Abstract
Purpose
To investigate the dosimetry and feasibility of carotid-sparing intensity-modulated radiotherapy (IMRT) for early glottic cancer and to report preliminary clinical experience.
Methods and Materials
Digital Imaging and Communications in Medicine radiotherapy (DICOM-RT) datasets from 6 T1–2 conventionally treated glottic cancer patients were used to create both conventional IMRT plans. We developed a simplified IMRT planning algorithm with three fields and limited segments. Conventional and IMRT plans were compared using generalized equivalent uniform dose and dose–volume parameters for in-field carotid arteries, target volumes, and organs at risk. We have treated 11 patients with this simplified IMRT technique.
Results
Intensity-modulated radiotherapy consistently reduced radiation dose to the carotid arteries (p < 0.05) while maintaining the clinical target volume coverage. With conventional planning, median carotid V35, V50, and V63 were 100%, 100%, and 69.0%, respectively. With IMRT planning these decreased to 2%, 0%, and 0%, respectively (p < 0.01). Radiation planning and treatment times were similar for conventional radiotherapy and IMRT. Treatment results have been excellent thus far.
Conclusions
Intensity-modulated radiotherapy significantly reduced unnecessary radiation dose to the carotid arteries compared with conventional lateral fields while maintaining clinical target volume coverage. Further experience and longer follow-up will be required to demonstrate outcomes for cancer control and carotid artery effects.
Keywords: Glottic cancer, Radiotherapy, IMRT, Larynx
INTRODUCTION
Early-stage (T1–2N0M0) glottic squamous cell carcinoma (SCC) is a highly curable disease (>90% to 80%) when treated with simple parallel opposed small-field (typically 4–6 cm2) radiotherapy (RT) (1-5). The low incidence of nodal spread (<5%) does not warrant elective nodal irradiation. Although reported acute and late treatment toxicity is low (6), there has been minimal attention devoted to vascular effects that can manifest more than 10 years after therapy. The carotid arteries are located eccentrically in the path of lateral beams so may receive higher radiation doses than those prescribed to the clinical treatment volume (CTV). The major consequence of carotid irradiation is direct potential injury, which may also limit future RT options in the case of a metachronous second primary head-and-neck cancer (HNC).
The excellent RT outcomes (2, 3, 5) for early glottic cancer imply that survivorship considerations merit greater consideration than previously afforded. Radiotherapy has emerged as an independent risk factor for accelerating carotid atherogenesis (7-13) and increased rates of cerebrovascular incidents (8, 9, 14-20) in HNC patients. Dorresteijn et al. (11) reported that the relative risk of ischemic stroke after radiation treatments to the neck was 10.1 times that of an age- and gender-matched population-based cohort of patients aged <60 years. This resulted in a 12.0% 15-year cumulative risk of stroke. Recently, Smith et al. (21) demonstrated using the Surveillance, Epidemiology and End Results Medicare dataset, for older patients, that the incidence of cerebrovascular events within 10 year of diagnosis was 33% for those treated with RT alone, compared with 25% for those treated with surgery (p < 0.001).
Treatment options for previously irradiated patients with metachronous HNC are potentially limited because of increased risk for carotid injury, occlusion, or hemorrhage (“blowout”) posed by reirradiation (22-25). Because most patients are cured from their index early glottic cancer, second primary cancers, commonly in the upper aerodigestive tract, represent their most threatening cancer mortality risk. From several available publications (26-31), the crude incidence of second malignancies in HNC patients ranges from 11% to 29%. Reported series show that between 4% and 31% of diagnosed second malignancies occur as second primary head-and-neck tumors (29, 32-35). These metachronous tumors contribute substantially to long-term mortality. Fujita et al. (28) reported that 15% of T1 glottic cancer patients died of second malignancies, primarily in the upper aerodigestive tract. Narayana et al. (30) noted that in a series of 144 T1 glottic HNC patients, second primary tumors were the leading cause of death.
The more recent implementation of intensity-modulated RT (IMRT) makes it possible to create radiation treatment plans with sharp dose gradients between the glottic CTV and the carotid arteries. We herein investigate the use of IMRT for treatment of early glottic cancer. In this report, we present the dosimetric and logistic feasibility of “carotid-sparing” IMRT for T1- and T2-stage glottic carcinoma along with an early clinical experience. The specific aims of this study include (1) development of a simplified IMRT planning and delivery technique; (2) virtual dosimetric comparison of conventional opposed lateral technique vs. standardized carotid-sparing IMRT, using patient-derived CT simulation Digital Imaging and Communications in Medicine (DICOM)-RT data from a series of patients with early-stage glottic cancers; and (3) evaluation of a pilot series of consecutive patients treated with carotid-sparing IMRT.
