al (12) demonstrated that matching appropriate sized nerves was key in obtaining the optimal functional outcome of nerve repair. In this animal model study, nerves were grafted in specific ratios of 1:1, 2:1, and 2.5:1. The results showed that the distal axon count actually increased with the 2.5:1 nerve ratio, but function was optimal in rats with the 1:1 donor to recipient axon ratio.
Given that the tibial nerve (TN) is often spared while the CPN can be severely injured, motor branches of the TN are considered to be reasonable donors based on the proximity and number of available, and potentially expendable, motor branches for CPN reanimation.
It is critical to select the appropriate and expendable motor nerves to reinnervate the ankle and toe extensors that will lead to more predictable and functional results in reconstructing the DPN compared with that achieved by nerve grafts. It is currently unknown, however, which branch is the best to restore function.
The purpose of the present anatomical study was to identify potential donors of motor axons from the TN that are comparable in size and total axons with the potential recipient motor branches of the DPN that require reconstruction (motor branch to tibialis anterior [TA] and to extensor hallucis longus [EHL]). TA and EHL are the two target muscles that we chose to investigate because these muscles are primarily involved in ankle and toe dorsiflexion and, thus, counteract foot drop.
METHODS
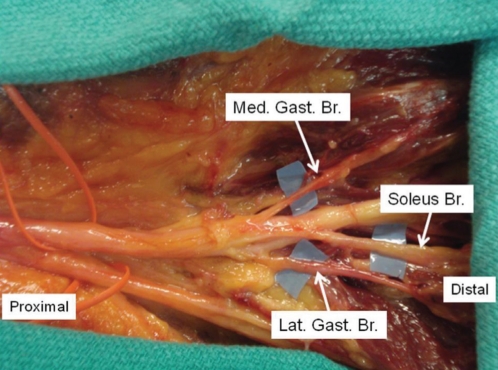
Using fresh, human cadaveric lower extremities, all motor nerve branches of the TN were identified, isolated and sectioned (Figure 1). To compare axon counts, sections of the motor branches of the DPN were also harvested.
Figure 1).
Branches of the tibial nerve. Lat Gast Br Lateral gastrocnemius branch; Med Gast Br Medial gastrocnemius branch; Soleus Br Soleus branch
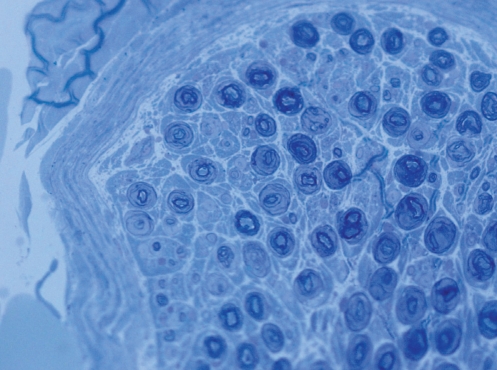
Excised nerves were fixed in Bilbao’s nerve fixative (2.5% glutaraldehyde in 0.025M sodium cacodylate buffer), then postfixed in 1% osmium tetroxide in 0.1M sodium cacodylate buffer. The nerves were dehydrated through a graded ethanol series (50%, 70%, 70%, 95%, 95%, 100% and 100%) and further dehydrated in two changes of propylene oxide. The samples were slowly infiltrated with increasing ratios of Epon/Araldite resin: propylene oxide, followed by several changes in 100% Epon/Araldite resin, oriented for transverse sectioning and polymerized overnight in an oven at 60°C. Sections (1 μm) were cut using a Leica Ultracut UCT microtome (Leica, Germany) and transferred onto glass slides. Dried sections were stained with Toluidine Blue O, and images of the entire transverse sections were collected using a Zeiss AxioCam (Carl Ziess Inc, Germany) high-resolution camera mounted on a Zeiss Axiovert 100 light microscope using a 63× water immersion lens (Figure 2). Cross-sectional area measurements and axon counts were performed using MetaMorph software (Universal Imaging Co, USA).
Figure 2).
Branch to the lateral gastrocnemius cross section. Toluoidine blue O stain, original magnification ×63
All axons in a maximum of three sections for each motor branch were manually counted. The mean and SD of axon counts for each nerve branch were calculated. Samples were taken from different sites. Nerve bundles were reported as area in square millimetres and number of axons per bundle.
