Abstract
Dengue viruses, primarily transmitted by the mosquito Aedes aegypti (L.), affect an estimated 50–100 million people yearly. Traditional approaches to control mosquito population numbers, such as the use of pesticides, have had only limited success. Atypical mosquito behavior may be one reason why current vector control efforts have been less efficacious than expected. In Puerto Rico, for example, adult Ae. aegypti have been observed emerging from septic tanks. Interestingly, adults emerging from septic tanks are larger on average than adults collected from surface containers. To determine whether adults colonizing septic tanks constitute a separate Ae. aegypti population, we used 12 previously validated microsatellite loci to examine adult mosquitoes collected from both septic tanks and surface containers, but found no evidence to suggest genetic differentiation. Size differences between septic tank and surface mosquitoes were reduced when nutrient levels were held constant across experimental groups. Despite the absence of evidence suggesting a genetic difference between experimental groups in this study, Ae. aegypti emerging from septic tanks may still represent a more dangerous phenotype and should be given special consideration when developing vector control programs and designing public health interventions in the future.
Keywords: Aedes aegypti, Puerto Rico, septic tanks, microsatellites, body size
With ≈50 million infections annually (World Health Organization [WHO] 2009), dengue fever remains a serious public health concern worldwide. After several decades of absence in the United States, dengue fever cases have recently been reported in Hawaii, along the Texas-Mexican border, in the Florida Keys, and in the commonwealth of Puerto Rico (Malachi et al. 2009, Centers for Disease Control and Prevention [CDC] 2010). Currently, >40% of the global population remains at risk of infection (WHO 2009). Furthermore, production of a vaccine has been complicated by the presence of multiple viral serotypes (Durbin and Whitehead 2010), while other prevention strategies, such as the use of bed nets, have been relatively ineffective because of the daytime feeding pattern of the principal mosquito vector, Aedes aegypti (L.). Other traditional control measures, such as insecticide spraying and source reduction, have also had limited success in reducing dengue prevalence in many areas (Ballenger-Browning and Elder 2009). Given these limitations, vector control measures based on inexpensive and reliable entomological surveillance are needed to improve efficacy of these programs in the future.
Relaxation of vector control programs in many regions globally has allowed the reemergence of Ae. aegypti populations in several countries where eradication efforts previously experienced some success. In regions where vector control practices are still actively used, questions remain regarding the efficacy of certain vector control efforts and the efficient allocation of limited resources (Ballenger-Browning and Elder 2009, Farrar et al. 2007).
Studies in Puerto Rico for example have suggested that the treatment of above ground water sources alone, either by insecticides or draining of containers, may be ineffective in controlling mosquito populations. The number of adults resting indoors within close proximity to humans was not significantly reduced after the use of these vector control practices, and may have been because of the colonization of underground septic tanks by Ae. aegypti (Barrera et al. 2008). These waste tanks are common throughout many residential areas in Puerto Rico. A study in 2005 within the southern Puerto Rican community of Playa-Playita estimated these septic tanks were capable of producing >18,000 Ae. aegypti adults per day (Barrera et al. 2008): well above the calculated density required to maintain an epidemic (Focks et al. 2000). In Playa-Playita, both larval presence and larval abundance in septic tanks were positively associated with uncovered access ports and other structural defects such as cracked walls (Burke et al. 2010).
The presence of Ae. aegypti in septic tanks is an interesting discovery, but the mechanism(s) influencing habitat selection remains unclear. Historically, Ae. aegypti mosquitoes have been thought to prefer clean water sources for oviposition and development, avoiding water contaminated with sewage (Christophers 1960, Burke et al. 2010). One potential explanation is that these mosquitoes may actually represent a distinct Ae. aegypti population, differing from other mosquito populations that prefer surface containers.