METHODS AND MATERIALS
Virtual plan comparison
Six consecutive patients with T1–2N0 glottic SCC were identified in our departmental database. All patients received conventional opposed lateral RT using three-dimensional (3D) CT-based treatment planning. This dosimetry study and retrospective chart review including waivers of consent were approved by the institutional review board of the University of Texas M.D. Anderson Cancer Center.
Archival treatment plans and CT simulation data were extracted as DICOM-RT files. The gross tumor volume (GTV) and CTV DICOM-RT structure sets were used. The GTV was contoured on the basis of clinical endoscopic examination and diagnostic CT when helpful. Additionally, post hoc contouring was performed for the left and right carotid arteries, thyroid cartilage, thyroid gland, postcricoid area, and spinal cord. A commercial treatment planning system software system was used (Pinnacle; Philips Medical Systems, Koninklijke Philips Electronics, Amsterdam, The Netherlands). Dose–volume data were then reconstructed for each listed structure using the clinically utilized beam characteristics from the initial plan and were exported for statistical analysis (JMP 6.1; SAS Institute, Cary, NC).
Subsequently, reconstructed DICOM-RT files were used to generate carotid-sparing IMRT virtual plans. The initial step was to develop a standard planning algorithm that was as simple as possible to provide desired target coverage and minimize carotid dose. The CTV was contoured as follows: the anterior limit was placed inside the skin as far as possible but to encompass thyroid cartilage with up to a 5-mm margin, and the posterior border was the posterior limit of the thyroid and cricoid cartilages. The field size and limits were modified to encompass each individual patient’s pre-defined GTV, and given the uncertainty of small field IMRT, a minimum 4 cm × 4 cm field size was used. Daily setup variations were minimized by using Aquaplast IMRT immobilization devices and image-guided RT imaging before treatments for setup verification. Given this approach, the fact that the glottis lies within a rigid cartilaginous framework, and the generous CTV, no planning target volume expansions were applied.
Priority IMRT planning goals included the following parameters. The skin goal dose was to be less than the prescribed dose except at the anterior border of the thyroid cartilage for anterior tumors. When using the IMRT planning algorithm, bolus was required even for anterior tumors. The spinal cord dose was limited to V90 <10 Gy and maximum point dose of 20 Gy,; this was readily achievable despite the dose–response profile for carotid changes noted at doses exceeding 35 Gy (vide infra). X-ray energy modeled was 6 MV, and dynamic multileaf collimation was used.
Multiple approaches with different numbers of beams, angles, and segments were evaluated. The final approach selected for standardized virtual planning was a three-field IMRT arrangement with preselected beam angles of 0°, 70°, and 290°. The 0° field was collimated so as to minimize exposure to the carotid arteries, whereas the oblique beams were angled to avoid inclusion of the ipsilateral carotid artery and only allow exit dose to the contralateral artery. Segment selection imposed a maximum number of 15 segments (approximately 5 segments per beam).
Clinical plan analysis
Additionally, DICOM-RT datasets from the first 11 patients with T1–2 glottic cancer treated with the aforementioned IMRT technique were analyzed. Dose–volume data for contoured structures were evaluated.
Emerging data suggest that there is a dose–response threshold for radiation effects on the carotid arteries. Martin et al. (36) observed that intimal–medial thickness was only statistically significant at doses of ≥35–50 Gy. Consequently, for this series, we selected the fractional volume of both carotids receiving 35 Gy (V35) and 50 Gy (V50) as reference dose–volume parameters that might reasonably approximate a “threshold dose” for a detectable subsequent pathologic response.
Dose–volume matrices were constructed and exported for statistical analysis into JMP statistical software. Dosimetric analysis was performed implementing the generalized equivalent uniform dose (gEUD) formalism developed by Niemierko (37), such that:
where Dref was 200 cGy, SF2 was set to 0.48, and Di represents the dose to the ith voxel. The gEUD serves as a representational parameter that accounts for dose inhomogeneity across a given volume and is an effective method of reporting complex dose distributions with a single parameter (38, 39). The gEUD conceptually represents the uniform dose to a volume in 2-Gy fractions that would achieve the same approximate biologic endpoint. The carotid V35, V50, and V63 were determined for each patient and compiled after stratification by RT technique for virtual as well as clinical plans. Owing to sample size limitations and the lack of sufficient cases for a determination of distributional fit, nonparametric statistical analyses were performed using the Wilcoxon rank test for continuous variable comparison. The calculated p values were uncorrected for multiple comparisons in this hypothesis-generating dataset, with statistical significance set at the p = 0.05 level for all analyses.
