Abstract
The performances of nine biosorbents derived from dead fungal biomass were investigated for their ability to remove Reactive Black 5 from aqueous solution. The biosorption data for removal of Reactive Black 5 were readily modeled using the Langmuir adsorption isotherm. Kinetic analysis based on both pseudo-second-order and Weber-Morris models indicated intraparticle diffusion was the rate limiting step for biosorption of Reactive Black 5 on to the biosorbents. Sorption capacities of the biosorbents were not correlated with the initial biosorption rates. Sensitivity analysis of the factors affecting biosorption examined by an artificial neural network model showed that pH was the most important parameter, explaining 22%, followed by nitrogen content of biosorbents (16%), initial dye concentration (15%) and carbon content of biosorbents (10%). The biosorption capacities were not proportional to surface areas of the sorbents, but were instead influenced by their chemical element composition. The main functional groups contributing to dye sorption were amine, carboxylic, and alcohol moieties. The data further suggest that differences in carbon and nitrogen contents of biosorbents may be used as a selection index for identifying effective biosorbents from dead fungal biomass.
Introduction
Large amounts of dyes are extensively used in the textile, leather, cosmetics, plastic, food, and pharmaceutical industries, in which many dyes are classified as both toxic to human health and harmful to aquatic ecosystems [1]. Therefore, many different treatment methods have been developed and used for dye removal, including chemical precipitation, reverse osmosis, ozonation, membrane filtration and photodegradation [2]. As many dye product industries are located in developing countries, high capital and operational costs or secondary sludge disposal problem has hindered the practical application of many of these methods [3]. Adsorption has been found to be one of the most efficient and low cost techniques for treating dye effluents. Adsorption can handle large flow rates, producing a high-quality effluent that does not result in the formation of harmful substances [4], [5]. Among the different materials studied to date, activated carbon is one of the most effective adsorbent for dyes. However, this adsorbent is relatively expensive and presents problems with regard to final disposal [6]. Alternative adsorbents have been proposed and are now being widely investigated. These include natural materials derived from waste materials from industry and agriculture, as well as biosorbents that are produced from microbial biomass. The latter are often found to be even more selective than traditional ion-exchange resins and activated carbons, and potentially provide an inexpensive method for dye removal [7].
Fungal biomass is a relatively inexpensive biosorbent that can be produced using simple fermentation techniques and inexpensive growth media. Prior studies with this material has shown that fungal biomass can be used been for removal of heavy metals, dyes and polycyclic aromatic hydrocarbons [8], [9], [10]. Dead fungal biomass could be further divided into two main forms in more specific: powdered fungal biomass [11] and fragmental fungal biomass [12]. Powdered fungal biomass loses almost all characteristics of fungal cell structures, whereas fungal cell structures could still be identified in fragmental fungal biomass. The mechanisms of biosorption involved surface adsorption/precipitation and intracellular accumulation depending on the location of the adsorbate [13]. With powdered fungal biomass, surface adsorption is likely mediated by ionic, chemical, and physical interactions.
Reactive Black 5 was selected in this study as a model anionic azo dye for adsorption experiment with its stability under different pH [14], [15], [16], which was abundantly used in textile industries for dyeing and found to be moderately toxic to Vibrio fischeri [17]. Nine strains of fungi were selected in this study, which belonged to ascomycota, basidiomycota and zygomycota, respectively and widely used as biosorbents for pollutants removal. In this research, the main aim was to investigate biosorption mechanisms for Reactive Black 5 on to powdered fungal biomass and the role of textural and chemical element compositions on adsorption capacities. Firstly, adsorption kinetics and isotherms were used to model the experimental data to discover the biosorption mechanisms. Then, a constructed artificial neural network was used to model the influence of differences in their textural and chemical element compositions on adsorption capacities and to identify the key physicochemical parameters that affected the biosorption process.
Materials and Methods
Materials and Microorganisms
Reactive Black 5 was purchased from Sigma-Aldrich Company and its detail information was shown in Table S1. Potato-dextrose broth was made in the laboratory according to descriptions for ATCC media number 336 without agar and adjusted to pH 5.5. Nine strains of fungi used in this study were isolated from soil and preserved in our laboratory and included Absidia coerulea 118 (abbreviation: F1), Aspergillus oryzae A4 (F2), Cunninghamella echinulata 102 (F3), Cunninghamella echinulata 2 (F4), Penicillium commune YW01 (F5), Penicillum griseofulvum A5 (F6); Phanerochete chrysosporium A3 (F7), Rhizopus nigricans A1 (F8), Trichoderma asperellum 72 (F9). They were all maintained on PDA plate.
Preparation of Biosorbents
Powdered fungal biomass from each of the strains was produced in flasks containing potato-dextrose broth at 30°C using a horizontal shaker at 200 rpm for aeration. The flasks were inoculated with 3.5×107 mL−1 spores and shaken for 5 days, except with Phanerochete chrysosporium for 8 days. After growth, the biomass was separated from the culture broth by filtration, and washed with generous amounts of distilled water. The washed biomass was killed by autoclaving at 121°C for 20 min and dried overnight at 50°C. The dried biomass was then ground to power in a disintegrator and sieved through a No. 60 standard sieve to obtain uniform size for the biosorption studies.