Pope and Wood (1981) have previously suggested genetics as a mechanism that may account for dissimilarities in resistance to different pollutant concentrations observed among some Ae. aegypti strains. These differences may ultimately facilitate the exploitation of new environmental niches, limiting competition for resources between other populations. In addition, previous studies in Kenya have shown that certain indoor-breeding Ae. aegypti populations are genetically distinct from their outdoor breeding counterparts despite being located within the same geographic locality (Trpis and Hausermann 1975, 1978; Scott and McClelland 1975; Tabachnick et al. 1979; Brown et al. 2011). Further evidence of microgeographic genetic differentiation among populations can be found in Phnom Penh, Cambodia where researchers restricted collection of Ae. aegypti larval and pupae samples to a single street. Not only were populations highly differentiated, but the pattern of genetic differentiation was dependent on the type of breeding site sampled, with the greatest level of differentiation occurring among samples collected from discarded containers (Paupy et al. 2004). Additional examples of microgeographic genetic differentiation have also been observed in Chiang Mai, Thailand (Mousson et al. 2002) and in Ho Chi Minh City, Vietnam (Huber et al. 2002), among other locations. Therefore, we were interested in determining whether genetic differences exist between Ae. aegypti mosquitoes colonizing septic tanks and those preferring surface containers in Puerto Rico. We sought to address this question by examining surface container and septic tank adults for genetic differences using 12 previously validated microsatellite loci.
Interestingly, size differences have also been noted among adult Ae. aegypti mosquitoes emerging from septic tanks and adults emerging from surface containers in Salinas, Puerto Rico (Mackay et al. 2009). The public health implications of this finding are important as adult mosquito size may influence a number characteristics related to vectorial capacity (Nasci 1986, Briegel 1990, Sumanochitrapon et al. 1998, Schneider et al. 2004).
Currently, it remains unclear whether adult mosquitoes emerging from septic tanks in other communities exhibit a similar trend toward increasing size comparable to that observed in Salinas (Mackay et al. 2009), and whether nutrient availability alone could be responsible for observed differences noted in previous studies. We sought to address this question by rearing the F1 generation to adulthood under constant laboratory conditions.
Materials and Methods
Site Selection
Patillas is a municipality located on the southern coast of Puerto Rico characterized by seasonal rainfall and a dry season that extends between December and April of each year (Fig. 1). The communities of Jagual and Centro, located within the municipality of Patillas, were selected as study locations based on differences in waste disposal infrastructure. Jagual relies solely on residential septic tanks as a means of facilitating disposal. Furthermore, surveillance efforts had previously confirmed the existence of Ae. aegypti adults in some septic tanks (R. Barrera, unpublished data). In contrast, Centro is a more urban community in which residential housing sections use a sewage system for waste disposal. Centro is free of any septic tank structures, but is otherwise similar to Jagual in terms of rainfall and other environmental factors. The two communities are located ≈2.5 km apart from one another.
Fig. 1.

Map of the study area within Puerto Rico.
Surveys were conducted at selected houses within each community between May and June of 2010. Well over 15 containers were sampled in each location. Within Jagual, residences were excluded if the septic tank was not accessible, lacked standing water, or if the owner could not be contacted or did not wish to participate in the study. Verbal informed consent was obtained at each house from a person aged 18 or older before conducting each survey. In Jagual, a total of three septic tanks were located that produced Ae. aegypti. Each was sampled and adults were combined for analysis. All septic tanks were within 1–2 km of each other.
Adult Sampling in Septic Tanks
After identifying candidate septic tanks, cracks were sealed in both the walls and ceiling of the structure using a commercially available polyurethane foam spray. Large openings were covered with a plastic lid and sealed with foam spray. Finally, vent pipes were screened to eliminate any potential uncontrolled entry or exit points from septic tanks. To ensure emergent adults actually developed in the septic tank and had not entered the tank before collection, we used an insecticide strip to first eliminate all adult mosquitoes residing within the structure. A commercially available 2,2-dichlorous impregnated strip (trade name: HotShot, Madison, WI) was suspended in the septic tank above the liquid and left in place for 24 h (Burke et al. 2010). After removal of the impregnated strip, previously constructed emergent traps were secured in place over the tank opening (Barrera et al. 2008). Traps were constructed from 3.8 liter plastic containers using a screen material to seal the open end and a fabric sleeve to cover a large diameter hole cut into the middle of the container wall. The fabric sleeve was placed either over the septic tank vent, or over a large inverted funnel that had been secured in place over an alternative septic tank opening. Traps were recovered and replaced daily until ≈150 adults were collected, ensuring adequate power for detecting differences during microsatellite analysis. Collection of Ae. aegypti was confirmed through visual identification. Adults were then transferred to large colony cages and fed a 10% sucrose solution. Adults were allowed to feed once through a membrane on swine blood and were kept until they laid eggs. Eggs were collected and dried for later use. Adults were killed by placing them in a −20°C freezer for 30 min. Wings were removed for measurement and carcasses were preserved in 80% ethanol for later DNA extraction.