RESULTS
Mean, standard deviation, range, and median of calculated gEUD for contoured structures of virtual plans are listed in Table 1. A nonparametric, statistically significant difference in gEUD was noted between groups using the Wilcoxon test, with binary cohort comparison revealing statistically significant dose decrement to carotids using IMRT compared with opposed laterals fields. No statistical difference was noted in terms of gEUD to tumor/target volumes or spinal cord.
Table 1.
Generalized effective uniform dose parameters for virtual plans
| Structure | Technique | Mean ± SD (Gy) | Range (Gy) | Median (Gy) | p |
|---|---|---|---|---|---|
| CTV | IMRT | 66.62 ± 0.70 | 65.28–67.39 | 66.76 | 0.22 |
| Opposed laterals | 64.59 ± 0.82 | 64.01–65.76 | 64.29 | ||
| Spinal cord | IMRT | 15.55 ± 4.98 | 12.01–25.44 | 14.21 | 0.44 |
| Opposed laterals | 9.49 ± 8.94 | 5.24–17.40 | 5.28 | ||
| Left carotid | IMRT | 18.64 ± 0.33 | 18.24–18.97 | 18.67 | 0.02* |
| Opposed laterals | 63.61 ± 0.67 | 62.98–64.57 | 63.45 | ||
| Right carotid | IMRT | 20.11 ± 1.63 | 17.97–21.40 | 20.54 | 0.03* |
| Opposed laterals | 63.95 ± 5.85 | 59.18–67.16 | 62.73 |
Values are generalized effective uniform dose.
Statistically significant.
Dose–volume parameters are tabulated for carotid volumes by treatment technique in Table 2, which shows statistically significant reduction in the volume of carotids artery receiving 35–63 Gy, as well as the dose delivered to 90% of carotid volumes (D90). Note that the relatively higher spinal cord dose for opposed lateral beams is skewed in the sample of actually treated patients by a single patient with a larger T2 tumor, who received a somewhat larger field treatment.
Table 2.
Bilateral carotid artery dosimetric parameters for virtual and clinical plans
| Parameter | Opposed laterals as treated | Virtual IMRT plan comparisons | p | Clinical IMRT (treated) |
|---|---|---|---|---|
| V35* | <0.001† | |||
| Mean ± SD | 0.99 ± 0.10 | 0.02 ± 0.05 | 0.02 ± 0.03 | |
| Range | 0.94–1.00 | 0.0–0.06 | 0.00–0.09 | |
| Median | 1.00 | 0.02 | 0.00 | |
| V50* | <0.001† | |||
| Mean ± SD | 0.93 ± 0.09 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Range | 0.75–1.00 | 0.00–0.00 | 0.00–0.00 | |
| Median | 1.00 | 0.00 | 0.00 | |
| V63* | <0.001† | |||
| Mean ± SD | 0.70 ± 0.17 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Range | 0.45–0.92 | 0.00–0.00 | 0.00–0.00 | |
| Median | 0.69 | 0.00 | 0.00 | |
| D90 (Gy) | <0.001† | |||
| Mean ± SD | 50.13 ± 6.36 | 9.74 ± 2.39 | 6.15 ± 4.14 | |
| Range | 42.48–62.95 | 6.11–14.19 | 1.22–15.91 | |
| Median | 50.94 | 9.70 | 5.53 |
Abbreviation: IMRT = intensity-modulated radiotherapy.
Values in normalized fractional volume.
Statistically significant.
The gEUD and dose–volume parameters are presented for the initial 11 patients treated using the IMRT technique (clinical IMRT) in Tables 2 and 3. Figure 1 shows the carotid artery dose–volume histogram for virtual plans. Figure 2 shows typical isodose plans for lateral vs. IMRT setups with the carotids contoured. We found that the IMRT planning time requirement was similar to that for the iterative 3D planning approach. The IMRT treatment times were similar to those required for two-field 6-MV X-ray fields using virtual wedges and approximately half the time required for two 60Co beams because of monitor unit output rate and the requirement for changing mechanical wedges.
Table 3.
Generalized effective uniform dose parameters for IMRT clinical plans
| Structure | Mean ± SD (Gy) | Range (Gy) | Median (Gy) |
|---|---|---|---|
| GTV | 65.22 ± 0.23 | 65.06–65.52 | 65.22 |
| CTV | 65.05 ± 0.43 | 64.41–65.38 | 65.03 |
| Spinal cord | 6.92 ± 2.04 | 4.02–8.42 | 7.26 |
| Left carotid | 19.43 ± 1.90 | 17.45–22.30 | 19.24 |
| Right carotid | 20.05 ± 1.97 | 17.94–23.38 | 19.39 |
Abbreviations: IMRT = intensity-modulated radiotherapy; GTV = gross tumor volume; CTV = clinical target volume.