Biosorbent Characterization
The surface areas of the biosorbents were determined using the Brunauer-Emmett-Teller (BET) method at 77°K using liquid nitrogen [18]. The measurements were carried out with an Autosorp-1-C instrument, Quantachrome. The total pore volume and average pore size were determined at a relative pressure (P/P0) of 1. Chemical analyses of nitrogen, carbon and hydrogen contents of biosorbents were carried out using a Flash EA 112 instrument (ThermoFinnigan). Biosorbents F1, F4, F5 and F7 were selected for surface morphologies and FTIR analysis based on the biosorption capacities for Reactive Black 5 and the extreme values of BET surface area and chemical element compositions. Surface morphologies of four selected biosorbents were determined by Scanning Electron Microscopy at 20 kV and 6,000 magnifications after coating with thin layer gold under reduced pressure [19]. Fourier transform infrared spectroscopy (FTIR) spectra of virgin and dye-loaded biosorbents F1, F4, F5 and F7 were recorded using a Nicolet 5700 spectrophotometer (Thermo, USA) in the 400 to 4,000 cm wavelength region.
Biosorption Experiments
Stock solutions (1000 mg L−1) of dye were prepared in deionized and double distilled water and diluted to get the desired concentration of dyes. Calibration curve for Reactive Black 5 were prepared by measuring the absorbance of different concentrations at 598 nm. Biosorption experiments were carried out with 100 ml dye solutions in 250 ml Erlenmyer flasks to which 0.1 g of biosorbent was added. The mixtures were shaken on a horizontal shaker at 200 rpm at 30°C for 6 h unless otherwise stated. The influence of hydrogen ion concentration on biosorption was first studied over the pH range from 1.0 to 9.0, with adjustments made using 0.1 M HCl or 0.1 M NaOH. Biosorption isotherms were then studied for dye concentrations ranging from 50 mg L−1 to 250 mg L−1 at the optimum pH determined for each biosorbent. The effect of contact time was investigated at different intervals over a 6 h period for each biosorbent. All the experiments were carried out in triplicate.
The biosorption capacity, Qe (mg g−1), was calculated as follows:
| (1) |
where Co and Ce are the initial and final concentrations (mg L−1), respectively, M is the adsorbent dosage (g) and V the volume of solution (L).
Mathematical Modeling Study
Langmuir [20] and Freundlich [21] adsorption isotherms were selected to explicate the dye-fungus system in this study. The pseudo-second-order [22] and Weber-Morris [23] kinetic models were applied to provide information that are important for evaluating the efficiency of the biosorption process. The pseudo-first-order model was discarded due to its poor fit with the experimental data (R2<0.80 for all the tested conditions, data were not shown).
Artificial neural network models (ANN) have been used in prior research to investigate the sensitivity analysis and model the effect of parameters that affect adsorption or degradation of dyes [1], [24], [25]. The data sets were randomized and divided into training, validation and test subsets, which included 135, 45 and 45 samples, respectively. Levenberg-Marquardt (LM) which was an algorithm for least-squares estimation of nonlinear parameters and had been proved to be the fastest and the most robust was used as training method in this study [26]. The sensitivity analysis were analyzed by Garson method [27] to calculate the relative importance of the different input variables on sorption capacity. Details of the adsorption isotherms, kinetic models and ANN model analysis are provided in experimental section S1.
Results and Discussion
Effect of pH
Table 1 shows the biosorption capacities of Reactive Black 5 onto biosorbents as a function of pH. Maximum biosorption capacities for Reactive Black 5 onto biosorbents F1, F3 and F8 was achieved at pH 2, whereas the maximum values for the other six biosorbents were obtained at pH 1. The biosorption capacities of biosorbents decreased as the pH increased to 7. The acidic conditions could be favorable for the biosorption between Reactive Black 5 and the biosorbents due to the electrostatic attraction that exists between the positively charged surfaces of the biosorbents under acidic conditions and the negatively charged anionic dyes [28]. Under basic conditions, the presence of excess OH− competed with the anionic dyes for biosorption sites, which led to low biosorption for all of the biosorbents. All the biomass materials exhibited relatively high biosorption capacities at pH 1.0–3.0. Biosorbent F1 was the most efficient for the removal of Reactive Black 5 over the tested pH range. In contrast, only 0.65 mg g−1 of dye could be adsorbed by biosorbent F2 at pH 9.0, which was the lowest value over the range of tested conditions.
Table 1. The effect of pH on the biosorption capacities of nine biosorbents for Reactive Black 5.