Pupal Sampling
Pupae were collected from multiple container types with standing water from within both community sites. Types of containers sampled included plant containers, tires, discarded containers, water storage containers, buckets, jars, and flower vases. Because feeding is discontinued when Ae. aegypti reach the pupal stage of development, collecting at this point minimized the developmental impacts on size that could have occurred if earlier larval stages were sampled and transferred to a laboratory setting where nutrient levels would likely not represent native habitats. Sampling of pupae also meant eliminating bias by preventing the cross contamination of sampling locations. Identification was confirmed using standard dichotomous keys (CDC, unpublished data). Pupae were placed into plastic containers filled with tap water inside large colony cages and allowed to emerge as adults. Ae. aegypti adults were fed a 10% sucrose solution, blood fed once, and allowed to deposit eggs. Eggs were collected on paper strips, dried and stored for later use. Again, adults were killed by placing them in a −20°C freezer for 30 min. Wings were removed for measurement and carcasses were preserved in 80% ethanol for later DNA extraction.
Lab Rearing of F1 Generation
Plastic trays of uniform size were sterilized by soaking with diluted bleach, thoroughly rinsing, and placing in a UV cross-linker for 10 min. Plastic trays were housed in an insectary and the temperature was maintained at 25°C with a relative humidity of 54%. A total of 500 ml of deoxygenated tap water was added to each of the trays. Strips containing the eggs deposited by field collected Ae. aegypti adults were placed into plastic containers and allowed to hatch. Containers were loosely covered with a plastic lid to prevent any contamination by falling debris. Egg strips were removed 24 h later and first instar densities were thinned to ≈90 individual mosquitoes per plastic container. Upon hatching, 100 mg of ground fish flakes were added to each container. Additional food and water was added to all containers equally either daily or every other day as needed. Mosquitoes were allowed to develop to 2-d old adults before being killed and preserved as described previously. Wings were removed for measurement purposes.
Wing Measurements
Wing length was used as an estimate of body size (Powell et al. 2009) and was obtained by measuring from the humeral cross vein to the wing tip, excluding fringe scales, using a Zeiss (Jena, Germany) AxioCam HRc live camera attached to a computer with AxioVision software. Wing length was first measured in pixels and then converted into absolute length (millimeters) for comparison (Powell et al. 2009). A total of between 86 and 122 female and between 6 and 43 male wing measurements were taken from each of the experimental groups collected in the Jagual and Centro communities. Additionally, a total of 41–54 female and 15–28 male wing measurements were taken from each of the laboratory reared experimental groups.
Genetic Methods
Whole body genomic DNA was extracted individually from adult mosquitoes from each comparison group [Jagual septic tanks (N = 140), Jagual surface containers (N = 138), and Centro surface containers (N = 103)] using DNeasy kits (Qiagen Inc., Valencia, CA) following the protocol provided by the manufacturer. Individual genotypes were scored at 12 previously published microsatellite loci. Detailed information regarding the development and screening of these loci are provided elsewhere (Brown et al. 2011).
Microsatellite loci were paired for multiplex reactions based on nonoverlapping size ranges. Each pair was amplified in a multiplex polymerase chain reaction (PCR) using a single fluorescent M13 primer, two forward primers containing M13 tails, and two reverse primers (Boutin-Ganache et al. 2001, Brown et al. 2011). Total volume of PCR reactions was 10 μl and contained 1× Type-it Multiplex PCR Master Mix (Qiagen), 25 nM of each forward primer, 250 nM of each reverse primer, and 500 nM of a fluorescently labeled M13 primer. Thermocycling conditions were established following those outlined in Slotman et al. (2007). PCR products were subsequently run on an Applied Biosystems 3730×l DNA Genetic Analyzer with a GS 500 Rox internal size standard available from Applied Biosystems (Carlsbad, CA). Genotype calls were automated using GeneMapper software and visually checked for errors. Additional microsatellite primer information can be found in Brown et al. (2011).
Analyses
A one-way analysis of variance (ANOVA) adjusted for multiple comparisons using the Tukey-Kramer method was used to compare wing length between groups for both field collected and lab reared generations.