Values are generalized effective uniform dose.
Fig. 1.
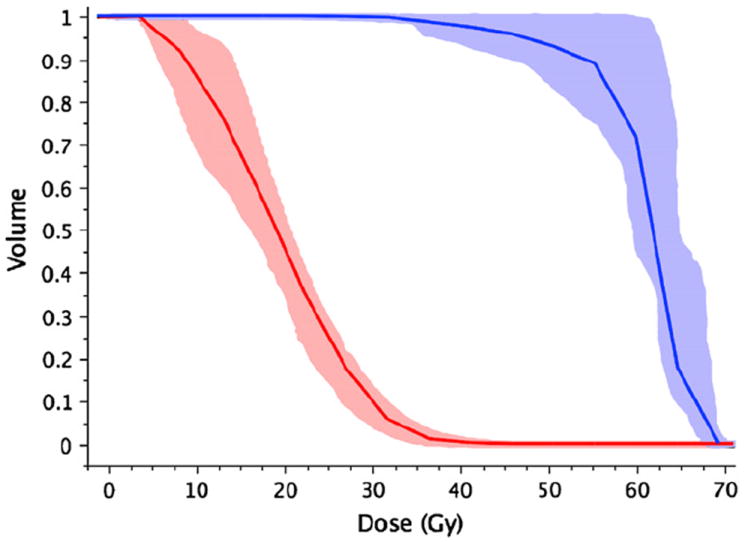
Composite carotid artery dose–volume histogram (n = 6) showing mean (solid line) and 95% confidence interval (shaded region) for virtual plans (red = intensity-modulated radiotherapy, blue = opposed laterals).
Fig. 2.
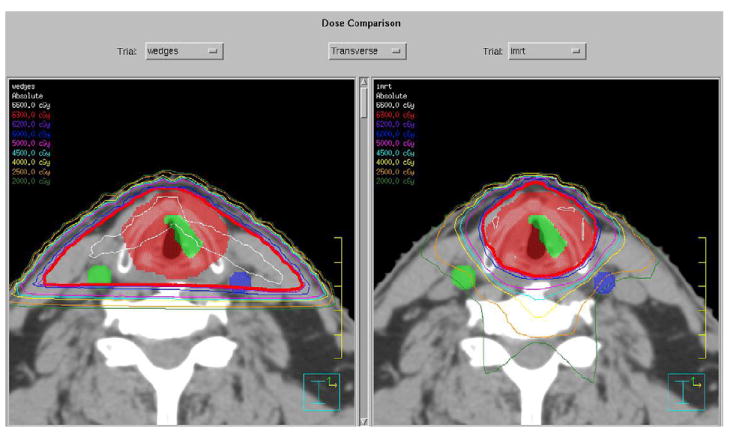
Stereotypic isodose plans for (a) lateral field setup and (b) intensity-modulated radiotherapy.
DISCUSSION
We developed an IMRT planning “algorithm” for early glottic cancer with limited beams and segments that reliably reduces carotid artery dose as compared with traditional lateral beam arrangements. This simplified planning has limited impact on resource use, and our early clinical experience is excellent. Despite the limited clinical experience and follow-up currently available, we believe that the important survivorship implications for carotid-sparing IMRT for early glottic cancer warrants introducing this new treatment approach now for academic discussion and further evaluation.
Intensity-modulated RT for T2 glottic cancer has previously been reported by Penagaricano et al. (40) in a pilot series of 3 patients showing reduction in dose to soft tissue and spinal cord. However, there are no previously published data on the effect of IMRT specifically for carotid dose reduction. The optimized 3D wedged oblique technique presented by Hardie et al. (41) to reduce arytenoids dose also reduces carotid dose; however, each carotid is at least in the path of the contralateral exit beam, leading to much higher carotid dose than can be achieved with IMRT, approximately half that of the dose prescribed to the CTV. Conceivably, there are additional strategies that might potentially reduce carotid dose without IMRT implementation. Radiation effects on the carotid arteries begin rather quickly after therapy and typically require several years to progress to clinical symptoms. Head-and-neck cancer RT patients in a small prospective series had a 21-fold increase in the thickness of the carotid wall at 1 year after therapy as compared with epidemiologically matched control cohorts (42). Cheng et al. (15) observed that the annualized progression rate from <50% to ≥50% stenosis in irradiated arteries was 15.4%, compared 4.8% in nonirradiated vessels, and noted time from RT (>6 years) as a significant risk factor. Relative conditional survival profiling for early glottic cancers demonstrates that laryngeal cancers, as a cohort, demonstrate a plateau in projected disease-specific mortality approximately 2 years after diagnosis (43).