A total of 10 legs were dissected, and the following TN branches were sampled: lateral gastrocnemius, medial gastrocnemius, popliteus, soleus, tibialis posterior, flexor hallucis longus. The recipient nerve branches of the CPN were also dissected, which included TA and EHL.
RESULTS
The motor branch to the TA muscle had a mean (± SD) cross sectional area of 0.255±0.111 mm and a mean of 3363±1997 axons. This was most similar in cross sectional area to the branch to lateral gastrocnemius (0.256±0.105 mm) and in total number of axons to the branch to popliteus (3317±1467) (Table 1).
TABLE 1.
Cross sectional area and total axon count of potential nerve donors
| Motor branch | Area, mm2 | Axons, n |
|---|---|---|
| Tibialis anterior | 0.255±0.111 | 3363±1997 |
| Extensor hallucis longus | 0.197±0.302 | 2062±2314 |
| Flexor hallucis longus | 0.234±0.147 | 1557±735 |
| Latissimus gastrocnemius | 0.256±0.105 | 2352±1249 |
| Medial gastrocnemius | 0.309±0.101 | 2834±718 |
| Popliteus | 0.425±0.421 | 3317±1467 |
| Soleus | 0.700±0.222 | 4941±1994 |
| Tibialis posterior | 0.348±0.253 | 3039±1528 |
Data presented as mean ± SD
The motor branch to EHL was found to have a mean cross sectional area of 0.197±0.302 mm and a mean of 2062±2314 axons. This was most similar in cross-sectional area to flexor hallucis longus (0.234±0.147 mm) and total number of axons to the branch to lateral gastrocnemius (2352±1249) (Table 1).
DISCUSSION
The present anatomical study identified potential donors of motor axons from the TN that could theoretically be transferred to branches of the CPN (specifically, motor branch to TA and to EHL, which are branches of the DPN). It has been shown that for the best functional outcome, it is imperative that nerve branches of similar size and axon count are used. Nerve donors and recipients comparable in size and total axons to the potential recipient motor branches of the DPN that require reconstruction is not something that has been extensively reported in the literature.
In 2009, a study explored the anatomical feasibility of using a transinterosseous nerve transfer between the tibia and fibula to restore motor function to the TA muscle, following injury to the common peroneal nerve (resulting in foot drop) (13). The authors found that the distance from the coaptation site to the TA muscle was shortest for the transfer using the nerve branch to the soleus. Histologically, the nerve branch to the soleus was most similar to the branch to the TA for both axonal count and cross-sectional area.
Based on our results, the ideal donor nerves were identified. The motor branch to TA was found to have an average cross-sectional area of 0.255±0.111 mm and an average of 3363±1997 axons. The motor branch to the TA muscle was most similar in cross-sectional area to the branch to lateral gastrocnemius (0.256±0.105 mm) and in total number of axons to the branch to popliteus (3317±1467).
The motor branch to EHL was found to have a mean cross-sectional area of 0.197±0.302 mm and an average of 2062±2314 axons. This was most similar in cross sectional area to flexor hallucis longus (0.234±0.147 mm) and total number of axons to the branch to lateral gastrocnemius (2352±1249).
Thus, any of these nerve branches should be an acceptable match for a nerve transfer; however, because the present study used a purely cadaveric model, if an actual transfer were to be performed, the feasibility of the transfer of either of these nerves must be examined and the donor selected based on shortest distance to the recipient branch, which would allow for shorter time to reinnervation of the target muscle.
The present study also demonstrated that potential secondary donors are available with similar axon counts. All potential donors should be considered when selecting branches for transfer. There is obvious benefit when a muscle has duel innervations (eg, gastrocnemius) and one nerve branch may be harvested without sacrificing function for the entire muscle group (eg, transfer soleus and lateral gastrocnemius branches while preserving the medial gastrocnemius branch).
Several variations in nerve anatomy with a variety of branching patterns were seen during the cadaveric dissections. Therefore, one must consider each transfer independently; however, these findings show trends toward the best matches, and provide options for donor nerves to reconstruct ankle and toe dorsiflexion.
Limited sample size and confounding issues with sample size, as well as comorbidities of patients and counting errors, were potential sources of error in the present study.
The present study may prompt other research needed in the clinical application of nerve transfers in the lower limb. This includes conducting objective measures in functional loss for various donor nerves/muscles in patients undergoing CPN reconstruction with nerve transfers. There is also a possibility to prospectively measure functional outcomes of patients undergoing nerve transfers.