Overall genetic differentiation among mosquito populations was assessed by calculating mean Fst values for all population pairs using Arlequin 3.5 software (Excoffier et al. 2010). Average allelic richness per locus was calculated for each population using HP-RARE (Kalinowski 2004, 2005). All microsatellite loci were tested for within-population deviations from Hardy-Weinberg equilibrium using the Web-version of the software GENEPOP (Raymond and Rousset 1995, Rousset 2008). Markov chain parameters were set at 1,000 dememorizations, 100 batches, and 1,000 iterations per batch. GENEPOP was also used to calculate allele frequencies for all loci across populations.
We evaluated patterns of population structure using the Bayesian clustering method implemented in the software program STRUCTURE v2.3 (Pritchard et al. 2000). STRUCTURE identifies genetic clusters and assigns all individuals to these clusters without any a priori information regarding sampling locations. The most likely number of clusters (K) was determined by conducting independent runs for each K = 1–5. For all runs we assumed an admixture model and correlated allele frequencies, and used a burn-in value of 100,000 iterations followed by 500,000 replications. The most likely number of clusters was determined following the guidelines in Pritchard et al. (2000). STRUCTURE results were visualized using the program DISTRUCT (Rosenberg 2004).
Finally, we assessed relatedness among the Puerto Rican populations studied here and other global populations (see Brown et al. 2011) using a nonmetric multidimensional scaling analysis based on the pairwise Fst values between populations (Hammer et al. 2001). We compared the Puerto Rican samples to those from Mexico (Pijijiapan), Texas (Houston), Dominica, two sites in Venezuela (Bolivar and Zulia), two sites in Thailand (Rayong and Prachuabkhirikan), French Polynesia (Tahiti), and three locations in Florida (Vaca Key, Conch Key, and Palm Beach County). See Brown et al. (2011) for data on these populations.
Results
Size of Emerging Mosquitoes From Septic Tanks and Surface Containers
For both females and males, average wing length did not statistically differ between adult Ae. aegypti emerging from septic tanks in Jagual and those originating from surface containers in Centro (Tukey-Kramer MSD > 0.002 (nonsignificant); Tukey-Kramer MSD > 0.194 (nonsignificant)) (Fig. 2). However, average wing length did statistically differ between experimental groups in Jagual (septic tank and surface container) for both female and male adult Ae. aegypti (Tukey-Kramer MSD < 0.195 (significant); Tukey-Kramer MSD < 0.188 (significant)), with septic tank adults having longer wing lengths than surface container adults on average (Fig. 2). Finally, average wing length statistically differed between mosquitoes originating from surface containers in Jagual and Centro in both female and male adult Ae. aegypti(Tukey-Kramer MSD < 0.193 (significant); Tukey-Kramer MSD < 0.382 (significant)). Wing length of adults originating from surface containers in Centro on average was larger than wing length of adults originating from surface containers in Jagual (Fig. 2). Among the field-collected mosquitoes of both sexes, there is notably less variation in wing length within the septic tank group than in the surface container collections from both localities.
Fig. 2.
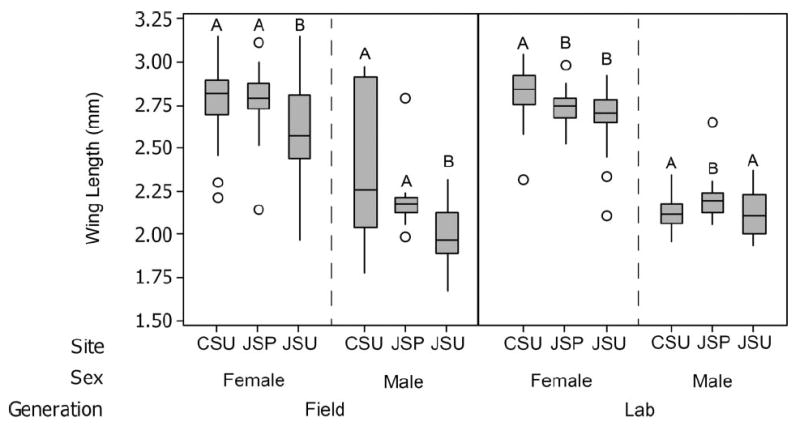
Wing length of adult Ae. aegypti from Centro surface containers (CSU), Jagual septic tanks (JSP), and Jagual surface containers (JSU). Field groups indicate mosquitoes taken straight from the field, while lab groups include adult Ae. aegypti reared under laboratory controlled conditions from eggs collected from the study sites. Box plots identify medians and quartiles, with whiskers representing the fifth and 95th percentiles. Outliers are indicated with open circles. Plots with the same letter are not statistically different from each other using one-way ANOVA (α = 0.05). Significance was analyzed separately within each group (i.e., between sites/container type, but within sex and generation).