Consequently, in early-stage larynx patients with expected disease-specific survival of >5 years, competing causes of mortality (including stroke and second cancers) will typically supersede the rate of recurrence for the index cancer.
Vascular event-probability modification is especially difficult given the typical concurrent health issues facing HNC patients, such as lifetime smoking history (7, 10, 13, 44, 45). Smoking cessation may reduce risks for vascular disease, as well as second primary cancers, and should be pursued with all patients. A dose–response relationship for carotid arterial effects raises the impetus to consider carotid artery radiation dose reduction when feasible (36). Surveillance vascular imaging has been recommended to detect and intervene for carotid lesions before clinical progression (13).
Given the tissue-sparing effects, IMRT has been increasingly adopted, especially for HNC (46). It is not infrequent for new technologies to be embraced for a variety of reasons before a benefit is proven, and sometimes the complexities of implementing new technology may undermine the potential gains as compared with more traditional techniques (47). To date, IMRT has been shown to increase recovery of parotid flow in patients with nasopharyngeal cancer in two randomized clinical trials (48, 49) and to be cost-effective only for prostate cancer (50).
Intensity-modulated RT planning for the vast majority of HNC is highly complex. It requires detailed knowledge of patterns of contiguous and lymphatic spread and the corresponding anatomy of tissue structures, dedicated time to delineate various target volumes (GTV, proper subclinical margins, draining nodal regions, all critical tissues to be spared), and logical specification of dose constraints to individual organs along with fractionation schemes with and without concurrent chemotherapy. Therefore, the professional component of the reimbursement is substantially undervalued despite the addition of special treatment procedure elements. An exception is the IMRT technique for the treatment of T1–2 glottic carcinoma as presented in this article, in which the workload is similar to that of conformal therapy and for which we do not bill differently.
Some have argued against the use of IMRT for early glottic cancer because there would be no practical way to prove benefit over standard therapy. Local control rates exceed 90% (51), and reported catastrophic complication rates are <1% (6). We espouse IMRT as a way to safely reduce the risk for late carotid events despite escalation of fractional dose (2 Gy to 2.25 Gy). There is also potential for using IMRT to reduce the dose to the arytenoids, cricopharyngeal inlet, and thyroid gland. A tradeoff, however, would be a small increase in dose to the spinal cord. There have been previous attempts to reduce potential acute treatment toxicity for early glottic cancer, primarily focusing on the reduction in arytenoid dose (52-54), and although this is also an important aim, there is not any published documented benefit to date.
Our clinical experience shows that IMRT optimization compensates for dosimetric shortcomings of 6-MV X-rays as compared with 60Co. Intensity-modulated RT planning gives the ability to direct more segments where required, which helps to reduce the penumbra effect to confine the effective field length to 4 cm for T1 cancers and may reduce the requirement for bolus for anteriorly located tumors, though dose modeling in the build-up region may require careful evaluation. Intensity-modulated RT additionally gives the potential for lateralizing therapy more precisely than lateral beam weighting. There is the potential for further dose escalation in the case of T2 tumors, for which control rates are still not optimal, but this notion requires further testing.
We launched a pilot trial based on the favorable outcome of the virtual dosimetry study, which confirmed the simplicity of planning and treatment delivery. The overall time in the treatment room is decreased, thereby improving patient throughput. We will continue to carefully monitor the incidence of cerebrovascular events in these patients. Our current approach treats the entire larynx, similar to the lateral technique. Future studies might serve to reduce dose further when appropriate to the arytenoids, the uninvolved contralateral cord, the cricopharyngeal inlet, and portions of the thyroid gland. Already, four-dimensional CT data have been reported from an investigation of the feasibility of single vocal cord radiation in early glottic cancer (55). Additional safe and effective small-volume dose escalation could reduce treatment duration. These refinements should be done in centers with excellent physics, dosimetry, and technical support and have implemented stringent quality control procedures.
In summary, limited-field and segmental IMRT techniques for the treatment of early glottic cancer significantly reduce unnecessary dose to the carotid arteries. The development of a template within the planning system allows plans to be generated, optimized, and delivered quickly. Given the high cure rate for early glottic cancer, these dosimetric advantages may be especially important for younger patients, those with extant carotid artery pathology, and those at risk for subsequent development of a metachronous second HNC. On the basis of these data, we are planning a prospective study to compare the conventional and IMRT radiation delivery approaches, specifically investigating posttreatment formation of carotid artery atherosclerotic plaques and clinical outcomes.
Fig. 3.