| Biosorbent | Biosorption capacity for pH (mg g−1) | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| F1 | 97.75±0.07 | 98.24±0.08 | 97.91±0.06 | 54.10±1.16 | 41.27±0.32 | 33.94±0.35 | 21.20±0.28 | 32.24±0.26 | 24.78±0.29 |
| F2 | 33.53±0.75 | 30.89±0.63 | 24.91±0.33 | 9.46±0.29 | 2.27±0.08 | 1.71±0.21 | 0.74±0.17 | 1.12±0.36 | 0.65±0.14 |
| F3 | 96.38±0.15 | 97.09±0.05 | 72.98±0.10 | 31.44±0.43 | 22.47±0.58 | 20.46±0.42 | 15.48±0.50 | 19.39±0.74 | 18.55±0.51 |
| F4 | 95.92±0.04 | 93.21±0.04 | 66.17±0.14 | 24.48±0.93 | 16.02±0.97 | 13.66±1.04 | 9.11±0.29 | 14.18±0.70 | 5.97±0.35 |
| F5 | 37.57±0.29 | 35.34±0.07 | 29.17±0.41 | 11.96±0.62 | 3.34±0.40 | 1.20±0.16 | 1.16±0.23 | 1.34±0.29 | 1.30±0.21 |
| F6 | 72.35±0.28 | 70.84±0.12 | 57.79±0.42 | 24.05±0.42 | 8.63±0.51 | 5.65±0.32 | 4.51±0.79 | 5.39±0.28 | 5.13±0.42 |
| F7 | 65.35±0.06 | 64.81±0.48 | 50.98±0.53 | 11.50±0.37 | 4.87±0.37 | 2.78±0.50 | 2.79±0.74 | 3.91±0.27 | 3.75±0.43 |
| F8 | 97.74±0.08 | 98.05±0.03 | 82.90±0.24 | 15.23±0.14 | 6.87±0.43 | 5.09±0.45 | 3.35±0.37 | 5.99±0.24 | 4.26±0.48 |
| F9 | 33.59±0.39 | 30.85±0.61 | 25.38±0.21 | 12.51±0.27 | 4.64±0.29 | 1.85±0.12 | 1.12±0.21 | 2.93±0.28 | 2.50±0.22 |
Biosorption Isotherms
The biosorption capacities of the powdered fungal biomass increased with increasing dye concentrations over the range from 0 to 250 mg L−1 (see Figure S1). The initial dye concentration provided the driving force to overcome mass transfer resistances of the dyes between the aqueous and solid phases [29]. Maximum biosorption capacity of Reactive Black 5 was 179.26 mg g−1 for F1 at an initial dye concentration 250 mg L−1, which was 5 times greater than that achieved with biosorbent F9. Biosorption isotherm models were evaluated for their ability to fit the equilibrium data. The parameters for each biosorbent obtained for the different isotherm models are listed in Table 2 along with their coefficients of determination. All the determination coefficients of Langmuir model were above 0.90, indicating that Reactive Black 5 formed a monolayer covering the biosorbents. Likewise, the determination coefficients using the Freundlich isotherm for F2, F5, F6, F7 and F9 were above 0.90, indicating both homogeneous and heterogeneous distribution of active sites on the surface of five of the biosorbents. However, sorption data generated for the biosorbents that had the highest sorption capacities (>100 mg g−1) failed to fit the Freundlich isotherm model (R2<0.80), indicating that homogeneous sorption only occurred for the sorbents with low binding capacity (F1, F3, F4 and F8). The R2 values for curves generated using the Langmuir model were higher than those obtained with the Freundlich isotherm model. Therefore, the Langmuir model was used for the final data analysis. All the values of Hall separation factor for the nine biosorbents were in the range from 0–1, indicating that the biosorption process was favorable. Similar patterns have been reported for adsorption of reactive dyes on to clinoptilolite [30] and for biosorption of acid dyes on to killed biomass of Penicillium [1].
Table 2. The Biosorption isotherm parameters for the biosorption of Reactive Black 5 onto biosorbents.
| Biosorption isotherms | Biosorbents | |||||||||
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | ||
| Langmuir constants | Qmax(mg g−1) | 179.26 | 35.21 | 125.95 | 140.84 | 38.41 | 77.62 | 70.52 | 157.82 | 34.18 |
| Kl (L mg−1) | 0.63 | 0.72 | 0.68 | 0.18 | 0.98 | 0.99 | 0.74 | 0.88 | 0.90 | |
| R2 | 0.920 | 0.973 | 0.920 | 0.943 | 0.963 | 0.929 | 0.941 | 0.981 | 0.980 | |
| Freundlich constants | n | 5.75 | 30.41 | 7.28 | 4.83 | 41.44 | 10.40 | 10.99 | 6.53 | 45.68 |
| KF(L g−1) | 86.68 | 35.76 | 69.12 | 53.12 | 33.92 | 50.65 | 46.42 | 84.51 | 30.46 | |
| R2 | 0.682 | 0.928 | 0.739 | 0.756 | 0.930 | 0.913 | 0.922 | 0.753 | 0.919 | |
Reactive Black 5 biosorption capacities of different kinds of adsorbents reported in the literature are summarized in Table 3. The biosorption capacity of fungal biomass in our study was found to be comparable to and moderately higher than those of many adsorbents such as powdered activated carbon, modified sepiolite and so on.
Table 3. Recent reported adsorption capacities (mg/g) for Reactive Black 5.
| Adsorbent | adsorption capacities (mg/g) | Literatures |
| Powdered activated carbon | 58.82 | [14] |
| Afsin-Elbistan fly ash | 7.94 | [14] |
| Penicillium restrictum | 142.04 | [15] |
| Surfactant-Modified Zeolite | 12.93 | [16] |
| Modified sepiolite | 120.5 | [37] |
| Modified zeolite | 60.5 | [37] |
| Sunflower seed shells | 0.87 | [38] |
| Mandarin peelings | 0.75 | [38] |
| seaweed Laminaria sp | 101.5 | [39] |
| Modified barley straw | 251.92 | [40] |
| Powdered Fungal biomass | 34.18–179.26 | This study |
Biosorption Kinetics
The rates of adsorption of Reactive Black 5 by the nine tested biosorbents were initially fast and then gradually decreased until equilibrium between bound and free dye was attained (See Figure S2). According to the determination coefficients (Table 4), the data were best fit using a pseudo-second-order model which gave R2>0.99 for all of the biosorbents. The Qmax values estimated from the pseudo-second-order kinetic model were also in accord with the experimental data (Qeq,exp values in Table 4). The results suggested that boundary layer resistance was not a rate limiting step since the dye biosorption followed pseudo-second order kinetics [11]. The initial biosorption rate for biosorbent F1 was 46.7 mg g−1 min−1, which was the fastest binding rate among the tested materials. This was followed by biosorbents F7 (43.1 mg g−1 min−1) and F8 (39.4 mg g−1 min−1) (See Table S2). In contrast, the initial biosorption rates for biosorbents F6 and F9 were only 10.5 and 7.3 mg g−1 min−1, respectively. There was no apparent relationship between biosorption rate and biosorption capacity, which had a Pearson correlation coefficient of 0.675. This phenomenon may be due to differences in the chemical and textural properties of the different biosorbents. Parameters derived from the Weber-Morris model indicated that the intraparticle diffusion of Reactive Black 5 within the biosorbents occurred in two stages involving macropore diffusion followed by micropore diffusion. Since the kw,2 values of nine biosorbents were smaller than the kw,1 values (Table 4), the intraparticle diffusion was predicted to be the rate limiting step for biosorption of Reactive Black 5 on to the biosorbents.