Size of F1 Generation Mosquitoes Reared Under Laboratory Conditions
When F1 mosquitoes were hatched and reared to adulthood in a controlled laboratory environment in which nutrient levels were kept the same between all experimental groups, both female and male differences in average wing length between groups were reduced. However, statistically significant differences still remained between some experimental groups (Fig. 2). On average, wing lengths of females descended from surface containers in Centro and males descended from septic tanks in Jagual were larger compared with other experimental groups. As demonstrated previously in this species (Schneider et al. 2011), variability in body size was greatly reduced in laboratory-reared mosquitoes as compared with those from the field (Fig. 2).
Genetic Analysis of Emerging Mosquitoes From Septic Tanks and Surface Containers
Allele frequency data for the 12 microsatellites in the Patillas collections appear online as supporting information (Supplemental Table S1 available online only). Mean pairwise Fst values showed that experimental groups differed (Table 1). However, because Fst values as high as 0.05 generally indicate negligible genetic differences (Olshen et al. 2008), and given that the average population pairwise Fst value for this subspecies in a previous study was 0.20 (Brown et al. 2011), we concluded that the differences observed in this study were not biologically meaningful and these experimental groups did not represent genetically separate Ae. aegypti populations. The Bayesian analysis of worldwide population structure indicated that the most likely number of clusters was one. All three experimental groups could not cleanly be separated into more than a single cluster (Fig. 3), indicating that mixing between groups was occurring.
Table 1.
Fst values between populations
| Jagual surface containers | Centro surface containers | |
|---|---|---|
| Jagual surface containers | — | 0.018 |
| Jagual septic tanks | 0.00733 | 0.01753 |
Values did not reach biological significance between experimental groups.
Fig. 3.

STRUCTURE bar plots. Each vertical bar depicted represents a single individual. The height of each color represents the probability of assignment to that cluster. Attempts were made to assign all sampled mosquitoes in all populations to K = 2 populations. However, no structure was detected for K = 2 populations and higher.
Average allelic richness per locus was 5.33 in Jagual surface container mosquitoes, 5.08 in Jagual septic tanks, and 5.67 in Centro surface containers. Overall, these values are slightly lower than the average of 7.46 across the subspecies (Brown et al. 2011). Five of 36 population-by-locus specific FIS values deviated significantly from Hardy Weinberg expectations after sequential Bonferonni correction (Supplemental Table S2 available online only).
Comparison of the Patillas populations to other Ae. aegypti populations using a nonmetric multidimensional scaling analysis based on the pairwise Fst values between populations revealed that these populations were most genetically similar to three mosquito populations in Florida that have previously been analyzed (Fig. 4).
Fig. 4.

Nonmetric multidimensional scaling analysis based on pairwise Fst values between populations.
Discussion
This study sought to contribute to the current knowledge surrounding an important vector mosquito, Ae. aegypti, in two ways. First, we examined Ae. aegypti mosquitoes collected from both subterranean septic tanks and surface containers for potential genetic differences using 12 previously validated microsatellite loci. Second, we examined and confirmed adult mosquito size differences between septic tank and surface containers. The absence of a clear genetic component demonstrated these size differences were largely because of the differential availability of nutrients. Size differences between septic tank and surface mosquitoes were reduced when nutrient levels were held constant across experimental groups. The importance of nutrients was further supported by the reduced variation in body size seen in septic tank mosquitoes from the field. These findings have important implications for public health and vector control, as increased adult mosquito size may influence susceptibility to dengue viral infection (Sumanochitrapon et al. 1998) and may increase reproductive capabilities as well as enhance a number of other characteristics related to vectorial capacity, such as longevity (Briegel 1990).