CONCLUSION
Potential donor nerves to restore ankle dorsiflexion by reinnervating the nerve branch to TA include the motor branches to lateral gastrocnemius and popliteus.
Potential donor nerves of EHL to restore great toe dorsiflexion include the motor branch to flexor hallucis longus and motor branch to lateral gastrocnemius.
We believe that nerve transfers should be included in the armamentarium for lower extremity reinnervation, as it is in the upper limb.
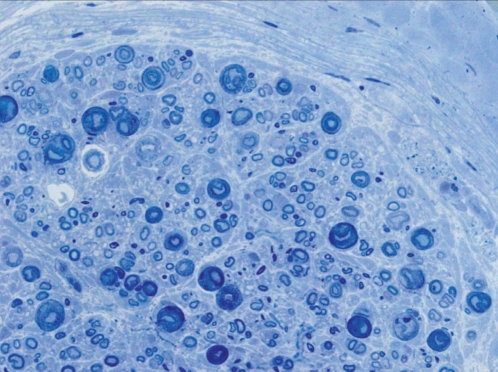
Figure 3).
Cross section of tibialis anterior. Toluoidine blue O stain, original magnification ×40
REFERENCES
- 1.Kim DH, Murovic JA, Tiel R, Kline DG. Management and outcomes in 353 surgically treated sciatic nerve lesions. J Neurosurg. 2004;101:8–17. doi: 10.3171/jns.2004.101.1.0008. [DOI] [PubMed] [Google Scholar]
- 2.de Bruijn IL, Geertzen JH, Dijkstra PU. Functional outcome after peroneal nerve injury. Int J Rehabil Res. 2007;30:333–7. doi: 10.1097/MRR.0b013e3282f14444. [DOI] [PubMed] [Google Scholar]
- 3.Thoma A, Fawcett S, Ginty M, Veltri K. Decompression of the common peroneal nerve: Experience with 20 consecutive cases. Plast Reconstr Surg. 2001;107:1183–9. doi: 10.1097/00006534-200104150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Humphreys DB, Novak CB, Mackinnon SE. Patient outcome after common peroneal nerve decompression. J Neurosurg. 2007;107:314–8. doi: 10.3171/JNS-07/08/0314. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson MC, Birch R. Repair of the common peroneal nerve. J Bone Joint Surg Br. 1995;77:501–3. [PubMed] [Google Scholar]
- 6.Ferraresi S, Garozzo D, Buffatti P. Common peroneal nerve injuries: Results with one-stage nerve repair and tendon transfer. Neurosurg Rev. 2003;26:175–9. doi: 10.1007/s10143-002-0247-4. [DOI] [PubMed] [Google Scholar]
- 7.De Marchi F, Malerba F, Montrasio Alfieri U, Ferrarin M, Rabuffetti M. Tibialis posterior tendon interosseal membrane in peroneal nerve transfer through the paralysis of the common. J Foot Ankle Surg. 2000;6:19–25. [Google Scholar]
- 8.Mackinnon SE, Colbert SH. Nerve transfers in the hand and upper extremity surgery. Tech Hand Up Extrem Surg. 2008;12:20–33. doi: 10.1097/BTH.0b013e31812714f3. [DOI] [PubMed] [Google Scholar]
- 9.Terzis JK, Konofaos P. Nerve transfers in facial palsy. Facial Plast Surg. 2008;24:177–93. doi: 10.1055/s-2008-1075833. [DOI] [PubMed] [Google Scholar]
- 10.Gousheh J, Babaei A. A new surgical technique for the treatment of high common peroneal nerve palsy. Plast Reconstr Surg. 2002;109:994–8. doi: 10.1097/00006534-200203000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Nath RK, Lyons AB, Paizi M. Successful management of foot drop by nerve transfers to the deep peroneal nerve. J Reconstr Microsurg. 2008;24:419–27. doi: 10.1055/s-0028-1082894. [DOI] [PubMed] [Google Scholar]
- 12.Mackinnon SE, Dellon AL, O’Brien JP, et al. Selection of optimal axon ratio for nerve regeneration. Ann Plast Surg. 1989;23:129–34. doi: 10.1097/00000637-198908000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Pirela-Cruz MA, Hansen U, Terreros DA, Rossum A, West P. Interosseous nerve transfers for tibialis anterior muscle paralysis (foot drop): A human cadaver-based feasibility study. J Reconstr Microsurg. 2009;25:203–11. doi: 10.1055/s-0028-1104548. [DOI] [PubMed] [Google Scholar]