Table 4. Parameters of pseudo-second-order and Weber-Morris model for adsorption of Reactive Black 5 onto biosorbents.
| Biosorbents | Qeq,exp (mg g−1) | Pseudo-second-order kinetic model | Initial linear portion (Weber-Morris) | Second Linear portion (Weber-Morris) | ||||||
| k2x10−2(g mg −1 min−1) | Qe,cal (mg g−1) | R2 | Kw,1 | I1 | R2 | Kw,2 | I2 | R2 | ||
| F1 | 98.981 | 0.467 | 100.000 | 1.000 | 5.349 | 58.584 | 0.906 | 0.047 | 98.148 | 0.902 |
| F2 | 33.849 | 1.170 | 34.130 | 0.999 | 1.432 | 22.403 | 0.913 | 0.069 | 32.594 | 0.922 |
| F3 | 95.859 | 0.197 | 97.087 | 0.999 | 6.657 | 40.492 | 0.947 | 0.316 | 90.228 | 0.908 |
| F4 | 95.908 | 0.265 | 97.087 | 0.999 | 7.807 | 36.748 | 0.905 | 0.126 | 93.636 | 0.935 |
| F5 | 37.327 | 1.267 | 37.453 | 1.000 | 1.716 | 24.421 | 0.901 | 0.068 | 36.063 | 0.985 |
| F6 | 72.793 | 0.191 | 74.074 | 0.999 | 4.050 | 35.001 | 0.965 | 0.550 | 63.078 | 0.904 |
| F7 | 64.928 | 1.022 | 64.935 | 1.000 | 1.210 | 54.476 | 0.911 | 0.112 | 62.910 | 0.916 |
| F8 | 98.754 | 0.394 | 100.000 | 1.000 | 5.266 | 58.376 | 0.904 | 0.152 | 96.051 | 0.915 |
| F9 | 33.816 | 0.627 | 34.129 | 0.999 | 1.922 | 17.915 | 0.903 | 0.174 | 30.649 | 0.936 |
Artificial Neural Network Modeling
The textural characteristics and chemical element composition of the biosorbents are shown in Table 5. To further investigate the degree to which different variables affected the biosorption capacities of the powdered fungal biomass, a background propagation artificial neural network (ANN) was used to conduct a sensitivity analysis on the individual variables (See Table S3). The ANN model showed satisfactory fits for the experimental data (See Figure S3). Table 6 showed that pH was the most influential parameter with a relative importance of 22.5%, followed by nitrogen content of biosorbents (15.7%), initial dye concentration (14.5%) and carbon content of biosorbents (10.1%). This indicated that biosorption is a complex process in which five factors each explained greater than 10% of the binding data. The sum total of operating parameters (pH, dye concentration, time) was 43.7%, whereas chemical element composition of biosorbents (Nitrogen content, carbon content, hydrogen content) determined 33.8% of binding capacity. Textural characteristics of the biosorbents (BET area, pore volume, pore diameter) explained 22.5%, indicating that chemical element compositions were more important than differences in textural characteristics.
Table 5. Textural characteristics and chemical element compositions of biosorbents.
| Properties | Biosorbents | ||||||||
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| Textural characteristics | |||||||||
| BET area (m2/g) | 0.1789 | 0.2282 | 0.1928 | 0.06979 | 0.4358 | 0.2971 | 0.7656 | 0.2214 | 0.2061 |
| Pore volume (m3/g) | 0.0007153 | 0.0006945 | 0.0004054 | 0.0001616 | 0.002344 | 0.001191 | 0.002361 | 0.0005918 | 0.0005641 |
| Pore diameter (nm) | 7.997 | 6.087 | 4.205 | 4.632 | 10.76 | 8.018 | 6.168 | 5.347 | 5.475 |
| Chemical element compositions | |||||||||
| Nitrogen content (%) | 4.43 | 2.38 | 2.77 | 2.29 | 3.16 | 4.02 | 4.70 | 4.05 | 2.71 |
| Carbon content (%) | 53.97 | 55.24 | 59.03 | 60.21 | 45.79 | 50.51 | 47.73 | 50.32 | 51.48 |
| Hydrogen content (%) | 8.36 | 8.68 | 8.99 | 9.18 | 7.19 | 7.75 | 7.36 | 7.87 | 7.96 |
Table 6. The relative importance of input variables by ANN.