As reported previously, small though statistically significant differences in adult wing length were noted between field collected experimental groups in Jagual. These findings support previous reports showing that Ae. aegypti adults emerging from septic tanks are larger than adults originating from surface container habitats on average (Mackay et al. 2009). Interestingly, however, are our experimental results showing that adults from septic tanks in Jagual and surface containers in Centro did not statistically differ in wing length. Why adult size differences were not observed between these two experimental groups remains unclear. However, it is possible that environmental conditions such as temperature and average nutritional content of larval breeding sites, the two most important environmental variables affecting holometabolous insect size, may have differed slightly between sampling locations and therefore could account for the experimental results described here (Barrera et al. 2006). Finally, when variation in nutrient levels between experimental groups was eliminated, differences in wing length between groups were largely reduced though not entirely eliminated. Size differences within these experimental groups appear too complex to be explained by nutrient availability alone.
Previous studies have shown genetic differences can exist between Ae. aegypti populations in close geographical proximity (Tabachnick et al. 1979, Paupy et al. 2004). Given this historical precedence, we considered whether a similar phenomenon was occurring in Puerto Rico, where differences in Ae. aegypti habitat selections have been noted. However, our findings indicate that gene flow is currently unrestricted between mosquitoes emerging from septic tanks and surface containers, or that not enough generations have passed for population genetic differences to emerge. Therefore, we currently do not consider these experimental groups as genetically distinct sympatric forms.
The size differences in adult mosquitoes emerging from septic tanks previously noted in Salinas, Puerto Rico (Mackay et al. 2009), and now here within the municipality of Patillas, is an interesting finding with important public health implications. Mosquito size has been shown to be related to a number of factors that may impact vectorial capacity including longevity, feeding frequency, reproductive capability, flight potential, and susceptibility to some viral infections (Nasci 1986, Briegel 1990, Sumanochitrapon et al. 1998, Schneider et al. 2004).
Studies examining the metabolic relationship between body size and reproductive fitness have shown that blood consumption by larger female Ae. aegypti mosquitoes was more than two times that of smaller mosquitoes, while fecundity of the larger mosquitoes increased by approximately a four-fold margin (Briegel 1990). Equally interesting were findings suggesting larger females also completed the gonotrophic cycle more rapidly compared with smaller adults, despite ingesting equal blood volumes (Briegel 1990). Taken together, these results indicate the potential for accelerated rates of reproduction and increased population numbers among larger Ae. aegypti mosquitoes, which could ultimately impact dengue virus transmission cycles. Research has also suggested an increased susceptibility to viral infections among larger adult mosquitoes. In one study examining the effect of size on oral infection, larger adult mosquitoes were approximately two times more susceptible to oral infection with the dengue-2 serotype compared with both small and medium sized adults (Sumanochitrapon et al. 1998, although see Alto et al. (2008) for a counter example.). Finally larger female adult mosquitoes with greater energy stores at eclosion have demonstrated a greater flight potential compared with smaller adults, suggesting a greater potential to contact a larger number of hosts (Nasci 1986, Briegel 1990).
Despite the absence of a genetic component in this study, Ae. aegypti mosquitoes emerging from septic tanks may still represent a more dangerous phenotype. In addition to producing larger adults on average, septic tanks have also been shown to enhance the production of mosquitoes to between three and nine times greater than that observed in other surface containers (Mackay et al. 2009). Furthermore, septic tanks may provide a sustainable habitat for year-round survival and proliferation. Given these observations, understanding and recognizing the importance of septic tanks and other similar structures on the development and productivity of these mosquitoes becomes critical in insuring the continued health of the communities in which these structures are still in use.
Finally, it has been suggested that the efficiency of current vector control methods could be improved by targeting control measures specifically toward mosquito populations most likely to transmit the pathogen (Sumanochitrapon et al. 1998). Because of the demonstrated potential for producing larger adult mosquitoes and greater population numbers, septic tanks should be given special consideration when designing control strategies and implementing public health programs in areas where these structures are still used. The above ground use of pesticides alone may be ineffective at controlling population size or limiting the incidence of disease. Septic tanks make a clear target, and focusing vector control efforts on these structures would have the additional benefit of impacting Culex populations in the area (Mackey et al. 2009). Further research related to improving vector control strategies is warranted, and should consider the impact of septic tanks and other similar subterranean structures on program design.