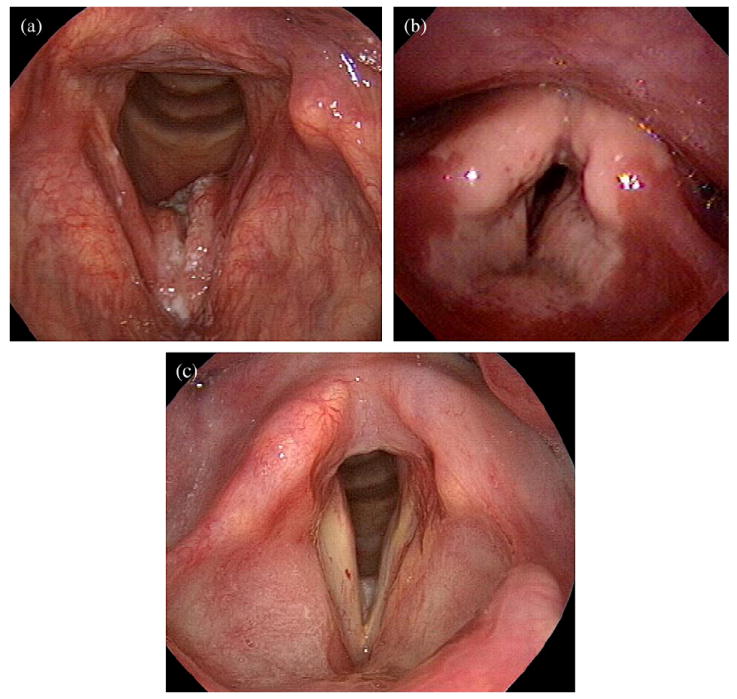
Pre- (a), during (b), and post–intensity-modulated radiotherapy (c) laryngoscopic examination, showing development and resolution of circular mucositis, in a distribution similar to the expected isodose volumes.
Acknowledgments
The authors thank Carl R. Bogardus, Jr., M.D., for his constructive comments.
C.D.F. is supported by a grant from the National Institutes of Health/National Institute of Biomedical Imaging and BioEngineering, “Multidisciplinary Training Program in Human Imaging” (5T32EB000817-04). D.L.S. is partially supported by the National Cancer Institute (CA132281). These funders had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest: none.
References
- 1.Halperin EC, Perez CA, Brady LW. Perez and Brady’s principles and practice of radiation oncology. 5. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 2.Chatani M, Matayoski Y, Masaki N, et al. Radiation therapy for early glottic carcinoma (T1N0M0). The final results of prospective randomized study concerning radiation field. Strahlenther Onkol. 1996;172:169–172. [PubMed] [Google Scholar]
- 3.Teshima T, Chatani M, Inoue T. Radiation therapy for early glottic cancer (T1N0M0): II. Prospective randomized study concerning radiation field. Int J Radiat Oncol Biol Phys. 1990;18:119–123. doi: 10.1016/0360-3016(90)90275-o. [DOI] [PubMed] [Google Scholar]
- 4.Teshima T, Chatani M, Hata K, et al. Radiation therapy of early glottic cancer (T1N0M0): Retrospective review of historical control. Rinsho Hoshasen. 1989;34:1603–1606. [PubMed] [Google Scholar]
- 5.Teshima T, Chatani M, Inoue T. Radiation therapy for early glottic cancer (T1N0M0): I. Results of conventional open field technique. Int J Radiat Oncol Biol Phys. 1989;17:1199–1202. doi: 10.1016/0360-3016(89)90526-9. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall WM, Amdur RJ, Morris CG, et al. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19:4029–4036. doi: 10.1200/JCO.2001.19.20.4029. [DOI] [PubMed] [Google Scholar]
- 7.So NM, Lam WW, Chook P, et al. Carotid intima-media thickness in patients with head and neck irradiation for the treatment of nasopharyngeal carcinoma. Clin Radiol. 2002;57:600–603. doi: 10.1053/crad.2001.0746. [DOI] [PubMed] [Google Scholar]
- 8.McGuirt WF, Feehs RS, Bond G, et al. Irradiation-induced atherosclerosis: A factor in therapeutic planning. Ann Otol Rhinol Laryngol. 1992;101:222–228. doi: 10.1177/000348949210100305. [DOI] [PubMed] [Google Scholar]
- 9.Feehs RS, McGuirt WF, Bond MG, et al. Irradiation. A significant risk factor for carotid atherosclerosis. Arch Otolaryngol Head Neck Surg. 1991;117:1135–1137. doi: 10.1001/archotol.1991.01870220083014. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SW, Wu LL, Ting AC, et al. Irradiation-induced extracranial carotid stenosis in patients with head and neck malignancies. Am J Surg. 1999;178:323–328. doi: 10.1016/s0002-9610(99)00184-1. [DOI] [PubMed] [Google Scholar]
- 11.Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282–288. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 12.Haynes JC, Machtay M, Weber RS, et al. Relative risk of stroke in head and neck carcinoma patients treated with external cervical irradiation. Laryngoscope. 2002;112:1883–1887. doi: 10.1097/00005537-200210000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Steele SR, Martin MJ, Mullenix PS, et al. Focused high-risk population screening for carotid arterial stenosis after radiation therapy for head and neck cancer. Am J Surg. 2004;187:594–598. doi: 10.1016/j.amjsurg.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Bilora F, Pietrogrande F, Petrobelli F, et al. Is radiation a risk factor for atherosclerosis? An echo-color Doppler study on Hodgkin and non-Hodgkin patients. Tumori. 2006;92:295–298. [PubMed] [Google Scholar]