| Input variables | Relative importance |
| pH | 22.5% |
| Initial dye concentration | 14.5% |
| Time | 6.7% |
| BET area | 10.0% |
| Pore volume | 6.2% |
| Pore diameter | 6.3% |
| Nitrogen content | 15.7% |
| Carbon content | 10.1% |
| Hydrogen content | 8.0% |
Modeling the Effect of Textural Characteristics and Chemical Element Composition on Biosorption
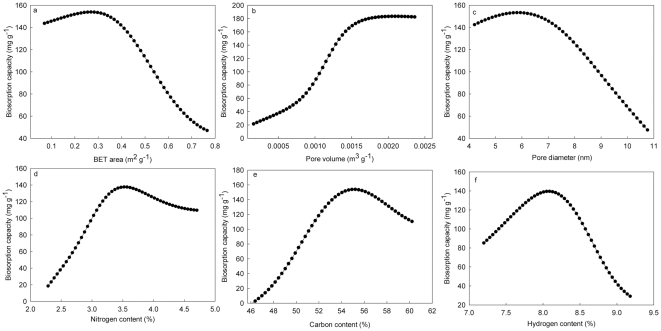
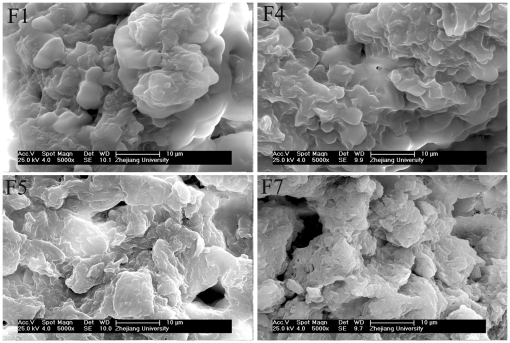
The effects of individual textural and chemical properties on biosorption capacity were examined using the ANN model. Figure 1a showed biosorption capacity first slightly increased and then decreased as the BET surface area increased from 0.238 to 0.78 m2 g−1. This was different from expectation in that adsorbents exhibiting a large surface are generally assumed to adsorb larger quantities of adsorbate than materials with lower surface areas. However, similar results have been reported in prior research [31], [32], in which surface area alone was not sufficient to predict dye adsorption capacity for biosorbents. Figure 1b showed that the biosorption capacity increased as pore volume of biosorbents increased and then reached maximum values. This infers that large pore volumes and a more porous structure favored the biosorption process. The effect of pore size on biosorption capacity is shown in Figure 1c, which shows when the average pore size ranged between 4.2–7.5 nm, the sorbents were effective for binding the dye. For larger pore sizes in the range from 7.5–11.0 nm, biosorption capacity was sharply decreased. This phenomenon may be due to the dimension of dyes aggregation in aqueous solution and the distribution of pore size of biosorbents [33]. In contrast to pore size, none of textural characteristics could be used alone to predict biosorption capacity. The SEM images of F1, F4, F5 and F7 are shown in Figure 2. Visual comparison of the images showed that the surfaces of F1 and F4 were smoother and more homogeneous than those of F5 and F7, such that the surface area and pore volumes of F1 and F4 were smaller than those of F5 and F7. Similar trend was also observed for poly (epicholorohydrin dimethylamine) modified bentonite [34] and chemical modified carbon adsorbents [35]. This is in accord with the experimental results (Table 2), which showed that the biosorption capacities for the different sorbents were not proportional to surface area.
Figure 1. The effects of textural characteristics and chemical element compositions on biosorption as predicted using an artificial neural network model by changing the values for the variable of interest while holding the other variables constant.
Figure 2. The SEM images of four selected biosorbents.
Chemical element compositions are also important for adsorption from aqueous solutions. Figure 1d showed that the biosorption capacity increased sharply as the nitrogen content of biosorbents increased from 2.2 to 3.6%. This may be explained by the increased numbers of nitrogenous functional groups existed in the biosorbents, such as −NH2 and −NH– groups, which bind the dye through electrostatic interactions:
| (2) |
Interestingly, for materials with higher nitrogen contents of biosorbents above 3.6, the biosorption capacity decreased, which may be differences in the abundance of other types of functional groups. Binding of the dye likely involves interactions between the sorbent and four 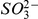 groups on the dye (Chemical structure of dye in Table S1). Similar trends were also observed for the effect of carbon content and hydrogen content of biosorbents on biosorption capacity (Figure 1e and 1f). The increase of bisorption capacity associated with increased carbon content of biosorbents may reflect differences in the abundance of carboxyl and carbonyl groups of the different biosorbents:
groups on the dye (Chemical structure of dye in Table S1). Similar trends were also observed for the effect of carbon content and hydrogen content of biosorbents on biosorption capacity (Figure 1e and 1f). The increase of bisorption capacity associated with increased carbon content of biosorbents may reflect differences in the abundance of carboxyl and carbonyl groups of the different biosorbents:
| (3) |
Since Reactive Black 5 has four 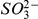 groups and only one NH2 group in the dye molecule, interactions between carboxyl and carbonyl groups of the biosorbents and NH2 group of the dyes maybe less important than that of NH2 groups of biosorbents and
groups and only one NH2 group in the dye molecule, interactions between carboxyl and carbonyl groups of the biosorbents and NH2 group of the dyes maybe less important than that of NH2 groups of biosorbents and 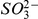 groups of Reactive Black 5. It was also observed that hydrogen content of biosorbents was inversely correlated with biosorption capacity. This may reflect decreased abundance of other functional groups in the materials with high hydrogen content. The FTIR spectra for four selected biosorbents confirmed changes in the availability of active functional groups and surface properties of the biosorbents after binding with the dye. All of the observed shifts in the FTIR spectra indicated that −NH2, carboxylic groups and −OH were the main functional groups that are responsible for binding Reactive Black 5 (Figure S4 and Tables S4, S5, S6, S7). Among the tested materials, biosorbent F1, which had the second highest nitrogen and fourth highest carbon content, exhibited the best binding capacity (179.26 mg g−1). Biosorbent F4, which had the third highest binding capacity (140.84 mg g−1), possessed the highest carbon content and lowest nitrogen content. Biosorbent F5, which had the lowest carbon content and the fourth lowest nitrogen content had a binding capacity of only 38.41 mg g−1. Biosorbent F7, which had the highest nitrogen content and the lowest carbon content exhibited 70.52 mg g−1 biosorption capacity for Reactive Black 5. Based on these data, carbon and nitrogen content of bisorbents as criteria for identifying superior biosorbents from fungi are better predictors of efficacy than BET specific surface area. Valix used an empirical model to find high total surface area and wider pores generally exhibited high dye adsorption capacities, and the surface chemistry of the carbon also influenced adsorption capacity significantly [36]. Compared to the results in this study, it also indicated that the selection criteria for choosing superior were significantly different from powdered activated carbon and fungal biomass.