Supplementary Material
Acknowledgments
We would like to acknowledge Manuel Amador, Jesus Flores, Jose Gonzalez, and Orlando Gonzalez for the many hours of field support provided. We also thank Maria Diuk-Wasser for the helpful suggestions and assistance given. Finally, we thank the residents of the municipality of Patillas for their hospitality and cooperation while conducting this study. This research was supported by a Yale Global Health Initiative Field Experience Award (G.S.), NIH predoctoral Genetics training grant T32 GM007499 (J.E.B.), Yale Institute for Biospheric Studies (YIBS), Center for Field Ecology pilot grant (J.E.B.), and by grant (RO1) AI046018 from the National Institutes of Health (J.R.P.).
References Cited
- Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg. 2008;79:688–695. [PMC free article] [PubMed] [Google Scholar]
- Ballenger-Browning K, Elder J. Multi-modal Aedes aegypti mosquito reduction interventions and dengue fever prevention. Trop Med Int Health. 2009;14:1542–1551. doi: 10.1111/j.1365-3156.2009.02396.x. [DOI] [PubMed] [Google Scholar]
- Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Barrera R, Amador M, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol. 2008;22:62–69. doi: 10.1111/j.1365-2915.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- Boutin-Ganache I, Raposo M, Raymond M, Deschepper C. M13-tailed primers improve the readability and usability of microsatellite analyses performed with two different allele-sizing methods. Biotechniques. 2001;31:24–26. [PubMed] [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol. 1990;36:165–172. [Google Scholar]
- Brown JE, McBride CS, Johnson P, Ritchie S, Paupy C, Bossin H, Lutomiah J, Fernandez-Salas I, Ponlawat A, Cornel AJ, et al. Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proc R Soc B. 2011 doi: 10.1098/rspb.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R, Barrera R, Lewis M, Kluchinsky T, Claborn D. Septic tanks as larval habitats for Aedes aegypti and Culex quinquefasciatus in Playa-Playita, Puerto Rico. Med Vet Entomol. 2010;24:117–123. doi: 10.1111/j.1365-2915.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- (CDC) Centers for Disease Control and Prevention. Locally acquired Dengue, Key West, Florida, 2009–2010. MMWR. 2010;59:577–581. [PubMed] [Google Scholar]
- Christophers S. Aedes aegypti (L): the yellow fever mosquito. Cambridge University Press; New York: 1960. [Google Scholar]
- Durbin AP, Whitehead SS. Dengue vaccine candidates in development. Curr Top Microbiol. 2010;338:129–143. doi: 10.1007/978-3-642-02215-9_10. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer H. Arlequin suite version 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Farrar J, Frocks D, Gubler D, Barrera R, Guzman MG, Simmons C, Kalayanarooj S, Lum L, McCall PJ, Lloyd L, et al. Towards a global dengue research agenda. Trop Med Int Health. 2007;12:695–699. doi: 10.1111/j.1365-3156.2007.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focks D, Brenner R, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg. 2000;62:11–18. [PubMed] [Google Scholar]
- Hammer O, Harper D, Ryan P. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9. [Google Scholar]
- Huber K, Le Loan L, Hoang TH, Ravel S, Rodhain F, Failloux AB. Genetic differentiation of the dengue vector, Aedes aegypti (Ho Chi Minh City, Vietnam) using microsatellite markers. Mol Ecol. 2002;11:1629–1635. doi: 10.1046/j.1365-294x.2002.01555.x. [DOI] [PubMed] [Google Scholar]
- Kalinowski ST. Counting alleles with rarefaction: private alleles and hierarchical sampling designs. Conserv Genet. 2004;5:539–543. [Google Scholar]
- Kalinowski ST. HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes. 2005;5:187–189. [Google Scholar]
- Mackay A, Amador M, Diaz A, Smith J, Barrera R. Dynamics of Aedes aegypti and Culex quinquefasciatus in septic tanks. J Am Mosq Control Assoc. 2009;25:409–416. doi: 10.2987/09-5888.1. [DOI] [PubMed] [Google Scholar]