- 15.Cheng SW, Ting AC, Ho P, et al. Accelerated progression of carotid stenosis in patients with previous external neck irradiation. J Vasc Surg. 2004;39:409–415. doi: 10.1016/j.jvs.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Call GK, Bray PF, Smoker WR, et al. Carotid thrombosis following neck irradiation. Int J Radiat Oncol Biol Phys. 1990;18:635–640. doi: 10.1016/0360-3016(90)90072-r. [DOI] [PubMed] [Google Scholar]
- 17.Freymiller EG, Sung EC, Friedlander AH. Detection of radiation-induced cervical atheromas by panoramic radiography. Oral Oncol. 2000;36:175–179. doi: 10.1016/s1368-8375(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 18.Loftus CM, Biller J, Hart MN, et al. Management of radiation-induced accelerated carotid atherosclerosis. Arch Neurol. 1987;44:711–714. doi: 10.1001/archneur.1987.00520190023011. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg GD, Britt RH, Goffinet DR. Radiation-induced carotid artery disease. Cancer. 1978;41:130–137. doi: 10.1002/1097-0142(197801)41:1<130::aid-cncr2820410121>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Chung TS, Yousem DM, Lexa FJ, et al. MRI of carotid angiopathy after therapeutic radiation. J Comput Assist Tomogr. 1994;18:533–538. doi: 10.1097/00004728-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26:5119–5125. doi: 10.1200/JCO.2008.16.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen EE, Rosine D, Haraf DJ, et al. Phase I trial of tirapazamine, cisplatin, and concurrent accelerated boost reirradiation in patients with recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;67:678–684. doi: 10.1016/j.ijrobp.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 23.De Crevoisier R, Bourhis J, Domenge C, et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol. 1998;16:3556–3562. doi: 10.1200/JCO.1998.16.11.3556. [DOI] [PubMed] [Google Scholar]
- 24.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: Results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25:4800–4805. doi: 10.1200/JCO.2006.07.9194. [DOI] [PubMed] [Google Scholar]
- 25.Salama JK, Vokes EE, Chmura SJ, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:382–391. doi: 10.1016/j.ijrobp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Cooper JS, Pajak TF, Rubin P, et al. Second malignancies in patients who have head and neck cancer: Incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989;17:449–456. doi: 10.1016/0360-3016(89)90094-1. [DOI] [PubMed] [Google Scholar]
- 27.Holland JM, Arsanjani A, Liem BJ, et al. Second malignancies in early stage laryngeal carcinoma patients treated with radiotherapy. J Laryngol Otol. 2002;116:190–193. doi: 10.1258/0022215021910500. [DOI] [PubMed] [Google Scholar]
- 28.Fujita M, Rudoltz MS, Canady DJ, et al. Second malignant neoplasia in patients with T1 glottic cancer treated with radiation. Laryngoscope. 1998;108:1853–1855. doi: 10.1097/00005537-199812000-00016. [DOI] [PubMed] [Google Scholar]
- 29.McDonald S, Haie C, Rubin P, et al. Second malignant tumors in patients with laryngeal carcinoma: Diagnosis, treatment, and prevention. Int J Radiat Oncol Biol Phys. 1989;17:457–465. doi: 10.1016/0360-3016(89)90095-3. [DOI] [PubMed] [Google Scholar]
- 30.Narayana A, Vaughan AT, Fisher SG, et al. Second primary tumors in laryngeal cancer: Results of long-term follow-up. Int J Radiat Oncol Biol Phys. 1998;42:557–562. doi: 10.1016/s0360-3016(98)00250-8. [DOI] [PubMed] [Google Scholar]
- 31.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 32.Lundgren J, Olofsson J. Multiple primary malignancies in patients treated for laryngeal carcinoma. J Otolaryngol. 1986;15:145–150. [PubMed] [Google Scholar]
- 33.Brown M. Second primaries in cases of cancer of the larynx. J Laryngol Otol. 1978;92:991–996. doi: 10.1017/s0022215100086412. [DOI] [PubMed] [Google Scholar]
- 34.Boice JD, Jr, Fraumeni JF., Jr Second cancer following cancer of the respiratory system in Connecticut, 1935-1982. Natl Cancer Inst Monogr. 1985;68:83–98. [PubMed] [Google Scholar]