groups of Reactive Black 5. It was also observed that hydrogen content of biosorbents was inversely correlated with biosorption capacity. This may reflect decreased abundance of other functional groups in the materials with high hydrogen content. The FTIR spectra for four selected biosorbents confirmed changes in the availability of active functional groups and surface properties of the biosorbents after binding with the dye. All of the observed shifts in the FTIR spectra indicated that −NH2, carboxylic groups and −OH were the main functional groups that are responsible for binding Reactive Black 5 (Figure S4 and Tables S4, S5, S6, S7). Among the tested materials, biosorbent F1, which had the second highest nitrogen and fourth highest carbon content, exhibited the best binding capacity (179.26 mg g−1). Biosorbent F4, which had the third highest binding capacity (140.84 mg g−1), possessed the highest carbon content and lowest nitrogen content. Biosorbent F5, which had the lowest carbon content and the fourth lowest nitrogen content had a binding capacity of only 38.41 mg g−1. Biosorbent F7, which had the highest nitrogen content and the lowest carbon content exhibited 70.52 mg g−1 biosorption capacity for Reactive Black 5. Based on these data, carbon and nitrogen content of bisorbents as criteria for identifying superior biosorbents from fungi are better predictors of efficacy than BET specific surface area. Valix used an empirical model to find high total surface area and wider pores generally exhibited high dye adsorption capacities, and the surface chemistry of the carbon also influenced adsorption capacity significantly [36]. Compared to the results in this study, it also indicated that the selection criteria for choosing superior were significantly different from powdered activated carbon and fungal biomass.
Conclusion
The biosorption capacities of powdered fungal biomass were in range from 34.18 to 179.26 mg g−1, which had competitive advantage compared to powdered activated carbon for adsorption capacity and cost. Among biosorbents in this study, the Absidia coerulea 118 biomass exhibited the highest biosorption capacity. The biosorption capacities were not proportional to surface areas of the sorbents, but were instead influenced by their chemical element compositions. And differences in carbon and nitrogen contents could be used as a selection index for identifying effective biosorbents. These results were significantly different from the selected criterion for selecting superior effective activated carbon. Sorption capacities of the biosorbents were not correlated with the initial biosorption rates.
Supporting Information Available
Additional details of experimental methods, effects of initial dye concentration and time on biosorption capaicites of nine biosorbents for Reactive Black 5, the FTIR Spectra of Reactive Black 5, Unloaded and Dye-loaded Biosorbents, and information of adsorption isotherms, kinetic models and ANN model analysis are available in the Supporting Information.
Supporting Information
Mathematical Modeling Study and Artificial Neural Network.
(DOC)
The effect of initial dye concentrations on biosorption capacities of biosorbents for Reactive Black 5 (30°C, 180 rpm).
(TIF)
The effect of contact time on biosorption capacities of biosorbents for Reactive Black 5 (30°C, 180 rpm).
(TIF)
The comparison between experimental values and predicted values using an ANN model. The value of the determination coefficient (R2) was 0.9855, indicating the ANN could be used to fit the experimental data and investigate the effect of input variables.
(TIF)
The FTIR spectrum of Reactive Black 5. 3454.4 cm−1: O–H stretching; 1633.6 cm−1: N–H bending; 1002.3–1227.7 cm−1: –SO3/C–N stretching; 739.4 cm−1: C–H bending.
(TIF)
The Chemical structure and characteristics of Reactive Black 5.
(DOC)
The initial biosorption rate of nine biosorbents for Reactive Black 5.
(DOC)
Model variables and their ranges for artificial neural network.
(DOC)
The FTIR Spectral Characteristics of Biosorbent F1 Before and After Biosorption of Reactive Black 5.
(DOC)
The FTIR Spectral Characteristics of Biosorbent F4 Before and After Biosorption of Reactive Black 5.
(DOC)
The FTIR Spectral Characteristics of Biosorbent F5 Before and After Biosorption of Reactive Black 5.