- Malachi C, Shetty A. Imported Dengue fever. Pediatr Emerg Care. 2009;25:769–772. doi: 10.1097/PEC.0b013e3181bec8c7. [DOI] [PubMed] [Google Scholar]
- Mousson L, Vazeille M, Chawprom S, Prajakwong S, Rodhain F, Failloux AB. Genetic structure of Aedes aegypti populations in Chiang Mai (Thailand) and relation with dengue transmission. Trop Med Int Health. 2002;7:865–872. doi: 10.1046/j.1365-3156.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- Nasci R. The size of emerging and host-seeking Aedes aegypti and the relation of size to blood-feeding success in the field. J Am Mosq Control Assoc. 1986;2:61–62. [PubMed] [Google Scholar]
- Olshen A, Gold B, Lohmueller KE, Struewing JP, Satagopan J, Stefanov SA, Eskin E, Kirchhoff T, Lautenberger JA, Klein RJ, et al. Analysis of genetic variation in Ashkenazi Jews by high density SNP genotyping. BMC Genet. 2008;9:14. doi: 10.1186/1471-2156-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupy C, Ngan C, Huber K, Lecoz N, Reynes J, Rodhain F, Failloux A. Influence of breeding sites features on genetic differentiation of Aedes aegypti populations analyzed on a local scale in Phnom Penh municipality of Cambodia. Am J Trop Med Hyg. 2004;71:73–81. [PubMed] [Google Scholar]
- Pope V, Wood J. Tolerance of Aedes aegypti larvae to synthetic sewage. Mosq News. 1981;4:732–736. [Google Scholar]
- Powell A, Davis M, Powell J. Phenotypic plasticity across 50 MY of evolution: Drosophila wing size and temperature. J Insect Physiol. 2009;56:380–382. doi: 10.1016/j.jinsphys.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Rousset F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. Heredity. 1995;86:248–249. [Google Scholar]
- Rosenberg NA. DISTRUCT: a program for the graphical display of population structure. Mol Ecol Notes. 2004;4:137–138. [Google Scholar]
- Rousset F. GENEPOP’ 007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Res. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- Schneider J, Morrison A, Astete H, Scott T, Wilson M. Adult size and distribution of Aedes aegypti (Diptera: Culicidae) associated with larval habitats in Iquitos, Peru. J Med Entomol. 2004;41:634–642. doi: 10.1603/0022-2585-41.4.634. [DOI] [PubMed] [Google Scholar]
- Schneider JR, Chadee DD, Mori A, Romero-Severson J, Severson DW. Heritability and adaptive phenotypic plasticity of adult body size in the mosquito Aedes aegypti with implications for dengue vector competence. Infect Genet Evol. 2011;11:11–16. doi: 10.1016/j.meegid.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, McClelland GAH. Electrophoretic differences between sympatric ecotypes. Nature. 1975;256:405–406. doi: 10.1038/256405a0. [DOI] [PubMed] [Google Scholar]
- Slotman M, Kelly N, Harrington L, Kitthawee S, Jones J, Scott T, Caccone A, Powell J. Polymorphic microsatellite markers for studies of Aedes aegypti (Diptera: Culicidae), the vector of dengue and yellow fever. Mol Ecol Notes. 2007;7:168–171. [Google Scholar]
- Sumanochitrapon W, Strickman D, Sithiprasasna R, Kittayapong P, Innis B. Effect of size and geographic origin of Aedes aegypti on oral infection with dengue-2 virus. Am J Trop Med Hyg. 1998;58:283–286. doi: 10.4269/ajtmh.1998.58.283. [DOI] [PubMed] [Google Scholar]
- Tabachnick W, Munstermann L, Powell J. Genetic distinctness of sympatric forms of Aedes aegypti in East Africa. Evolution. 1979;33:287–295. doi: 10.1111/j.1558-5646.1979.tb04682.x. [DOI] [PubMed] [Google Scholar]
- Trpis M, Hausermann W. Demonstration of differential domesticity of Aedes aegypti (L.) (Diptera, Culicidae) in Africa by mark–release–recapture. B Entomol Res. 1975;65:199–208. [Google Scholar]
- Trpis M, Hausermann W. Genetics of house-entering behaviour in East African populations of Aedes aegypti (L.) (Diptera: Culicidae) and its relevance to speciation. B Entomol Res. 1978;68:521–532. [Google Scholar]
- (WHO) World Health Organization. Dengue and dengue hemorrhagic fever. WHO Fact Sheet No. 117. 2009 http://www.who.int/mediacentre/factsheets/fs117/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