- 35.de Vries N, Snow GB. Multiple primary tumours in laryngeal cancer. J Laryngol Otol. 1986;100:915–918. doi: 10.1017/s0022215100100313. [DOI] [PubMed] [Google Scholar]
- 36.Martin JD, Buckley AR, Graeb D, et al. Carotid artery stenosis in asymptomatic patients who have received unilateral head-and-neck irradiation. Int J Radiat Oncol Biol Phys. 2005;63:1197–1205. doi: 10.1016/j.ijrobp.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 38.Choi B, Deasy JO. The generalized equivalent uniform dose function as a basis for intensity-modulated treatment planning. Phys Med Biol. 2002;47:3579–3589. doi: 10.1088/0031-9155/47/20/302. [DOI] [PubMed] [Google Scholar]
- 39.Wu Q, Mohan R, Niemierko A, et al. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. Int J Radiat Oncol Biol Phys. 2002;52:224–235. doi: 10.1016/s0360-3016(01)02585-8. [DOI] [PubMed] [Google Scholar]
- 40.Penagaricano JA, Ratanatharathorn V, Papanikolaou N, et al. Intensity-modulated radiation therapy reduces the dose to normal tissue in T2N0M0 squamous cell carcinoma of the glottic larynx. Med Dosim. 2004;29:254–257. doi: 10.1016/j.meddos.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Hardie CL, McKenna A, Przeslak AJ, et al. Minimising carotid artery dose in the radiotherapy of early glottic cancer. Clin Oncol (R Coll Radiol) 2007;19:800. doi: 10.1016/j.clon.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Muzaffar K, Collins SL, Labropoulos N, et al. A prospective study of the effects of irradiation on the carotid artery. Laryngoscope. 2000;110:1811–1814. doi: 10.1097/00005537-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Fuller CD, Wang SJ, Thomas CR, Jr, et al. Conditional survival in head and neck squamous cell carcinoma: Results from the SEER dataset 1973-1998. Cancer. 2007;109:1331–1343. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 44.Abayomi OK. Neck irradiation, carotid injury and its consequences. Oral Oncol. 2004;40:872–878. doi: 10.1016/j.oraloncology.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Halak M, Fajer S, Ben-Meir H, et al. Neck irradiation: A risk factor for occlusive carotid artery disease. Eur J Vasc Endovasc Surg. 2002;23:299–302. doi: 10.1053/ejvs.2001.1555. [DOI] [PubMed] [Google Scholar]
- 46.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer. 2005;104:1296–1303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 47.Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:747–755. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 50.Konski A, Watkins-Bruner D, Feigenberg S, et al. Using decision analysis to determine the cost-effectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:408–415. doi: 10.1016/j.ijrobp.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 51.Feigenberg SJ, Lango M, Nicolaou N, et al. Intensity-modulated radiotherapy for early larynx cancer: Is there a role? Int J Radiat Oncol Biol Phys. 2007;68:2–3. doi: 10.1016/j.ijrobp.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Wang CC. Keeping the arytenoid dose low. Int J Radiat Oncol Biol Phys. 1990;18:1535. doi: 10.1016/0360-3016(90)90334-g. [DOI] [PubMed] [Google Scholar]
- 53.Zouhair A, Azria D, Coucke P, et al. Decreased local control following radiation therapy alone in early-stage glottic carcinoma with anterior commissure extension. Strahlenther Onkol. 2004;180:84–90. doi: 10.1007/s00066-004-1164-y. [DOI] [PubMed] [Google Scholar]
- 54.Allal AS, Miralbell R, Lehmann W, et al. Effect of arytenoid sparing during radiation therapy of early stage glottic carcinoma. Radiother Oncol. 1997;43:63–65. doi: 10.1016/s0167-8140(97)01903-8. [DOI] [PubMed] [Google Scholar]
- 55.Osman SO, de Boer HC, Heijmen BJ, et al. Four-dimensional CT analysis of vocal cords mobility for highly focused single vocal cord irradiation. Radiother Oncol. 2008;89:19–27. doi: 10.1016/j.radonc.2008.05.016. [DOI] [PubMed] [Google Scholar]