(DOC)
The FTIR Spectral Characteristics of Biosorbent F7 Before and After Biosorption of Reactive Black 5.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the Science and Technology Project of Zhejiang Province (2010C13G2010074,http://www.zjkjt.gov.cn), National Natural Science Foundation of China (31070079,http://www.nsfc.gov.cn), the Science and Technology Project of Zhejiang Province (2008C13014-3,http://www.zjkjt.gov.cn/) and the International Cooperation Project in Science and Technology of Zhejiang Province (No. 2008C14038,http://www.zjkjt.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yang Y, Wang G, Wang B, Li Z, Jia X, et al. Biosorption of Acid Black 172 and Congo Red from aqueous solution by nonviable Penicillium YW 01: Kinetic study, equilibrium isotherm and artificial neural network modeling. Bioresour Technol. 2011;102:828–834. doi: 10.1016/j.biortech.2010.08.125. [DOI] [PubMed] [Google Scholar]
- 2.Mondal S. Methods of Dye Removal from Dye House Effluent—An Overview. Environ Eng Sci. 2008;25:383–396. [Google Scholar]
- 3.Kaushik P, Malik A. Fungal dye decolourization: Recent advances and future potential. Environ Int. 2009;35:127–141. doi: 10.1016/j.envint.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Forgacs E, Cserháti T, Oros G. Removal of synthetic dyes from wastewaters: a review. Environ Int. 2004;30:953–971. doi: 10.1016/j.envint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoodi NM, Hayati B, Arami M, Mazaheri F. Single and Binary System Dye Removal from Colored Textile Wastewater by a Dendrimer as a Polymeric Nanoarchitecture: Equilibrium and Kinetics. J Chem Eng Data. 2010;55:4660–4668. [Google Scholar]
- 6.Bhatnagar A, Vilar VJP, Botelho CMS, Boaventura RAR. Coconut-based biosorbents for water treatment – A review of the recent literature. Adv Colloid Interface Sci. 2010;160:1–15. doi: 10.1016/j.cis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Crini G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour Technol. 2006;97:1061–1085. doi: 10.1016/j.biortech.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Akar T, Divriklioglu M. Biosorption applications of modified fungal biomass for decolorization of Reactive Red 2 contaminated solutions: Batch and dynamic flow mode studies. Bioresour Technol. 2010;101:7271–7277. doi: 10.1016/j.biortech.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 9.Chen B, Wang Y, Hu D. Biosorption and biodegradation of polycyclic aromatic hydrocarbons in aqueous solutions by a consortium of white-rot fungi. J Hazard Mater. 2010;179:845–851. doi: 10.1016/j.jhazmat.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 10.Das SK, Das AR, Guha AK. A Study on the Adsorption Mechanism of Mercury on Aspergillus versicolor Biomass. Environ Sci Technol. 2007;41:8281–8287. doi: 10.1021/es070814g. [DOI] [PubMed] [Google Scholar]
- 11.Xiong X-J, Meng X-J, Zheng T-L. Biosorption of C.I. Direct Blue 199 from aqueous solution by nonviable Aspergillus niger. J Hazard Mater. 2010;175:241–246. doi: 10.1016/j.jhazmat.2009.09.155. [DOI] [PubMed] [Google Scholar]
- 12.Aksu Z, Tezer S. Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: effect of temperature. Process Biochem. 2000;36:431–439. [Google Scholar]
- 13.Das SK, Bhowal J, Das AR, Guha AK. Adsorption Behavior of Rhodamine B on Rhizopus oryzae Biomass. Langmuir. 2006;22:7265–7272. doi: 10.1021/la0526378. [DOI] [PubMed] [Google Scholar]
- 14.Eren Z, Acar FN. Adsorption of Reactive Black 5 from an aqueous solution: equilibrium and kinetic studies. Desalination. 2006;194:1–10. [Google Scholar]
- 15.Iscen CF, Kiran I, Ilhan S. Biosorption of Reactive Black 5 dye by Penicillium restrictum: The kinetic study. J Hazard Mater. 2007;143:335–340. doi: 10.1016/j.jhazmat.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Karadag D, Turan M, Akgul E, Tok S, Faki A. Adsorption Equilibrium and Kinetics of Reactive Black 5 and Reactive Red 239 in Aqueous Solution onto Surfactant-Modified Zeolite. J Chem Eng Data. 2007;52:1615–1620. [Google Scholar]
- 17.Ramsay JA, Nguyen T. Decoloration of textile dyes by Trametes versicolor and its effect on dye toxicity. Biotechnol Lett. 2002;24:1756–1760. [Google Scholar]
- 18.Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–319. [Google Scholar]
- 19.Bayramoğlu G, Yakup Arıca M. Biosorption of benzidine based textile dyes “Direct Blue 1 and Direct Red 128” using native and heat-treated biomass of Trametes versicolor. J Hazard Mater. 2007;143:135–143. doi: 10.1016/j.jhazmat.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–1403. [Google Scholar]
- 21.Freundlich HMF. Über die adsorption in lösungen. Z Phys Chem. 1906;57:385–470. [Google Scholar]
- 22.Ho YS, McKay G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ. 1998;76:183–191. [Google Scholar]
- 23.Özer A, Akkaya G, Turabik M. Biosorption of Acid Blue 290 (AB 290) and Acid Blue 324 (AB 324) dyes on Spirogyra rhizopus. J Hazard Mater. 2006;135:355–364. doi: 10.1016/j.jhazmat.2005.11.080. [DOI] [PubMed] [Google Scholar]
- 24.Khataee AR, Dehghan G, Ebadi A, Zarei M, Pourhassan M. Biological treatment of a dye solution by Macroalgae Chara sp.: Effect of operational parameters, intermediates identification and artificial neural network modeling. Bioresour Technol. 2010;101:2252–2258. doi: 10.1016/j.biortech.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 25.Zarei M, Niaei A, Salari D, Khataee AR. Removal of four dyes from aqueous medium by the peroxi-coagulation method using carbon nanotube-PTFE cathode and neural network modeling. J Electroanal Chem. 2010;639:167–174. [Google Scholar]
- 26.Marquardt DW. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. SIAM J Appl Math. 1963;11:431–441. [Google Scholar]
- 27.Garson GD. Interpreting neural-network connection weights. AI Expert. 1991;6:46–51. [Google Scholar]
- 28.Aksu Z, Donmez G. A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere. 2003;50:1075–1083. doi: 10.1016/s0045-6535(02)00623-9. [DOI] [PubMed] [Google Scholar]
- 29.Aksu Z, KarabayIr G. Comparison of biosorption properties of different kinds of fungi for the removal of Gryfalan Black RL metal-complex dye. Bioresour Technol. 2008;99:7730–7741. doi: 10.1016/j.biortech.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 30.Sismanoglu T, Kismir Y, Karakus S. Single and binary adsorption of reactive dyes from aqueous solutions onto clinoptilolite. J Hazard Mater. 2010;184:164–169. doi: 10.1016/j.jhazmat.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Patdhanagul N, Srithanratana T, Rangsriwatananon K, Hengrasmee S. Ethylene adsorption on cationic surfactant modified zeolite NaY. Microporous Mesoporous Mater. 2010;131:97–102. [Google Scholar]
- 32.Stavropoulos GG, Samaras P, Sakellaropoulos GP. Effect of activated carbons modification on porosity, surface structure and phenol adsorption. J Hazard Mater. 2008;151:414–421. doi: 10.1016/j.jhazmat.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Walker GM, Weatherley LR. Adsorption of dyes from aqueous solution – the effect of adsorbent pore size distribution and dye aggregation. Chem Eng J. 2001;83:201–206. [Google Scholar]
- 34.Kang Q, Zhou W, Li Q, Gao B, Fan J, et al. Adsorption of anionic dyes on poly(epicholorohydrin dimethylamine) modified bentonite in single and mixed dye solutions. Applied Clay Science. 2009;45:280–287. [Google Scholar]
- 35.Nadeem M, Mahmood A, Shahid SA, Shah SS, Khalid AM, et al. Sorption of lead from aqueous solution by chemically modified carbon adsorbents. J Hazard Mater. 2006;138:604–613. doi: 10.1016/j.jhazmat.2006.05.098. [DOI] [PubMed] [Google Scholar]
- 36.Valix M, Cheung WH, McKay G. Roles of the Textural and Surface Chemical Properties of Activated Carbon in the Adsorption of Acid Blue Dye. Langmuir. 2006;22:4574–4582. doi: 10.1021/la051711j. [DOI] [PubMed] [Google Scholar]
- 37.Ozdemir O, Armagan B, Turan M, Çelik MS. Comparison of the adsorption characteristics of azo-reactive dyes on mezoporous minerals. Dyes Pigments. 2004;62:49–60. [Google Scholar]
- 38.Osma JF, Saravia V, Toca-Herrera JL, Couto SR. Sunflower seed shells: A novel and effective low-cost adsorbent for the removal of the diazo dye Reactive Black 5 from aqueous solutions. J Hazard Mater. 2007;147:900–905. doi: 10.1016/j.jhazmat.2007.01.112. [DOI] [PubMed] [Google Scholar]
- 39.Vijayaraghavan K, Yun Y-S. Biosorption of C.I. Reactive Black 5 from aqueous solution using acid-treated biomass of brown seaweed Laminaria sp. Dyes Pigments. 2008;76:726–732. [Google Scholar]
- 40.Oei BC, Ibrahim S, Wang SB, Ang HM. Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution. Bioresour Technol. 2009;100:4292–4295. doi: 10.1016/j.biortech.2009.03.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mathematical Modeling Study and Artificial Neural Network.
(DOC)
The effect of initial dye concentrations on biosorption capacities of biosorbents for Reactive Black 5 (30°C, 180 rpm).
(TIF)
The effect of contact time on biosorption capacities of biosorbents for Reactive Black 5 (30°C, 180 rpm).
(TIF)
The comparison between experimental values and predicted values using an ANN model. The value of the determination coefficient (R2) was 0.9855, indicating the ANN could be used to fit the experimental data and investigate the effect of input variables.
(TIF)
The FTIR spectrum of Reactive Black 5. 3454.4 cm−1: O–H stretching; 1633.6 cm−1: N–H bending; 1002.3–1227.7 cm−1: –SO3/C–N stretching; 739.4 cm−1: C–H bending.
(TIF)
The Chemical structure and characteristics of Reactive Black 5.
(DOC)
The initial biosorption rate of nine biosorbents for Reactive Black 5.
(DOC)
Model variables and their ranges for artificial neural network.
(DOC)
The FTIR Spectral Characteristics of Biosorbent F1 Before and After Biosorption of Reactive Black 5.
(DOC)
The FTIR Spectral Characteristics of Biosorbent F4 Before and After Biosorption of Reactive Black 5.
(DOC)
The FTIR Spectral Characteristics of Biosorbent F5 Before and After Biosorption of Reactive Black 5.
(DOC)
The FTIR Spectral Characteristics of Biosorbent F7 Before and After Biosorption of Reactive Black 5.
(DOC)